Abstract
Tumor necrosis factor (TNF) has pleiotropic effects on a wide variety of cell types. In vitro studies have demonstrated that TNF has antiviral properties and is induced in response to viral infections. However, a role for TNF in the antiviral immune response of the host has yet to be demonstrated. Here we describe the construction of and studies using a recombinant vaccinia virus that encodes the gene for murine TNF-alpha. By comparing the replication of and immune responses elicited by the TNF-encoding virus to a similarly constructed control virus, we hoped to observe immunobiological effects of TNF in the host. The in vivo experiments with this recombinant virus demonstrate that the localized production of TNF-alpha during a viral infection leads to the rapid and efficient clearance of the virus in normal mice and attenuates the otherwise lethal pathogenicity of the virus in immunodeficient animals. This attenuation occurs early in the infection (by postinfection hour 24) and is not due to the enhancement of cellular or antibody responses by the vaccinia virus-encoded TNF. This evidence suggests that attenuation of the recombinant virus is due to a direct antiviral effect of TNF on cells at the site of infection. Therefore, these results support the suggestion that TNF produced by immune cells may be an important effector mechanism of viral clearance in vivo.
Full text
PDF
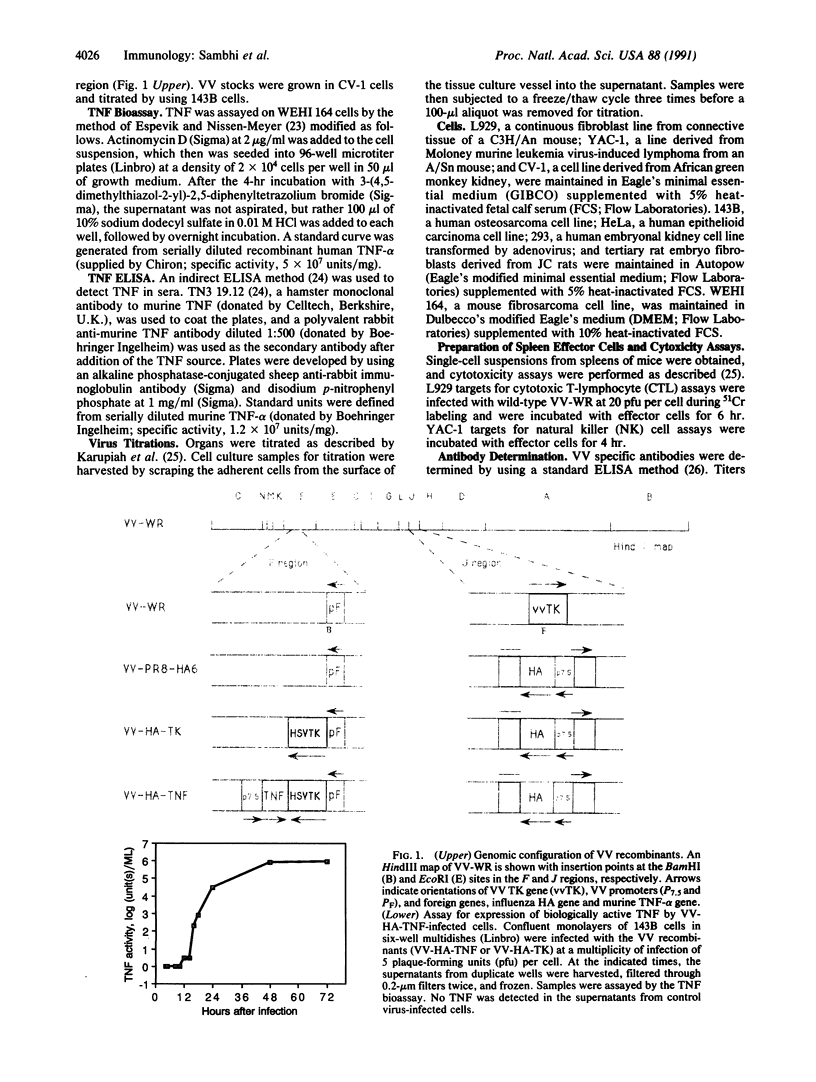
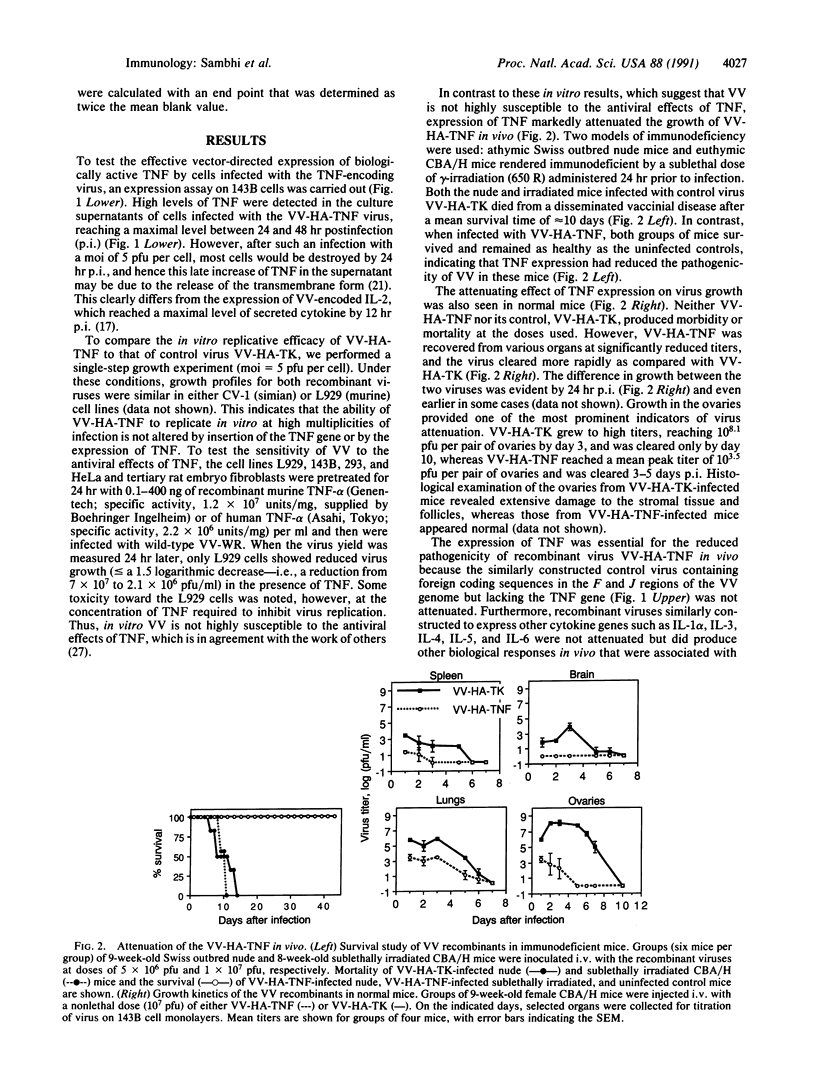


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Aderka D., Novick D., Hahn T., Fischer D. G., Wallach D. Increase of vulnerability to lymphotoxin in cells infected by vesicular stomatitis virus and its further augmentation by interferon. Cell Immunol. 1985 May;92(2):218–225. doi: 10.1016/0008-8749(85)90003-6. [DOI] [PubMed] [Google Scholar]
- Ammann A. J., Palladino M. A., Volberding P., Abrams D., Martin N. L., Conant M. Tumor necrosis factors alpha and beta in acquired immunodeficiency syndrome (AIDS) and aids-related complex. J Clin Immunol. 1987 Nov;7(6):481–485. doi: 10.1007/BF00915059. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupar B. E., Andrew M. E., Boyle D. B. A general method for the construction of recombinant vaccinia viruses expressing multiple foreign genes. Gene. 1988 Aug 15;68(1):1–10. doi: 10.1016/0378-1119(88)90593-8. