Abstract
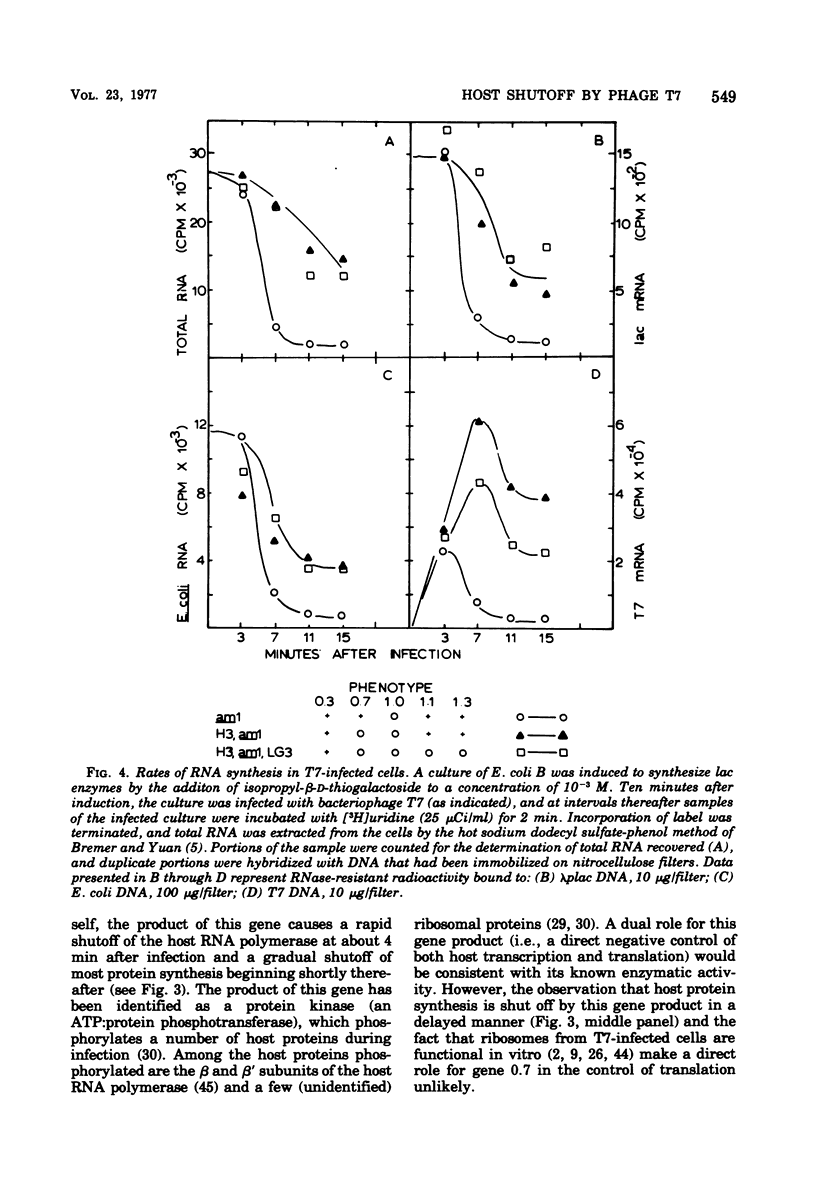
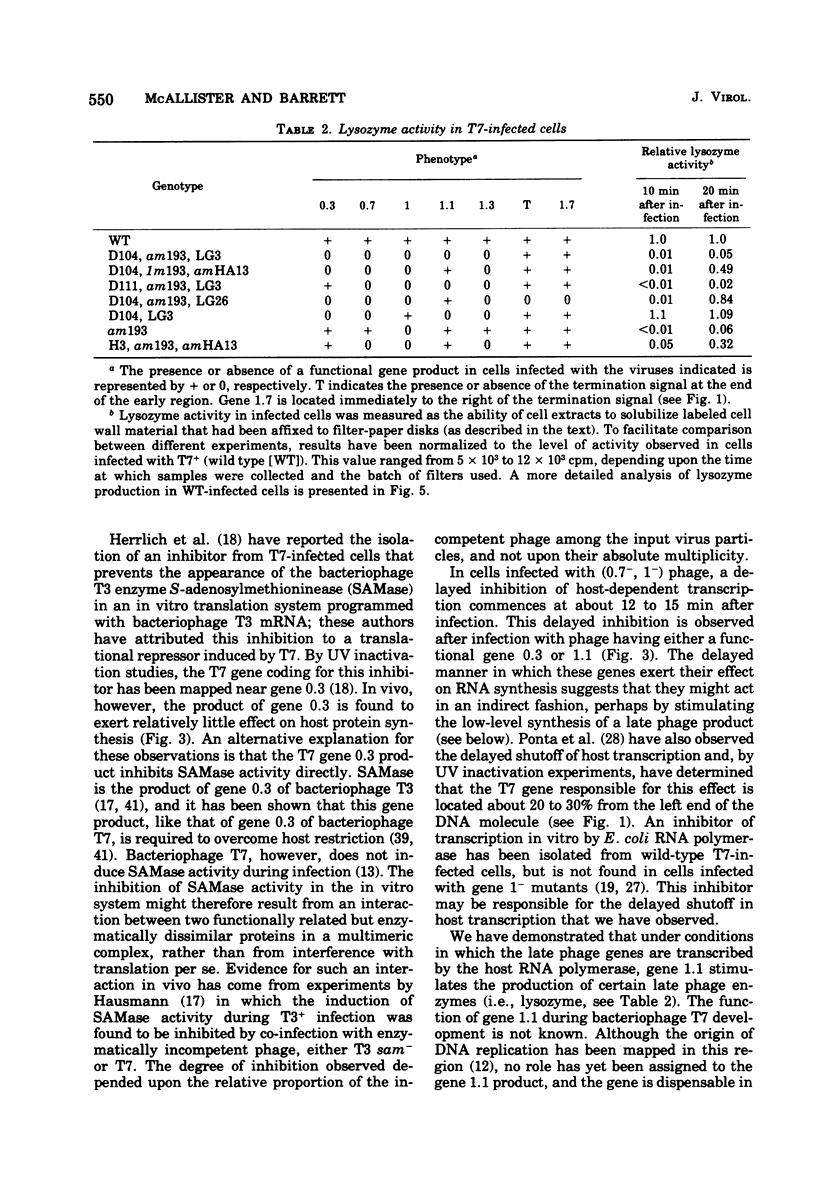
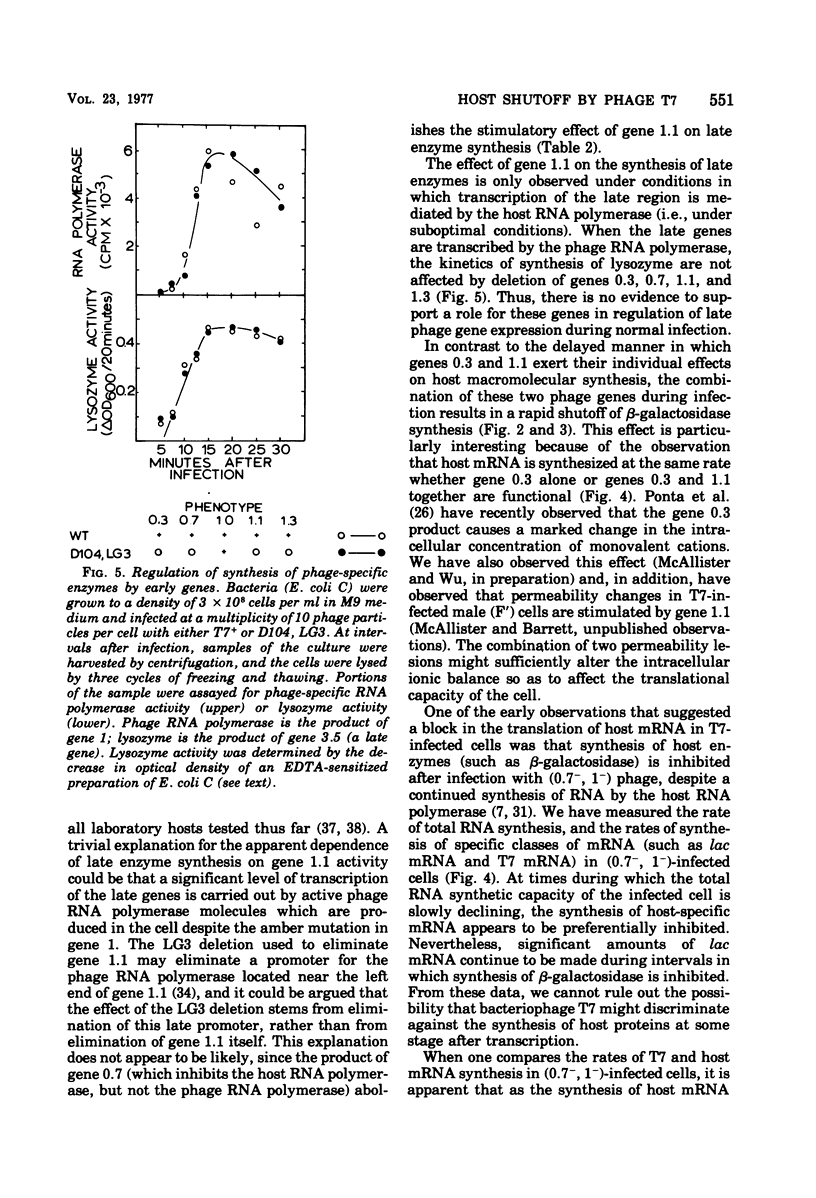
Through the use of phage mutants in which various combinations of the early genes are active, and in which late gene expression is blocked, we have examined the roles of each of the five early gene products of bacteriophage T7 in regulating the synthesis of host RNA and proteins. At least two independent transcriptional controls operate during bacteriophage T7 development. The product of gene 0.7, acting alone, leads to a rapid (by 5 min) shutoff of host transcription. In the absence of gene 0.7 function, and in the absence of the phage-specified RNA polymerase, a delayed shutoff of host-dependent transcription begins at approximately 15 min after infection. This secondary control element requires either a functional gene 0.3 or gene 1.1. In the absence of any early gene products, host shutoff is not observed until much later in infection (>30 min). The delayed manner in which the products of genes 0.3 and 1.1 exert their effect suggests that their mode of action is indirect. Under conditions in which the late genes are transcribed (inefficiently) by the host RNA polymerase, gene 1.1 is observed to stimulate the synthesis of lysozyme (the product of a late phage gene). In contrast, when the late genes are transcribed by the phage-specified RNA polymerase (the product of gene 1), the kinetics of synthesis of the phage RNA polymerase itself, and of lysozyme, are not affected by the deletion of genes 0.3, 0.7, 1.1, and 1.3. We conclude that under these conditions, the products of these genes are required neither for regulation of expression of the late genes nor for the shutoff of early phage gene expression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G., WEIGLE J. J. Host controlled variation in bacterial viruses. J Bacteriol. 1953 Feb;65(2):113–121. doi: 10.1128/jb.65.2.113-121.