Abstract
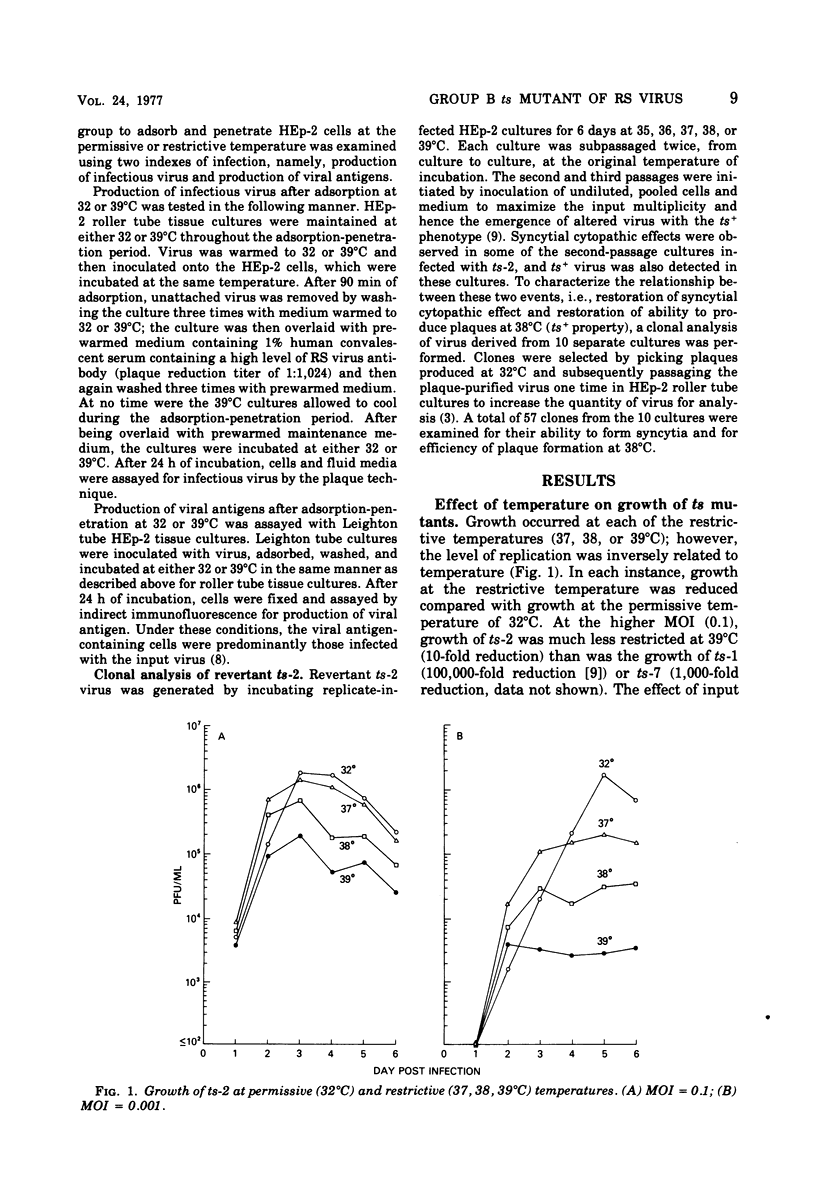
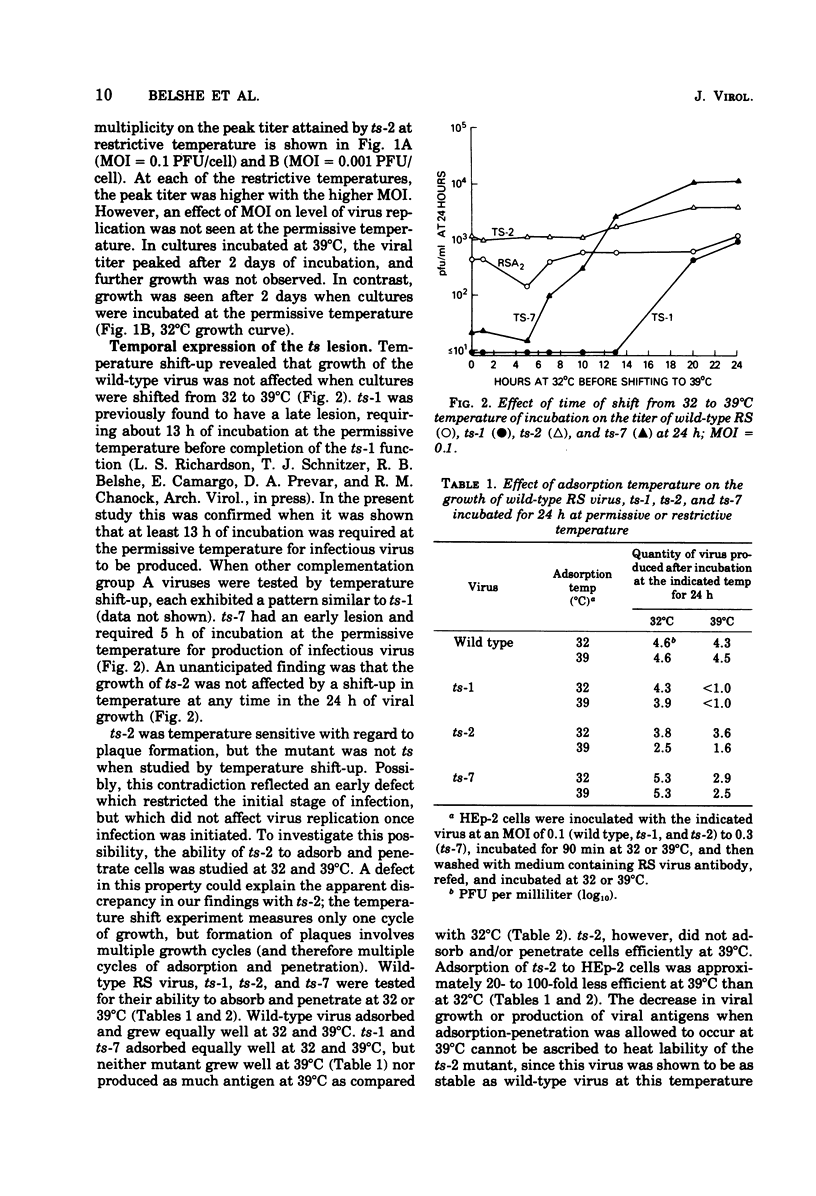
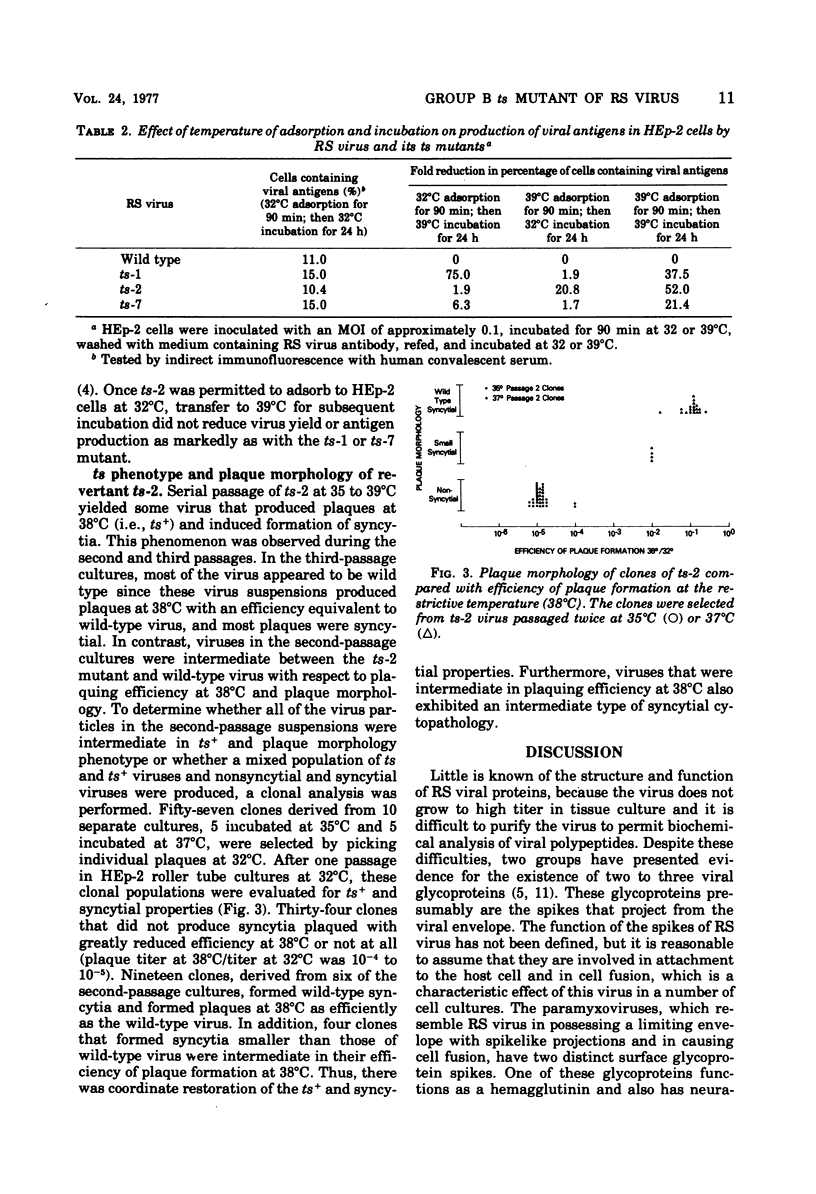
ts-2, a temperature-sensitive and plaque morphology mutant of respiratory syncytial virus and sole representative of complementation group B, was compared with members of the other complementation groups of respiratory syncytial virus (group A [ts-1] and group C [ts-7]). ts-2 was found to be 10- to 1,000-fold more restricted in growth and ability to spread at restrictive temperatures (37, 38, and 39 degrees C) than at the permissive temperature (32 degrees C). In temperature shift-up experiments, the ts defect of ts-1 and other members of complementation group A was found to effect a late function that was required for at least 13 h in the replicative cycle. The ts lesion of ts-7 affected a function early in the replication cycle. In contrast, ts-2 was not temperature sensitive when studied by the shift-up technique. The discrepancy between the ts plaque property and failure to detect temperature sensitivity during the shift-up experiment was resolved when it was shown that ts-2 had a defect in adsorption or penetration or both at the restrictive