Abstract
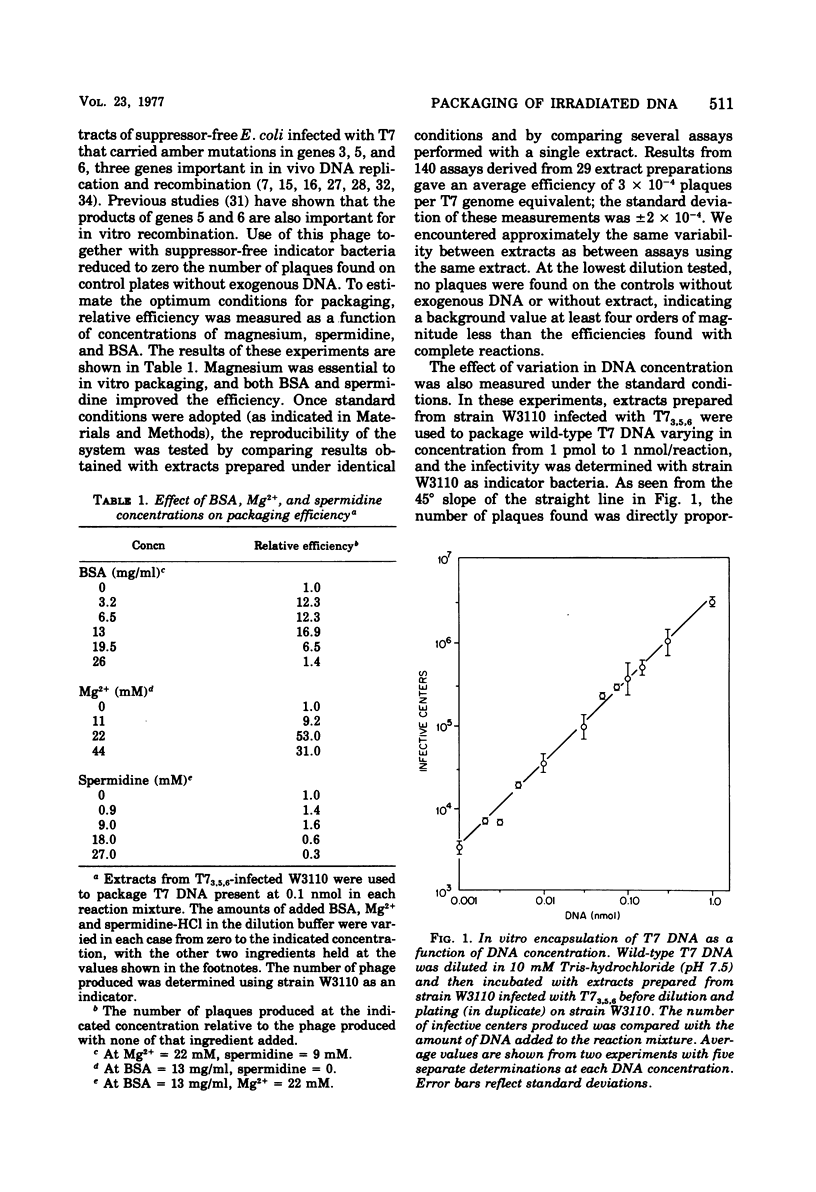
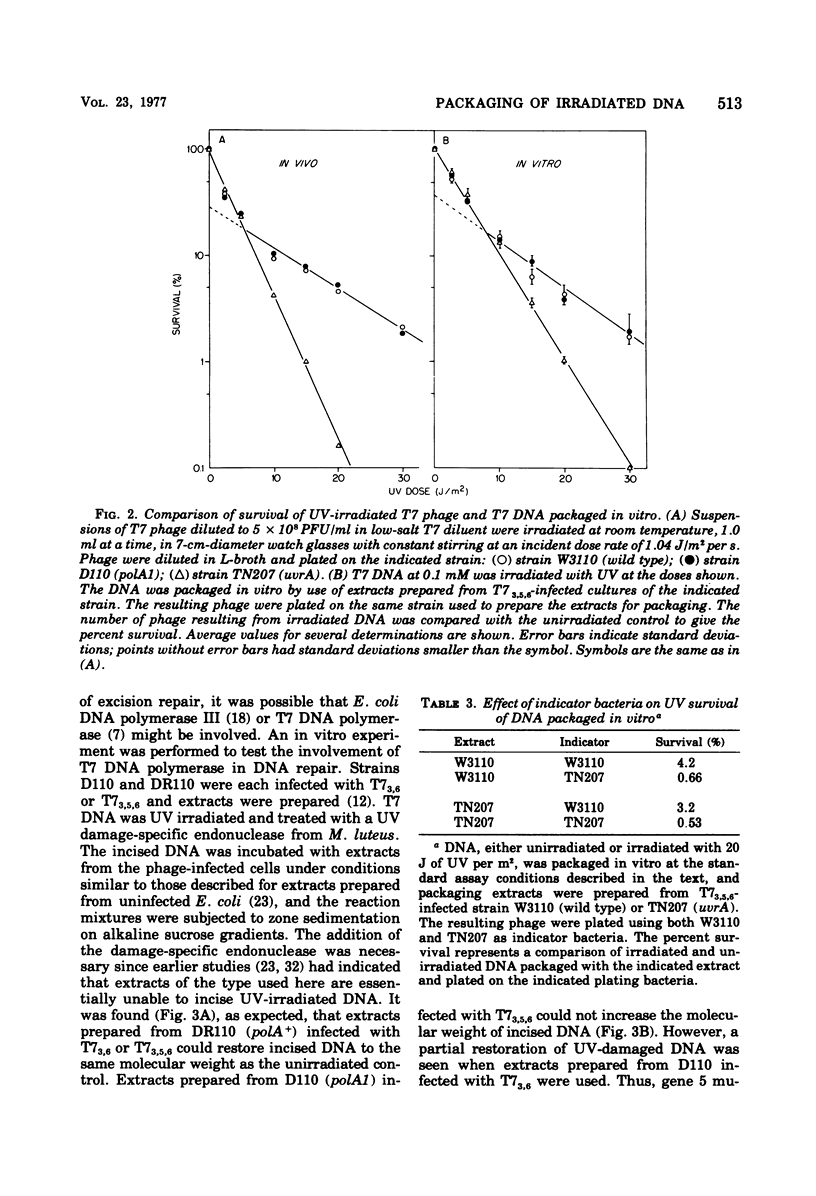
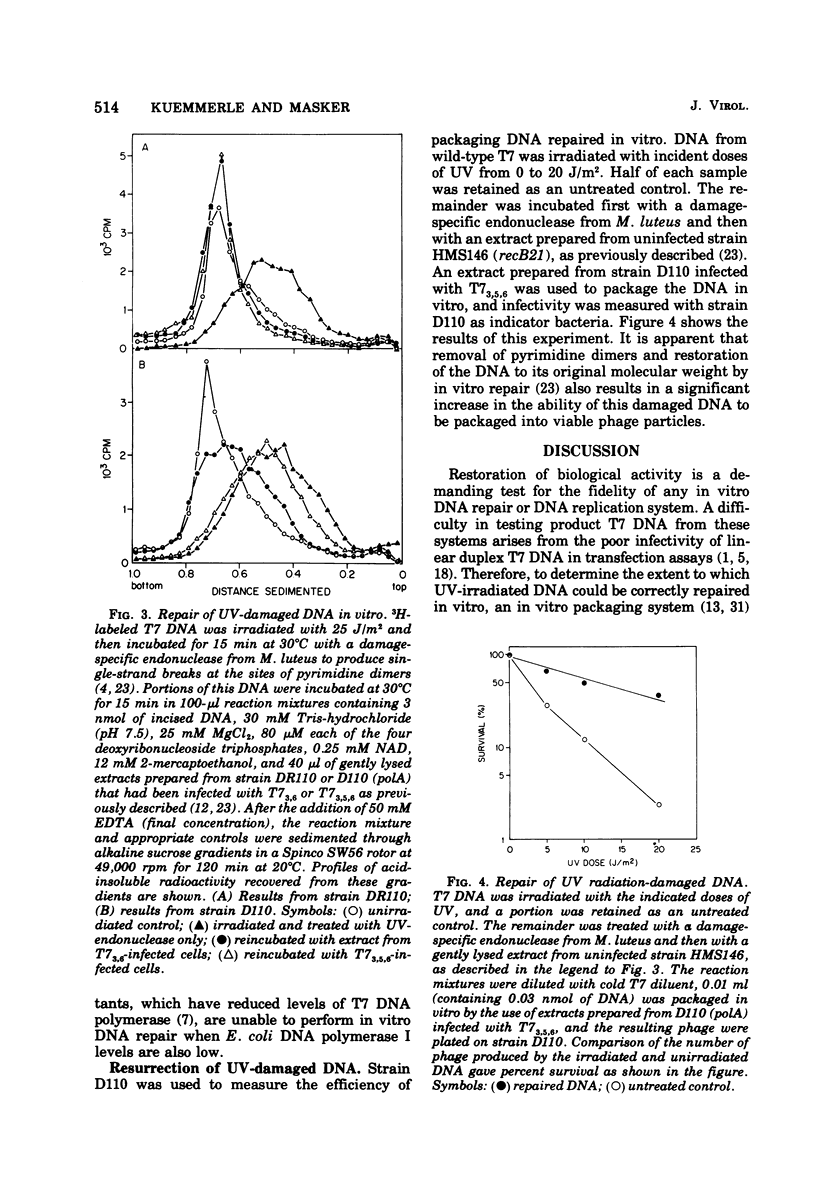
When DNA from bacteriophage T7 is irradiated with UV light, the efficiency with which this DNA can be packaged in vitro to form viable phage particles is reduced. A comparison between irradiated DNA packaged in vitro and irradiated intact phage particles shows almost identical survival as a function of UV dose when Escherichia coli wild type or polA or uvrA mutants are used as the host. Although uvrA mutants perform less host cell reactivation, the polA strains are identical with wild type in their ability to support the growth of irradiated T7 phage or irradiated T7 DNA packaged in vitro into complete phage. An examination of in vitro repair performed by extracts of T7-infected E.coli suggests that T7 DNA polymerase may substitute for E. coli DNA polymerase I in the resynthesis step of excision repair. Also tested was the ability of a similar in vitro repair system that used extracts from uninfected cells to restore biological activity of irradiated DNA. When T7 DNA damaged by UV irradiation was treated with an endonuclease from Micrococcus luteus that is specific for pyrimidine dimers and then was incubated with an extract of uninfected E. coli capable of removing pyrimidine dimers and restoring the DNA of its original (whole genome size) molecular weight, this DNA showed a higher packaging efficiency than untreated DNA, thus demonstrating that the in vitro repair system partially restored the biological activity of UV-damaged DNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLISON S. A., FEINER R. R., HILL R. F. A host effect on bacteriophage survival after ultraviolet irradiation. Virology. 1960 May;11:294–296. doi: 10.1016/0042-6822(60)90069-6. [DOI] [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Hamilton L., Mahler I., Grossman L. Enzymatic repair of deoxyribonucleic acid; the biochemical and biological repair properties of a deoxyribonucleic acid polymerase from micrococcus luteus. Biochemistry. 1974 Apr 23;13(9):1886–1896. doi: 10.1021/bi00706a017. [DOI] [PubMed] [Google Scholar]