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Mabie C. T., Malamy M. H. T7 protein synthesis in F-factor-containing cells: evidence for an episomally induced impairment of translation and relation to an alteration in membrane permeability. J Virol. 1975 Jan;17(1):94–105. doi: 10.1128/jvi.17.1.94-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Yuan D. Chain growth rate of messenger RNA in Escherichia coli infected with bacteriophage T4. J Mol Biol. 1968 Jun 28;34(3):527–540. doi: 10.1016/0022-2836(68)90178-2. [DOI] [PubMed] [Google Scholar]
- Brunovskis I., Summers W. C. The process of infection with coliphage 17. VI. A phage gene controlling shutoff of host RNA synthesis. Virology. 1972 Nov;50(2):322–327. doi: 10.1016/0042-6822(72)90383-2. [DOI] [PubMed] [Google Scholar]
- Brunovskis I., Summers W. C. The process of infection with coliphage T7. V. Shutoff of host RNA synthesis by an early phage function. Virology. 1971 Jul;45(1):224–231. doi: 10.1016/0042-6822(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Dambly C., Couturier M., Thomas R. Control of development in temperate bacteriophages. II. Control of lysozyme synthesis. J Mol Biol. 1968 Feb 28;32(1):67–81. doi: 10.1016/0022-2836(68)90146-0. [DOI] [PubMed] [Google Scholar]
- Dausse J. P., Sentenac A., Fromageot P. Interaction of RNA polymerase from Escherichia coli with DNA. Effect of temperature and ionic strength on selection of T7 DNA early promoters. Eur J Biochem. 1976 Jun 1;65(2):387–393. doi: 10.1111/j.1432-1033.1976.tb10352.x. [DOI] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M., Hausmann R., Gold M., Hurwitz J. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. X. Bacteriophage T3-induced S-adenosylmethionine cleavage. J Biol Chem. 1966 May 10;241(9):1995–2006. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. Positive control of endolysin synthesis in vitro by the gene N protein of phage lambda. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3606–3610. doi: 10.1073/pnas.69.12.