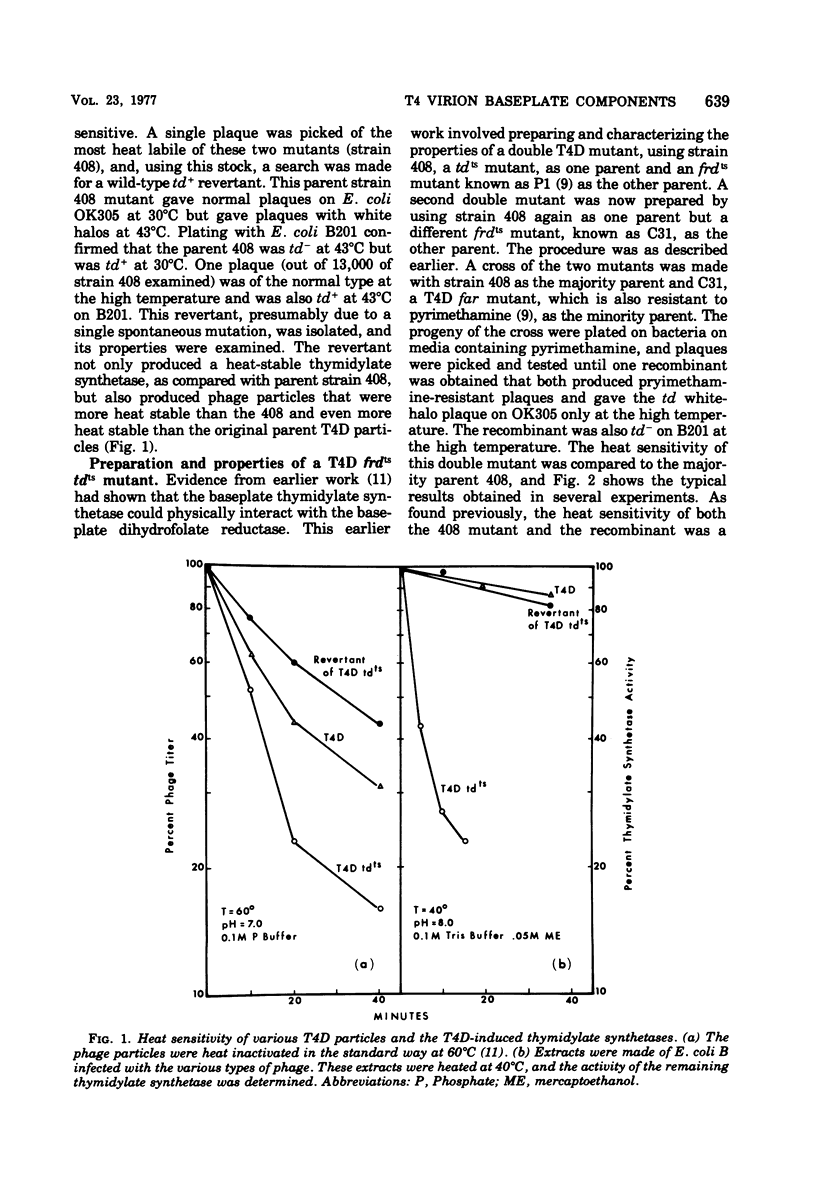
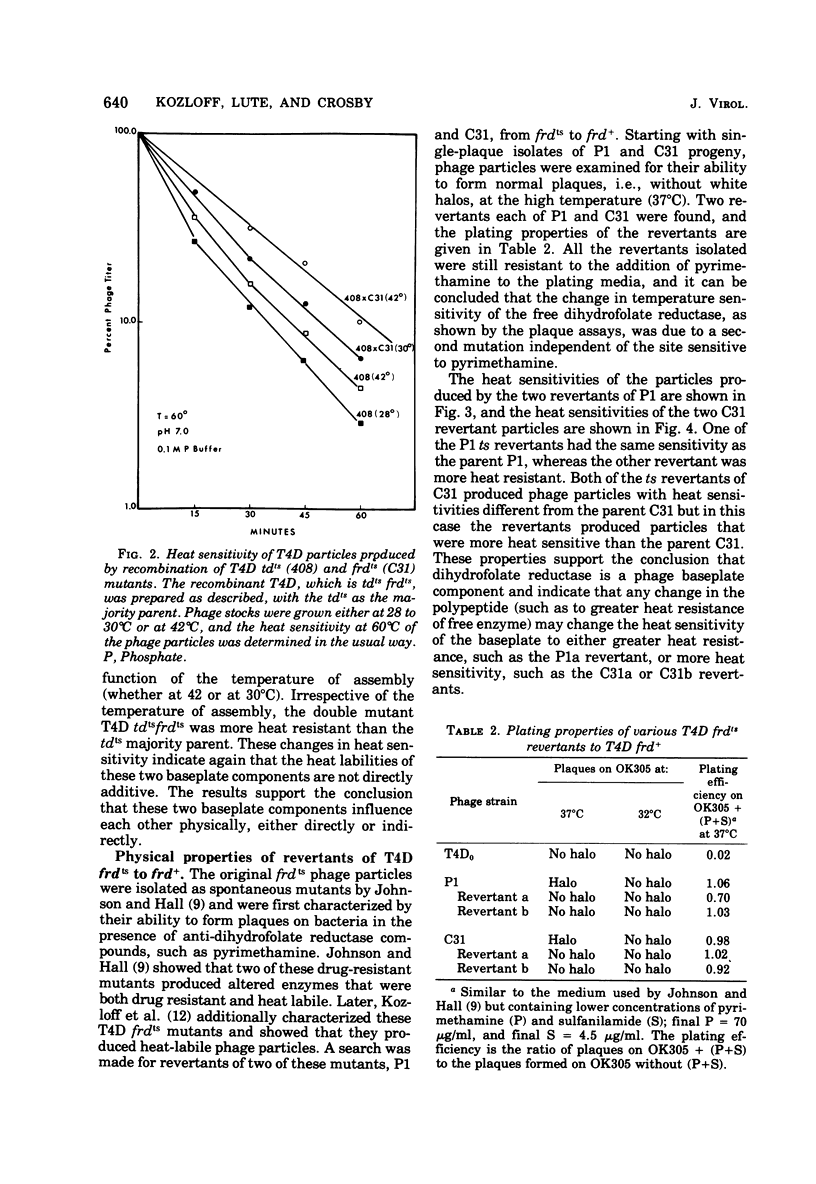
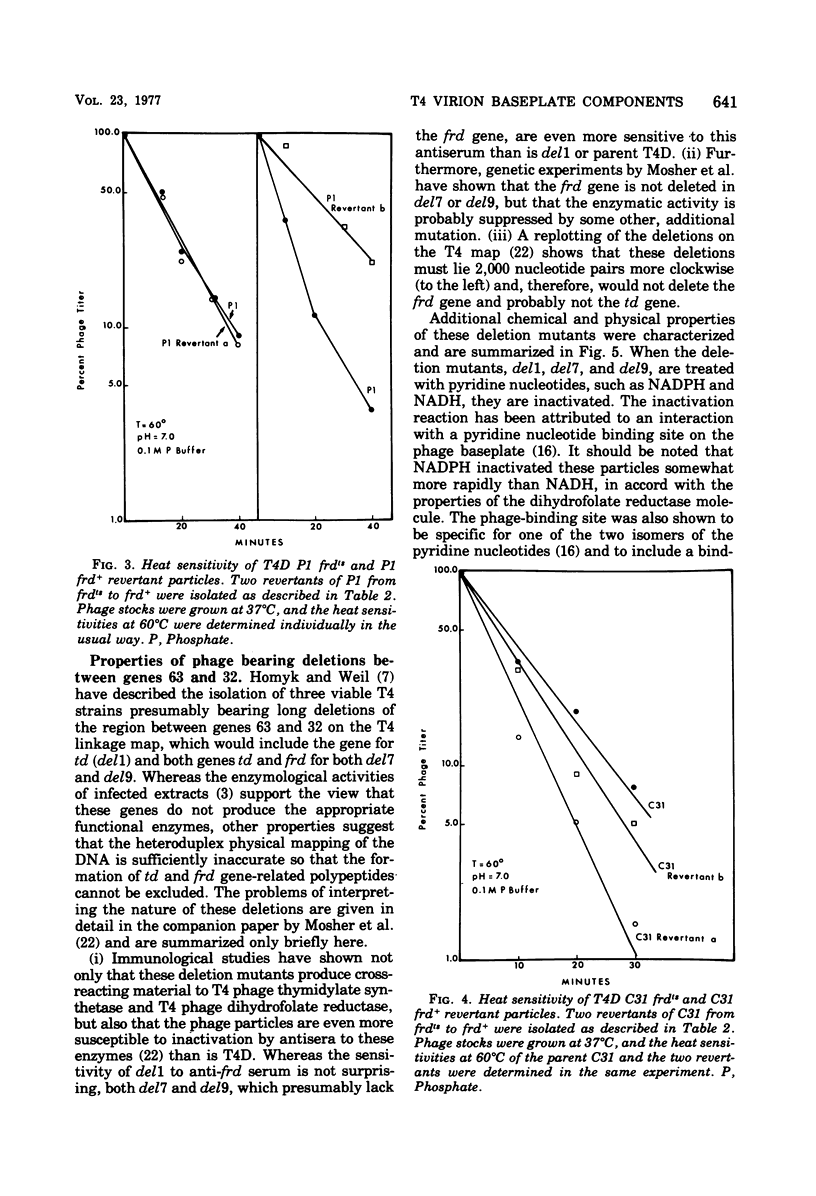
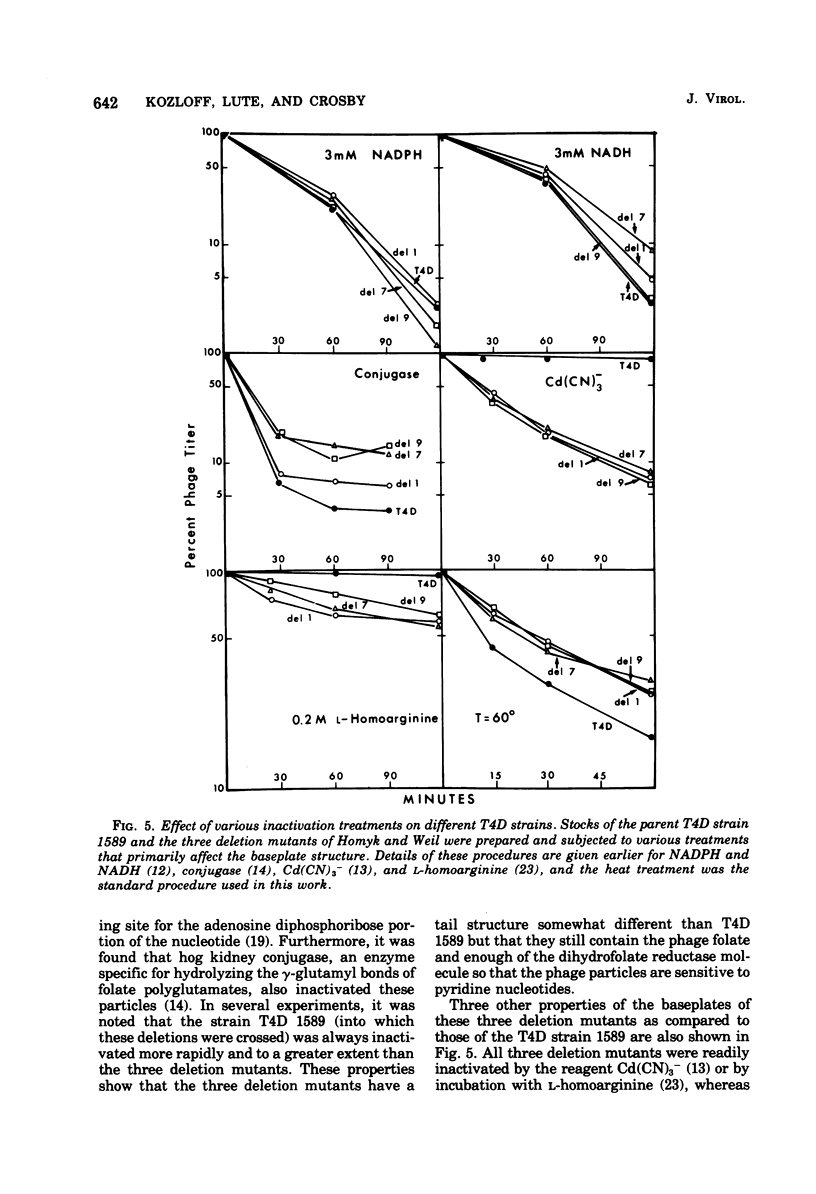
Abstract
Additional evidence is presented that both the phage T4D-induced thymidylate synthetase (gp td) and the T4D-induced dihydrofolate reductase (gp frd) are baseplate structural components. With regard to phage td it has been found that: (i) low levels of thymidylate synthetase activity were present in highly purified preparations of T4D ghost particles produced after infection with td+, whereas particles produced after infection with td− had no measurable enzymatic activity; (ii) a mutation of the T4D td gene from tdts to td+ simultaneously produced a heat-stable thymidylate synthetase enzyme and heat-stable phage particles (it should be noted that the phage baseplate structure determines heat lability); (iii) a recombinant of two T4D mutants constructed containing both tdts and frdts genes produced particles whose physical properties indicate that these two molecules physically interact in the baseplate. With regard to phage frd it has been found that two spontaneous revertants each of two different T4D frdts mutants to frd+ not only produced altered dihydrofolate reductases but also formed phage particles with heat sensitivities different from their parents. Properties of T4D particles