temperature. Clonal analysis of revertant ts-2 showed a coordinate restoration of ts+ phenotype ans syncytium-forming capacity. It appears that ts-2 has a defect in a protein that is involved in adsorption and/or penetration of virus and is also responsible for cell fusion activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gharpure M. A., Wright P. F., Chanock R. M. Temperature-sensitive mutants of respiratory syncytial virus. J Virol. 1969 Apr;3(4):414–421. doi: 10.1128/jvi.3.4.414-421.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes D. S., Kim H. W., Parrott R. H., Camargo E., Chanock R. M. Genetic alteration in a temperature-sensitive mutant of respiratory syncytial virus after replication in vivo. Proc Soc Exp Biol Med. 1974 Apr;145(4):1158–1164. doi: 10.3181/00379727-145-37972. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Wright P. F., Hetrick F. M., Chanock R. M. Electron microscopic studies of respiratory syncytial temperature-sensitive mutants. Arch Gesamte Virusforsch. 1973;41(3):248–258. doi: 10.1007/BF01252772. [DOI] [PubMed] [Google Scholar]
- Levine S. Polypeptides of respiratory syncytial virus. J Virol. 1977 Jan;21(1):427–431. doi: 10.1128/jvi.21.1.427-431.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman A. V., Pedreira F. A., Tauraso N. M. Attempts to demonstrate hemagglutination and hemadsorption by respiratory syncytial virus. Appl Microbiol. 1971 Jun;21(6):1099–1100. doi: 10.1128/am.21.6.1099-1100.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schieble J. H., Kase A., Lennette E. H. Fluorescent cell counting as an assay method for respiratory syncytial virus. J Virol. 1967 Jun;1(3):494–499. doi: 10.1128/jvi.1.3.494-499.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Richardson L. S., Chanock R. M. Growth and genetic stability of the ts-1 mutant of respiratory syncytial virus at restrictive temperatures. J Virol. 1976 Feb;17(2):431–438. doi: 10.1128/jvi.17.2.431-438.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Gharpure M. A., Hodes D. S., Chanock R. M. Genetic studies of respiratory syncytial virus temperature-sensitive mutants. Arch Gesamte Virusforsch. 1973;41(3):238–247. doi: 10.1007/BF01252771. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Respiratory syncytial virus proteins. Virology. 1976 Aug;73(1):228–243. doi: 10.1016/0042-6822(76)90077-5. [DOI] [PubMed] [Google Scholar]