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R. Synthesis of an S-adenosylmethionine-cleaving enzyme in T3-infected Escherichia coli and its disturbance by co-infection with enzymatically incompetent bacteriophage. J Virol. 1967 Feb;1(1):57–63. doi: 10.1128/jvi.1.1.57-63.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Rahmsdorf H. J., Pai S. H., Schweigher M. Translational control induced by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1088–1092. doi: 10.1073/pnas.71.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbach B. A., Yamada Y., Nakada D. Isolation of an inhibitor protein of E. coli RNA polymerase from T7 phage infected cell. Nature. 1974 Nov 1;252(5478):71–74. doi: 10.1038/252071b0. [DOI] [PubMed] [Google Scholar]
- Hyman R. W. Physical mapping of T7 messenger RNA. J Mol Biol. 1971 Oct 28;61(2):369–376. doi: 10.1016/0022-2836(71)90386-x. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Ponta H., Altendorf K. H., Schweiger M., Hirsch-Kaufmann M., Pfennig-Yeh M. L., Herrlich P. E. coli membranes become permeable to ions following T7-virus-infection. Mol Gen Genet. 1976 Dec 8;149(2):145–150. doi: 10.1007/BF00332882. [DOI] [PubMed] [Google Scholar]
- Ponta H., Pon C. L., Herrlich P., Gualerzi C., Hirsch-Kauffmann M., Pfennig-Yeh M. l., rahmsdorf H. J., Schweiger M. The sex-factor-dependent exclusion of coli virus T7. Eur J Biochem. 1975 Nov 1;59(1):261–270. doi: 10.1111/j.1432-1033.1975.tb02450.x. [DOI] [PubMed] [Google Scholar]
- Ponta H., Rahmsdorf H. J., Pai S. H., Herrlich P., Schweiger M. Control of gene expression in bacteriophage T7. Isolation of a new control protein and mechanism of action. Mol Gen Genet. 1974;134(1):29–38. doi: 10.1007/BF00332810. [DOI] [PubMed] [Google Scholar]
- Ponta H., Rahmsdorf H. J., Pai S. H., Hirsch-Kauffmann M., Herrlich P., Schweiger M. Control of gene expression in bacteriophage T7: transcriptional controls. Mol Gen Genet. 1974;134(4):281–297. doi: 10.1007/BF00337463. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Herrlich P., Pai S. H., Schweiger M., Wittmann H. G. Ribosomes after infection with bacteriophage T4 and T7. Mol Gen Genet. 