produced after infection with parental T4D mutants presumed to have a deletion of the td gene and/or the frd gene indicate that these particles still retain some characteristics associated with the presence of both the td and the frd molecules. Furthermore, the particles produced by the deletion mutants have been found to be physically different from the parent particles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccanari D., Phillips A., Smith S., Sinski D., Burchall J. Purification and properties of Escherichia coli dihydrofolate reductase. Biochemistry. 1975 Dec 2;14(24):5267–5273. doi: 10.1021/bi00695a006. [DOI] [PubMed] [Google Scholar]
- Capco G. R., Krupp J. R., Mathews C. K. Bacteriophage-coded thymidylate synthetase: characteristics of the T4 and T5 enzymes. Arch Biochem Biophys. 1973 Oct;158(2):726–735. doi: 10.1016/0003-9861(73)90567-5. [DOI] [PubMed] [Google Scholar]
- Capco G. R., Mathews C. K. Bacteriophage-coded thymidylate synthetase. Evidence that the T4 enzyme is a capsid protein. Arch Biochem Biophys. 1973 Oct;158(2):736–743. doi: 10.1016/0003-9861(73)90568-7. [DOI] [PubMed] [Google Scholar]
- Dawes J., Goldberg E. B. Functions of baseplate components in bacteriophage T4 infection. I. Dihydrofolate reductase and dihydropteroylhexaglutamate. Virology. 1973 Oct;55(2):380–390. doi: 10.1016/0042-6822(73)90178-5. [DOI] [PubMed] [Google Scholar]