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Feduchi E., Alonso M. A., Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989 Mar;63(3):1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexner C., Hügin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987 Nov 19;330(6145):259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- Fransen L., Müller R., Marmenout A., Tavernier J., Van der Heyden J., Kawashima E., Chollet A., Tizard R., Van Heuverswyn H., Van Vliet A. Molecular cloning of mouse tumour necrosis factor cDNA and its eukaryotic expression. Nucleic Acids Res. 1985 Jun 25;13(12):4417–4429. doi: 10.1093/nar/13.12.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Nahmias A. J., Murphy F. A., Kramer J. H. Cellular immunity in vaccinia infection of mice. Anti-thymocyte serum effects on primary and secondary responsiveness. J Exp Med. 1968 Jul 1;128(1):121–132. doi: 10.1084/jem.128.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen H., Mestan J., Mittnacht S., Dieffenbach C. W. Beta interferon subtype 1 induction by tumor necrosis factor. Mol Cell Biol. 1989 Jul;9(7):3037–3042. doi: 10.1128/mcb.9.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G., Coupar B. E., Andrew M. E., Boyle D. B., Phillips S. M., Müllbacher A., Blanden R. V., Ramshaw I. A. Elevated natural killer cell responses in mice infected with recombinant vaccinia virus encoding murine IL-2. J Immunol. 1990 Jan 1;144(1):290–298. [PubMed] [Google Scholar]
- Kirkpatrick C. H., Davis K. C., Horsburgh C. R., Jr, Cohn D. L., Penley K., Judson F. N. Interleukin-2 production by persons with the generalized lymphadenopathy syndrome or the acquired immune deficiency syndrome. J Clin Immunol. 1985 Jan;5(1):31–37. doi: 10.1007/BF00915166. [DOI] [PubMed] [Google Scholar]
- Klavinskis L. S., Geckeler R., Oldstone M. B. Cytotoxic T lymphocyte control of acute lymphocytic choriomeningitis virus infection: interferon gamma, but not tumour necrosis factor alpha, displays antiviral activity in vivo. J Gen Virol. 1989 Dec;70(Pt 12):3317–3325. doi: 10.1099/0022-1317-70-12-3317. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Fann A. V. Human tumor necrosis factor-alpha kills herpesvirus-infected but not normal cells. Lymphokine Res. 1986 Summer;5(3):215–221. [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kohonen-Corish M. R., King N. J., Woodhams C. E., Ramshaw I. A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-gamma. Eur J Immunol. 1990 Jan;20(1):157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L. Vaccinia virus expression vectors. J Gen Virol. 1986 Oct;67(Pt 10):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- Mestan J., Brockhaus M., Kirchner H., Jacobsen H. Antiviral activity of tumour necrosis factor. Synergism with interferons and induction of oligo-2',5'-adenylate synthetase. J Gen Virol. 1988 Dec;69(Pt 12):3113–3120. doi: 10.1099/0022-1317-69-12-3113. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Andrew M. E., Phillips S. M., Boyle D. B., Coupar B. E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987 Oct 8;329(6139):545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- Reis L. F., Ho Lee T., Vilcek J. Tumor necrosis factor acts synergistically with autocrine interferon-beta and increases interferon-beta mRNA levels in human fibroblasts. J Biol Chem. 1989 Oct 5;264(28):16351–16354. [PubMed] [Google Scholar]
- Reis L. F., Le J. M., Hirano T., Kishimoto T., Vilcek J. Antiviral action of tumor necrosis factor in human fibroblasts is not mediated by B cell stimulatory factor 2/IFN-beta 2, and is inhibited by specific antibodies to IFN-beta. J Immunol. 1988 Mar 1;140(5):1566–1570. [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Soike K. F., Czarniecki C. W., Baskin G., Blanchard J., Liggitt D. Enhancement of simian varicella virus infection in African green monkeys by recombinant human tumor necrosis factor alpha. J Infect Dis. 1989 Feb;159(2):331–335. doi: 10.1093/infdis/159.2.331. [DOI] [PubMed] [Google Scholar]
- Turtinen L. W., Assimacopoulos A., Haase A. T. Increased monokines in cytomegalovirus infected myelomonocytic cell cultures. Microb Pathog. 1989 Aug;7(2):135–145. doi: 10.1016/0882-4010(89)90032-6. [DOI] [PubMed] [Google Scholar]
- Van Damme J., De Ley M., Van Snick J., Dinarello C. A., Billiau A. The role of interferon-beta 1 and the 26-kDa protein (interferon-beta 2) as mediators of the antiviral effect of interleukin 1 and tumor necrosis factor. J Immunol. 1987 Sep 15;139(6):1867–1872. [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]