- Harm W. Dark recovery of UV-irradiated phage. Ti. II. Interpretation of the survival kinetics obtained under conditions of host-cell reactivation. Mutat Res. 1974 Oct;25(1):3–14. doi: 10.1016/0027-5107(74)90212-7. [DOI] [PubMed] [Google Scholar]
- Heijneker H. L. Physico-chemical and biological study of excision-repair of UV--irradiated phiX174 RF DNA in vitro. Nucleic Acids Res. 1975 Nov;2(11):2147–2161. doi: 10.1093/nar/2.11.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Kleber I., Benzinger R. Transfection of Escherichia coli spheroplasts. 3. Facilitation of transfection and stabilization of spheroplasts by different basic polymers. J Virol. 1973 Oct;12(4):741–747. doi: 10.1128/jvi.12.4.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro. Requirements for deoxyribonucleic acid synthesis and characterization of the product. J Biol Chem. 1974 May 10;249(9):2974–2980. [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Packaging and maturation of DNA of bacteriophage T7 in vitro. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3545–3549. doi: 10.1073/pnas.71.9.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laipis P. J., Ganesan A. T. In vitro repair of x-irradiated DNA extracted from Bacillus subtilis deficient in polymerase I. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3211–3214. doi: 10.1073/pnas.69.11.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Miller R. C., Jr, Scraba D., Paetkau V. The essential role of bacteriophage T7 endonuclease (gene 3) in molecular recombination. J Mol Biol. 1976 Jul 15;104(4):883–888. doi: 10.1016/0022-2836(76)90189-3. [DOI] [PubMed] [Google Scholar]