1973 Dec 31;127(3):259–271. doi: 10.1007/BF00333766. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Pai S. H., Ponta H., Herrlich P., Roskoski R., Jr, Schweiger M., Studier F. W. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Feb;71(2):586–589. doi: 10.1073/pnas.71.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M., Herrlich P., Scherzinger E., Rahmsdorf H. J. Negative control of protein synthesis after infection with bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2203–2207. doi: 10.1073/pnas.69.8.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- Skare J., Niles E. G., Summers W. C. Localization of the leftmost initiation site for T7 late transcription, in vivo and in vitro. Biochemistry. 1974 Sep 10;13(19):3912–3916. doi: 10.1021/bi00716a015. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Movva N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J Virol. 1976 Jul;19(1):136–145. doi: 10.1128/jvi.19.1.136-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Summers W. C. Regulation of RNA metabolism of T7 and related phages. Annu Rev Genet. 1972;6:191–202. doi: 10.1146/annurev.ge.06.120172.001203. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Transcription of late phage RNA by T7 RNA polymerase. Nature. 1970 Dec 19;228(5277):1160–1162. doi: 10.1038/2281160a0. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nakada D. F-Factor-mediated restriction of bacteriophage T7: protein synthesis in cell-free systems from T7-infected Escherichia coli F- and F+ cells. J Virol. 1975 Dec;16(6):1483–1491. doi: 10.1128/jvi.16.6.1483-1491.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Fujiki H., Blum W., Janeković D., Schweiger M., Rahmsdorf H., Ponta H., Hirsch-Kauffmann M. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage-T7-induced protein kinase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2506–2510. doi: 10.1073/pnas.72.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]



