Abstract
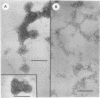
Analysis of polyoma virions by X-ray fluorometry demonstrated that calcium (Ca2+) was associated with the purified virion. Treatment of purified virions with ethyleneglycol-bis-N,N'-tetraacetic acid (EGTA), which chelates Ca2+, and the reducing agent dithiothreitol caused the virions to dissociate. Electron microscopy revealed that the virions were dissociated to the capsomere level. Incubation of polyoma virions with 150 mM NaCl, 10 mM EGTA, and 3 mM dithiothreitol was optimum for the dissociation reaction. The pH for the dissociation reaction ranged from 7.5 to 10.5. Cesium chloride density gradient centrifugation indicated that both EGTA and dithiothreitol were necessary for dissociation to occur; neither reagent alone dissociated the virus. The major protein product of the dissociated viral particles sedimented at 12S. Relationships between these experiments and the alkaline carbonate-bicarbonate dissociation of polyoma are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderer F. A., Koch M. A., Schlumberger H. D. Structure of simian virus 40. 3. Alkaline degradation of the virus particle. Virology. 1968 Mar;34(3):452–458. doi: 10.1016/0042-6822(68)90065-2. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. Multiplication of polyoma virus. II. Source of constituents for viral deoxyribonucleic acid and protein synthesis. J Bacteriol. 1966 Sep;92(3):789–791. doi: 10.1128/jb.92.3.789-791.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Zabielski J., Weil R. Plaque assay for polyoma virus on primary mouse kidney cell cultures. Appl Microbiol. 1973 Oct;26(4):627–628. doi: 10.1128/am.26.4.627-628.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham A. C., Hendry D. A., Von Wechmar M. B. Does calcium ion binding control plant virus disassembly? Virology. 1977 Apr;77(2):524–533. doi: 10.1016/0042-6822(77)90478-0. [DOI] [PubMed] [Google Scholar]
- Etchison D., Walter G. Subunit interactions in polyoma virus structure. Virology. 1977 Apr;77(2):783–796. doi: 10.1016/0042-6822(77)90499-8. [DOI] [PubMed] [Google Scholar]
- Favre M., Breitburd F., Croissant O., Orth G. Chromatin-like structures obtained after alkaline disruption of bovine and human papillomaviruses. J Virol. 1977 Mar;21(3):1205–1209. doi: 10.1128/jvi.21.3.1205-1209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., David D. Structural roles of polyoma virus proteins. J Virol. 1972 Oct;10(4):776–782. doi: 10.1128/jvi.10.4.776-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- Hare J. D., Chan J. C. Role of hydrogen and disulfide bonds in polyoma capsid structure. Virology. 1968 Mar;34(3):481–491. doi: 10.1016/0042-6822(68)90068-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Characterization of polyoma DNA-protein complexes. I. Electrophoretic identification of the proteins in a nucleoprotein complex isolated from polyoma-infected cells. J Virol. 1974 Dec;14(6):1326–1336. doi: 10.1128/jvi.14.6.1326-1336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. In vitro radioisotopic labeling of proteins associated with purified polyoma virions. J Virol. 1974 Dec;14(6):1627–1629. doi: 10.1128/jvi.14.6.1627-1629.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., To C. M., Consigli R. A. Alkaline degradation of polyoma virus. J Gen Virol. 1969 Apr;4(3):403–411. doi: 10.1099/0022-1317-4-3-403. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Walter G., Deppert W. Intermolecular disulfide bonds: an important structural feature of the polyoma virus capsid. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):255–257. doi: 10.1101/sqb.1974.039.01.033. [DOI] [PubMed] [Google Scholar]