- Lee M., Miller R. C., Jr T7 exonuclease (gene 6) is necessary for molecular recombination of bacteriophage T7. J Virol. 1974 Nov;14(5):1040–1048. doi: 10.1128/jvi.14.5.1040-1048.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman I. R., Chien J. R. Persistence of deoxyribonucleic acid polymerase I and its 5'--3' exonuclease activity in PolA mutants of Escherichia coli K12. J Biol Chem. 1973 Nov 25;248(22):7717–7723. [PubMed] [Google Scholar]
- Livingston D. M., Hinkle D. C., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Purification and properties. J Biol Chem. 1975 Jan 25;250(2):461–469. [PubMed] [Google Scholar]
- Luria S. E. Reactivation of Irradiated Bacteriophage by Transfer of Self-Reproducing Units. Proc Natl Acad Sci U S A. 1947 Sep;33(9):253–264. doi: 10.1073/pnas.33.9.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Masker W. E. Deoxyribonucleic acid repair in vitro by extracts of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1415–1423. doi: 10.1128/jb.129.3.1415-1423.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Ultraviolet-stimulated DNA synthesis in toluenzied Escherichia coli deficient in DNA polymerase I. Proc Natl Acad Sci U S A. 1973 Jan;70(1):129–133. doi: 10.1073/pnas.70.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E. The ATP dependence of the incision and resynthesis steps of excision repair. Biochim Biophys Acta. 1976 Aug 18;442(2):162–173. doi: 10.1016/0005-2787(76)90487-1. [DOI] [PubMed] [Google Scholar]
- Masker W., Hanawalt P., Shizuya H. Role of DNA polymerase II in repair replication in Escherichia coli. Nat New Biol. 1973 Aug 22;244(138):242–243. doi: 10.1038/newbio244242a0. [DOI] [PubMed] [Google Scholar]
