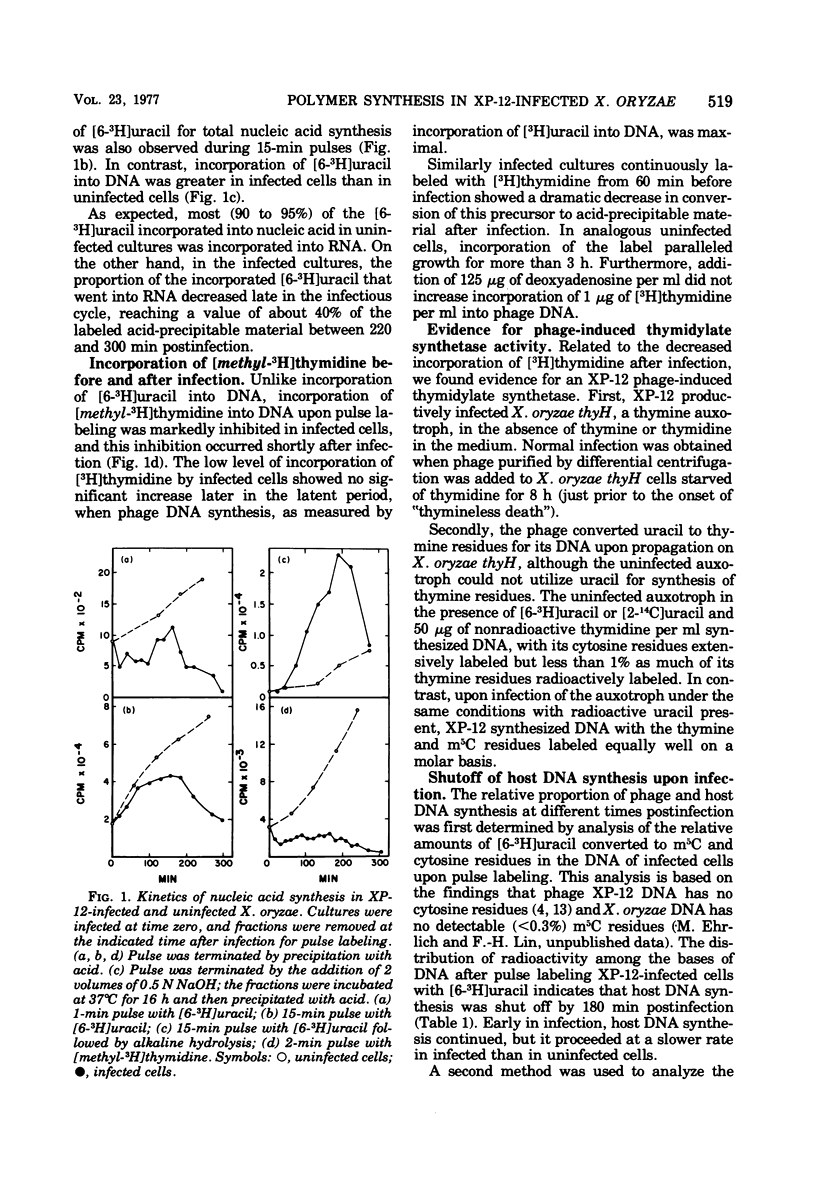
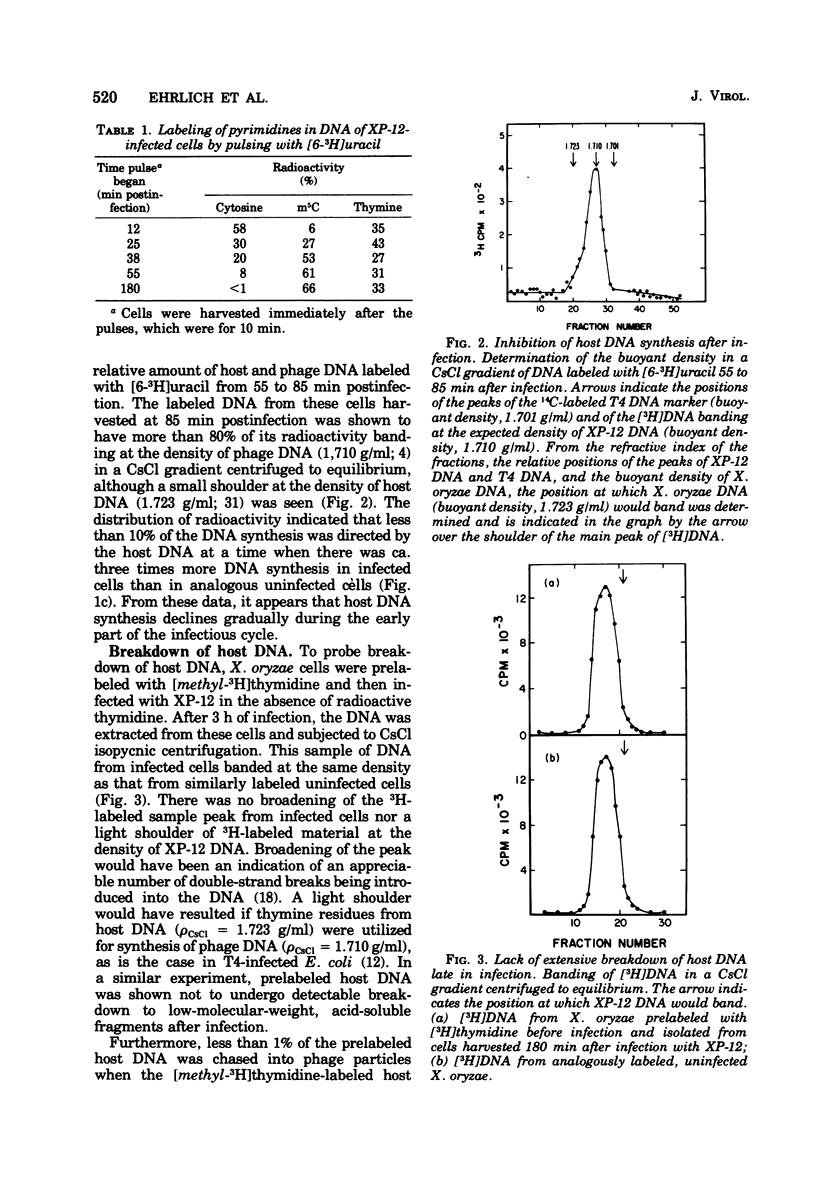
Abstract
Phage XP-12, which has complete substitution of the cytosine residues in its DNA with 5-methylcytosine residues, was shown to inhibit incorporation of uracil into host DNA and RNA during the latent period. This apparent inhibition of host macromolecular synthesis was not accompanied by extensive degradation of the host chromosome. Phage DNA synthesis in infected cells occurred at a faster rate than host DNA synthesis in analogous uninfected cells. However, phage DNA synthesis could not be accurately monitored by incorporation of [methyl-3H]thymidine into DNA because, soon after infection, there was a marked inhibition of utilization of exogenous thymidine for DNA synthesis. Phage infection conferred upon a thymine auxotrophic host the ability to synthesize thymine nucleotides for phage DNA synthesis. It is suggested that a phage-induced thymidylate synthetase activity is partially responsible for the inhibition of thymidine incorporation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNER H. D., COHEN S. S. Virus-induced acquisition of metabolic function. IV. Thymidylate synthetase in thymine-requiring Escherichia coli infected by T2 and T5 bacteriophages. J Biol Chem. 1959 Nov;234:2987–2991. [PubMed] [Google Scholar]
- BREITMAN T. R. The feedback inhibition of thymidine kinase. Biochim Biophys Acta. 1963 Jan 8;67:153–155. doi: 10.1016/0006-3002(63)91807-9. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Ehrlich K., Mayo J. A. Unusual properties of the DNA from Xanthomonas phage XP-12 in which 5-methylcytosine completely replaces cytosine. Biochim Biophys Acta. 1975 Jun 16;395(2):109–119. doi: 10.1016/0005-2787(75)90149-5. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. Virus-induced acquisition of metabolic function. III. Formation and some properties of thymidylate synthetase of bacteriophage-infected Escherichia coli. J Biol Chem. 1959 Nov;234:2981–2986. [PubMed] [Google Scholar]