- Hall D. H. Mutants of bacteriophage T4 unable to induce dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1967 Aug;58(2):584–591. doi: 10.1073/pnas.58.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I., Karlström O. Linkage of T4 genes controlling a series of steps in pyrimidine biosynthesis. Virology. 1967 Mar;31(3):442–448. doi: 10.1016/0042-6822(67)90224-3. [DOI] [PubMed] [Google Scholar]
- Homyk T., Jr, Weil J. Deletion analysis of two nonessential regions of the T4 genome. Virology. 1974 Oct;61(2):505–523. doi: 10.1016/0042-6822(74)90286-4. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J Mol Biol. 1977 Feb 5;109(4):487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- Johnson J. R., Hall D. H. Isolation and characterization of mutants of bacteriophage T4 resistant to folate analogs. Virology. 1973 Jun;53(2):413–426. doi: 10.1016/0042-6822(73)90221-3. [DOI] [PubMed] [Google Scholar]
- KOZLOFF L. M., LUTE M. Viral invasion. III. The release of viral nucleic acid from its protein covering. J Biol Chem. 1957 Sep;228(1):537–546. [PubMed] [Google Scholar]
- Kikuchi Y., King J. Genetic control of bacteriophage T4 baseplate morphogenesis. II. Mutants unable to form the central part of the baseplate. J Mol Biol. 1975 Dec 25;99(4):673–694. doi: 10.1016/s0022-2836(75)80179-3. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Crosby L. K., Lute M. Bacteriophage T4 baseplate components. III. Location and properties of the bacteriophage structural thymidylate synthetase. J Virol. 1975 Dec;16(6):1409–1419. doi: 10.1128/jvi.16.6.1409-1419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Crosby L. K., Lute M., Hall D. H. Bacteriophage T4 baseplate components. II. Binding and location of bacteriophage-induced dihydrofolate reductase. J Virol. 1975 Dec;16(6):1401–1408. doi: 10.1128/jvi.16.6.1401-1408.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Crosby L. K. Bacteriophage T4 baseplate components. I. Binding and location of the folic acid. J Virol. 1975 Dec;16(6):1391–1400. doi: 10.1128/jvi.16.6.1391-1400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M. Folic acid, a structural component of T4 bacteriophage. J Mol Biol. 1965 Jul;12(3):780–792. doi: 10.1016/s0022-2836(65)80327-8. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Verses C., Lute M., Crosby L. K. Bacteriophage tail components. II. Dihydrofolate reductase in T4D bacteriophage. J Virol. 1970 Jun;5(6):740–753. doi: 10.1128/jvi.5.6.740-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S. W., Stollar B. D., Friedkin M. Genetic and immunological studies of bacteriophage T4 thymidylate synthetase. J Virol. 1973 May;11(5):783–791. doi: 10.1128/jvi.11.5.783-791.