Abstract
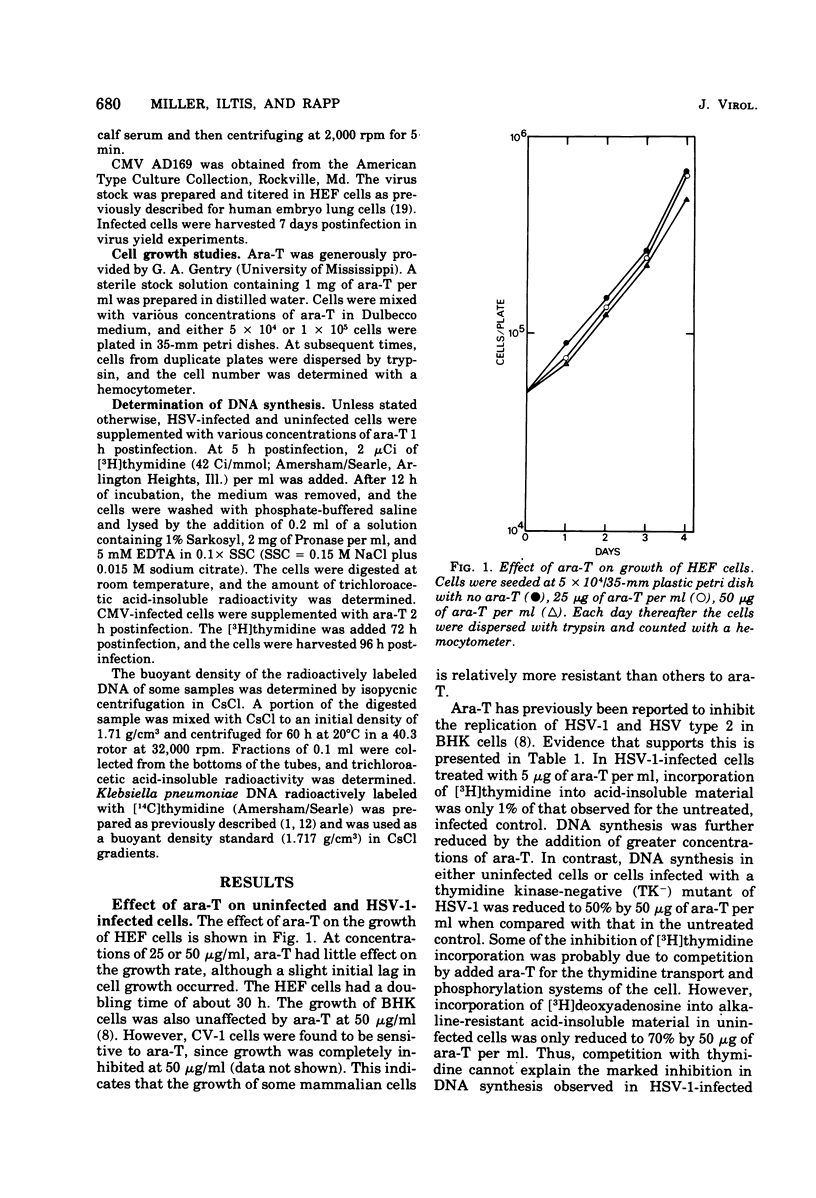
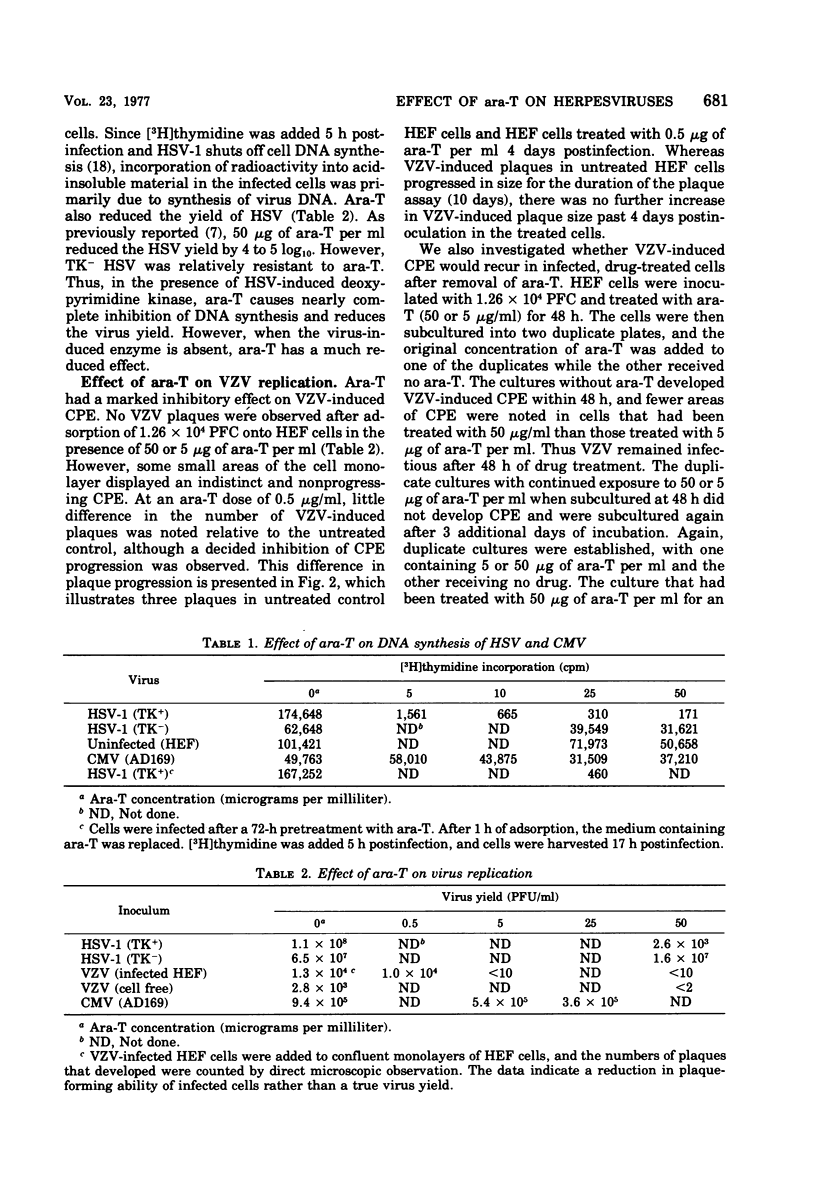
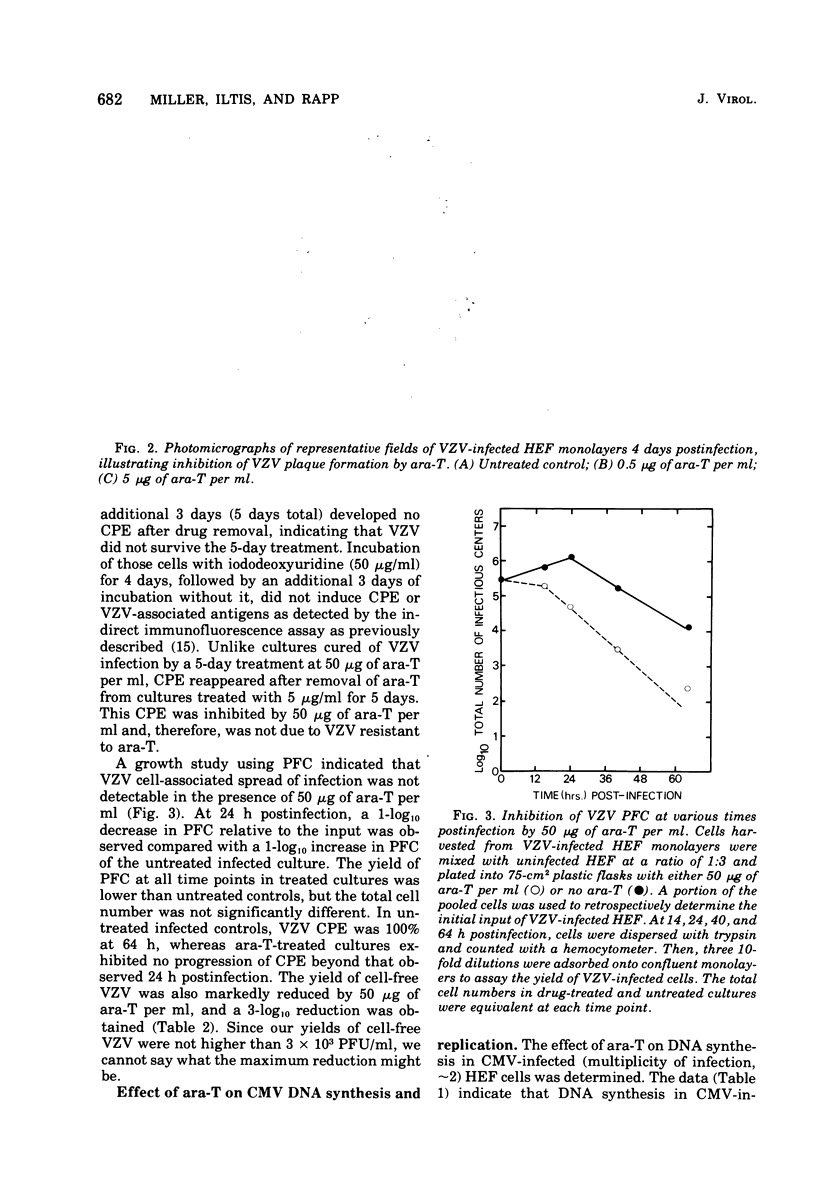
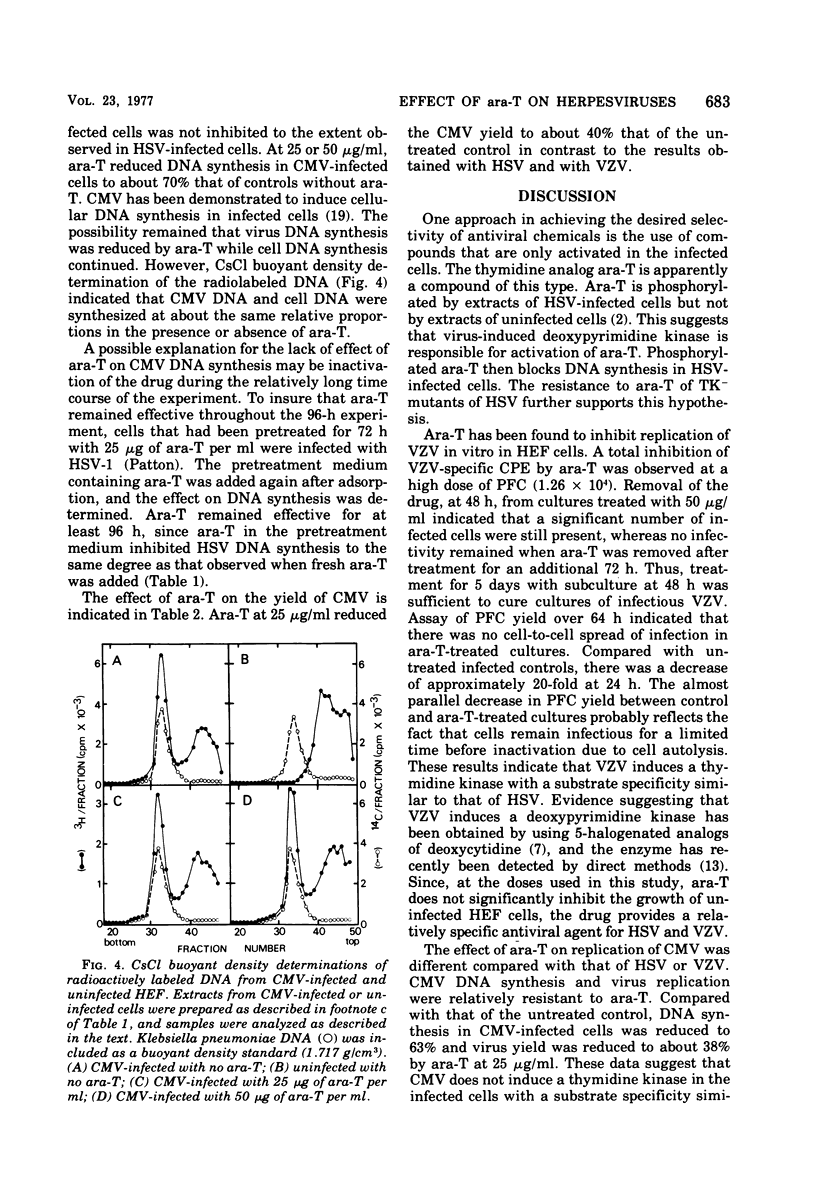
The thymidine analog 1-beta-arabinofuranosylthymine (ara-T) has previously been found to selectively inhibit herpes simplex virus replication. At a relatively nontoxic conentration (50 microgram/ml), ara-T reduced herpes simplex virus yields by 4 to 5 log10. Ara-T was also effective in inhibiting the replication of varicellazoster virus (VZV) in vitro in human embryo fibroblasts, completely preventing VZV-specific cytopathic effects. The inhibition of VZV was reversible upon drug removal at 48 h after addition but was not reversible after 5 days of treatment. ara-T also reduced cell-free virus infectivity and the plaque-forming cell yield of VZV. Compared with the untreated controls, which demonstrated a 1-log10 increase over input plaque-forming cells at 24 h after infection, 50 microgram of ara-T per ml resulted in a 1-log10 decrease. In contrast to herpes simplex virus and VZV, cytomegalovirus