- McKee R. A., Hart M. G. Effects of the Escherichia coli K12 recA56, uvrB and polA mutations on UV reactivation in bacteriophage T7. Mutat Res. 1975 May;28(2):305–308. doi: 10.1016/0027-5107(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr, Lee M. The role of bacteriophage T7 exonuclease (gene 6) in genetic recombination and production of concatemers. J Mol Biol. 1976 Feb 25;101(2):223–234. doi: 10.1016/0022-2836(76)90374-0. [DOI] [PubMed] [Google Scholar]
- Powling A., Knippers R. Some functions involved in bacteriophage T7 genetic recombination. Mol Gen Genet. 1974;134(2):173–180. doi: 10.1007/BF00268418. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. The 5'-terminal nucleotides of T7 bacteriophage deoxyribonucleic acid. J Mol Biol. 1966 Jan;15(1):49–61. doi: 10.1016/s0022-2836(66)80208-5. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Vetter D. Genetic recombination of bacteriophage T7 DNA in vitro. Proc Natl Acad Sci U S A. 1976 Mar;73(3):692–696. doi: 10.1073/pnas.73.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Nissen-Meyer J., Strike P. Incision of ultraviolet-irradiated DNA by extracts of E. coli requires three different gene products. Nature. 1976 Oct 7;263(5577):524–526. doi: 10.1038/263524a0. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Strike P. Excision repair of ultraviolet-irradiated deoxyribonucleic acid in plasmolyzed cells of Escherichia coli. J Bacteriol. 1976 Mar;125(3):787–795. doi: 10.1128/jb.125.3.787-795.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Harris A. L., Smith D. W. DNA repair in Escherichia coli mutants deficient in DNA polymerases I, II and-or 3. Proc Natl Acad Sci U S A. 1974 Mar;71(3):675–679. doi: 10.1073/pnas.71.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transfection of restrictionless Escherichia coli by bacteriophage T7 dna: effect of in vitro erosion of DNA by gamma exonuclease. J Mol Biol. 1976 Aug 25;105(4):603–609. doi: 10.1016/0022-2836(76)90238-2. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]