- Hayward J. Inhibition of bacterial DNA and protein synthesis in Bacillus subtilis by phage SP82. Effect of changes of temperature on the inhibition. Virology. 1969 Aug;38(4):538–549. doi: 10.1016/0042-6822(69)90174-3. [DOI] [PubMed] [Google Scholar]
- IVES D. H., MORSE P. A., Jr, POTTER V. R. Feedback inhibition of thymodine kinase by thymodine triphosphate. J Biol Chem. 1963 Apr;238:1467–1474. [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- KOZLOFF L. M. Origin and fate of bacteriophage material. Cold Spring Harb Symp Quant Biol. 1953;18:209–220. doi: 10.1101/sqb.1953.018.01.032. [DOI] [PubMed] [Google Scholar]
- Kammen H. O., Strand M. Thymine metabolism in Escherichia coli. II. Altered uptake of thymine after bacteriophage infection. J Biol Chem. 1967 Apr 25;242(8):1854–1863. [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. I. Continued synthesis of host ribonucleic acid. J Virol. 1968 Nov;2(11):1262–1271. doi: 10.1128/jvi.2.11.1262-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. T., Huang T. C., Teng M. H. 5-Methylcytosine replacing cytosine in the deoxyribonucleic acid of a bacteriophage for Xanthomonas oryzae. J Mol Biol. 1968 Jul 14;34(2):373–375. doi: 10.1016/0022-2836(68)90263-5. [DOI] [PubMed] [Google Scholar]
- Markewych O., Boghosian A., Dosmar M., Ende D., Witmer H. SP-10 bacteriophage-specific nucleic acid and enzyme synthesis in Bacillus subtilis W23. J Virol. 1977 Jan;21(1):84–95. doi: 10.1128/jvi.21.1.84-95.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of DNA-defective mutants of bacteriophage T4. Thymine nucleotide pool dynamics. Arch Biochem Biophys. 1976 Jan;172(1):178–187. doi: 10.1016/0003-9861(76)90064-3. [DOI] [PubMed] [Google Scholar]