replication was relatively resistant to ara-T. Neither cytopathic effects nor the incorporation of [3H]thymidine into acid-insoluble material in cytomegalovirus-infected cells was markedly affected. Analysis of the newly synthesized labeled DNA by CsCl buoyant density determinations indicated that the same relative proportions of cell and virus DNA were synthesized with or without added drug. Interpretation of these results with regard to virus-induced deoxypyrimidine kinase is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Lindahl T. Epstein-Barr virus DNA in human lymphoid cell lines: in vitro conversion. Virology. 1976 Aug;73(1):96–105. doi: 10.1016/0042-6822(76)90064-7. [DOI] [PubMed] [Google Scholar]
- Aswell J. F., Gentry G. A. Cell-dependent antiherpesviral activity of 5-methylarabinosylcytosine, an intracellular ara-T donor. Ann N Y Acad Sci. 1977 Mar 4;284:342–350. doi: 10.1111/j.1749-6632.1977.tb21969.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. S. Introduction to the biochemistry of D-arabinosyl nucleosides. Prog Nucleic Acid Res Mol Biol. 1966;5:1–88. doi: 10.1016/s0079-6603(08)60231-7. [DOI] [PubMed] [Google Scholar]
- Dobersen M. J., Jerkofsky M., Greer S. Enzymatic basis for the selective inhibition of varicella-zoster virus by 5-halogenated analogues of deoxycytidine. J Virol. 1976 Nov;20(2):478–486. doi: 10.1128/jvi.20.2.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry G. A., Aswell J. F. Inhibition of herpes simplex virus replication by araT. Virology. 1975 May;65(1):294–296. doi: 10.1016/0042-6822(75)90034-3. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R. Arabinofuranosylcytosine-induced stimulation of thymidine kinase and deoxycytidylic deaminase activities of mammalian cultures. Cancer Res. 1966 Sep;26(9):1859–1866. [PubMed] [Google Scholar]
- Ogino T., Otsuka T., Takahashi M. Induction of deoxypyrimidine kinase activity in human embryonic lung cells infected with varicella-zoster virus. J Virol. 1977 Mar;21(3):1232–1235. doi: 10.1128/jvi.21.3.1232-1235.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., VANDERSLICE D. SPREAD OF ZOSTER VIRUS IN HUMAN EMBRYONIC LUNG CELLS AND THE INHIBITORY EFFECT OF LODODEOXYURIDINE. Virology. 1964 Mar;22:321–330. doi: 10.1016/0042-6822(64)90023-6. [DOI] [PubMed] [Google Scholar]
- RAPP F. VARIANTS OF HERPES SIMPLEX VIRUS: ISOLATION, CHARACTERIZATION, AND FACTORS INFLUENCING PLAQUE FORMATION. J Bacteriol. 1963 Nov;86:985–991. doi: 10.1128/jb.86.5.985-991.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. II. THE RELATION BETWEEN PROTEIN SYNTHESIS AND THE DUPLICATION OF VIRAL DNA IN INFECTED HEP-2 CELLS. Virology. 1964 Feb;22:262–269. doi: 10.1016/0042-6822(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Renis H. E., Buthala D. A. Development of resistance to antiviral drugs. Ann N Y Acad Sci. 1965 Jul 30;130(1):343–354. doi: 10.1111/j.1749-6632.1965.tb12568.x. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD G. E., WISNER C. A., WEED S. D. CYTOSINE ARABINOSIDE (CA) AND OTHER NUCLEOSIDES IN HERPES VIRUS INFECTIONS. Arch Ophthalmol. 1964 Oct;72:505–512. doi: 10.1001/archopht.1964.00970020505014. [DOI] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]