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax M. I., Greenberg G. R. An exchange between the hydrogen atom on carbon 5 of deoxyuridylate and water catalyzed by thymidylate synthetase. J Biol Chem. 1967 Mar 25;242(6):1302–1306. [PubMed] [Google Scholar]
- Male C. J., Kozloff L. M. Function of T4D structural dihydrofolate reductase in bacteriophage infection. J Virol. 1973 Jun;11(6):840–847. doi: 10.1128/jvi.11.6.840-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K., Crosby L. K., Kozloff L. M. Inactivation of T4D bacteriophage by antiserum against bacteriophage dihydrofolate reductase. J Virol. 1973 Jul;12(1):74–78. doi: 10.1128/jvi.12.1.74-78.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K. Identity of genes coding for soluble and structural dihydrofolate reductases in bacteriophage T4. J Virol. 1971 Apr;7(4):531–533. doi: 10.1128/jvi.7.4.531-533.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher R. A., DiRenzo A. B., Mathews C. K. Bacteriophage T4 virion dihydrofolate reductase: approaches to quantitation and assessment of function. J Virol. 1977 Sep;23(3):645–658. doi: 10.1128/jvi.23.3.645-658.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D., Kozloff L. M. A C-terminal arginine residue necessary for bacteriophage T4D tail assembly. J Mol Biol. 1970 Jul 28;51(2):185–201. doi: 10.1016/0022-2836(70)90136-1. [DOI] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



