Abstract
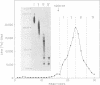
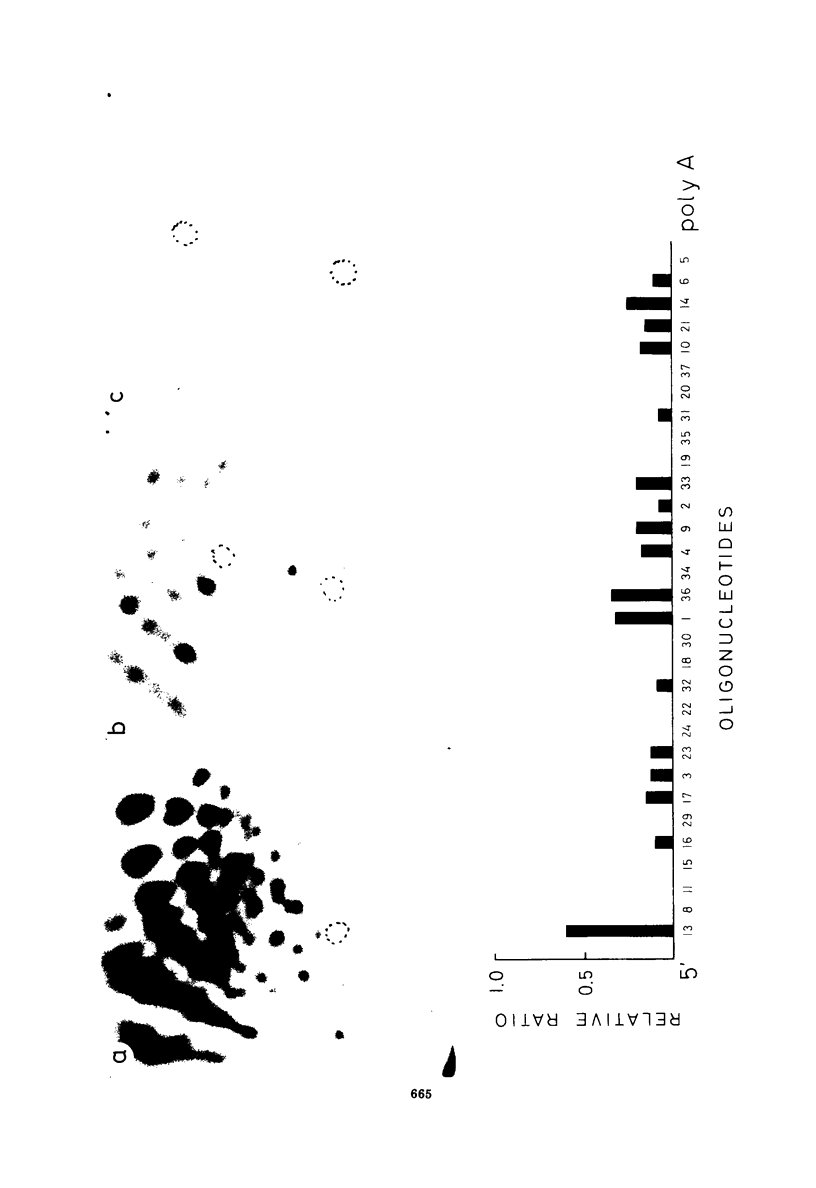
Conditions are described that promote the efficient reverse transcription of most of Rous sarcoma virus (RSV) RNA sequences by avian myeloblastosis virus DNA polymerase in vitro. A detailed analysis of the reverse transcription reaction was carried out using two procedures: in situ analysis of the RNA sequences transcribed and DNA-RNA annealing studies. Under optimal conditions, after 1 h of reaction, practically all RSV RNA sequences were transcribed with a frequency varying from 30 to 90%. The DNA product was at least 95% single stranded, had a chain length ranging from a few hundred up to 5,000 necleotide residues, half of it being larger than 1,000 residues, and, after hybridization at RNA excess, protected the entire RSV genome from RNase digestion, as monitored by the large T1 oligonucleotides of RSV RNA. Analysis of the product of a very short reaction time (5 min) showed that DNA synthesis occurs mainly at three sites, one near the 5' end and two near the center of the subunit RNA. This in in agreement with our previous analysis of a much less efficient reverse transcription reaction. Under optimal conditions of reverse transcription, we find now that the RNase H associated with the avian myeloblastosis virus DNA polymerase is active in degrading the RNA moiety of the RNA-DNA hybrids synthesized.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashion L. M., Joho R. H., Planitz M. A., Billeter M. A., Weissmann C. Initiation sites of Rous sarcoma virus RNA-directed DNA synthesis in vitro. Nature. 1976 Jul 15;262(5565):186–190. doi: 10.1038/262186a0. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Evidence for circularization of the avian oncornavirus RNA genome during proviral DNA synthesis from studies of reverse transcription in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1329–1332. doi: 10.1073/pnas.73.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of 70S RNA by the RNA-directed DNA polymerase of Rouse sarcoma virus: lack of influence of RNase H. J Virol. 1975 Jan;17(1):291–295. doi: 10.1128/jvi.17.1.291-295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. New procedure for the direct analysis of in vitro reverse transcription of Rous sarcoma virus RNA. J Virol. 1977 Apr;22(1):118–129. doi: 10.1128/jvi.22.1.118-129.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T. Recovery of a new virus from apparently normal chick cells by infection with avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1797–1803. doi: 10.1073/pnas.67.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. B., Bromley P. A. Molecular weight of RNA subunits of Rous sarcoma virus determined by electron microscopy. J Virol. 1975 Jan;15(1):161–166. doi: 10.1128/jvi.15.1.161-166.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Spiegelman S. Purification and detection of reverse transcriptase in viruses and cells. Methods Enzymol. 1974;29:150–173. doi: 10.1016/0076-6879(74)29018-9. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M., Kodama M., Wells R. T. DNA ligase and exonuclease activities in virions of rous sarcoma virus. Nat New Biol. 1971 Apr 21;230(16):232–235. doi: 10.1038/newbio230232a0. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Zamecnik P. C., Weith H. L. Rous sarcoma virus genome is terminally redundant: the 3' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):994–998. doi: 10.1073/pnas.74.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. Characterization of the products of DNA-directed DNA polymerases in oncogenic RNA viruses. Nature. 1970 Aug 8;227(5258):563–567. doi: 10.1038/227563a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Faras A. J., Varmus H. E., Levinson W. E., Bishop J. M. Ribonucleic acid directed deoxyribonucleic acid synthesis by the purified deoxyribonucleic acid polymerase of Rous sarcoma virus. Characterization of the enzymatic product. Biochemistry. 1972 Jun 6;11(12):2343–2351. doi: 10.1021/bi00762a021. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]