- Mathews C. K. Phage Growth and Deoxyribonucleic Acid Synthesis in Escherichia coli Infected by a Thymine-Requiring Bacteriophage. J Bacteriol. 1965 Sep;90(3):648–652. doi: 10.1128/jb.90.3.648-652.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., YANAGISAWA K., RYAN F. J. A method for securing thymineless mutants from strains of E. coli. Z Vererbungsl. 1961;92:403–412. doi: 10.1007/BF00890061. [DOI] [PubMed] [Google Scholar]
- Price A. R., Frabotta M. Resistance of bacteriophage PBS2 infection to rifampicin, an inhibitor of Bacillus subtilis RNA synthesis. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1578–1585. doi: 10.1016/0006-291x(72)90894-7. [DOI] [PubMed] [Google Scholar]
- Pène J. J. Host macromolecular synthesis in bacteriophage-infected Bacillus subtilis. Bacteriol Rev. 1968 Dec;32(4 Pt 1):379–386. [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. A., Jamieson A. T., White F. E. The induction of deoxythymidine kinase by bacteriophage T4. J Gen Virol. 1974 Jul;24(1):115–127. doi: 10.1099/0022-1317-24-1-115. [DOI] [PubMed] [Google Scholar]
- Roscoe D. H. Synthesis of DNA in phage-infected Bacillus subtilis. Virology. 1969 Aug;38(4):527–537. doi: 10.1016/0042-6822(69)90173-1. [DOI] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P., Conroy L. M. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption. I. Isolation and genetic characterization. J Mol Biol. 1974 Nov 15;89(4):663–673. doi: 10.1016/0022-2836(74)90043-6. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Parson K. A., Warner H. R., Tutas D. J., Wehner J. M., Koerner J. F. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption. II. Physiological state of the host nucleoid in infected cells. J Mol Biol. 1974 Nov 15;89(4):675–687. doi: 10.1016/0022-2836(74)90044-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]



