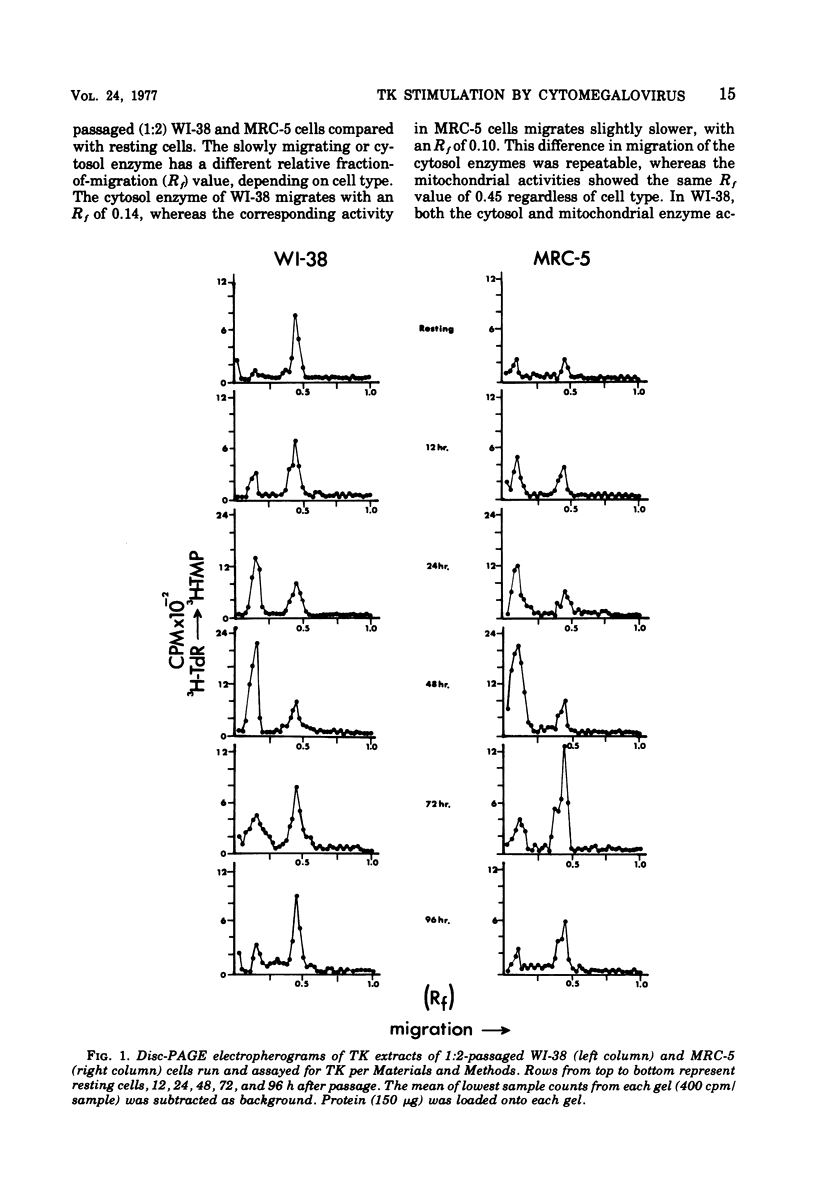
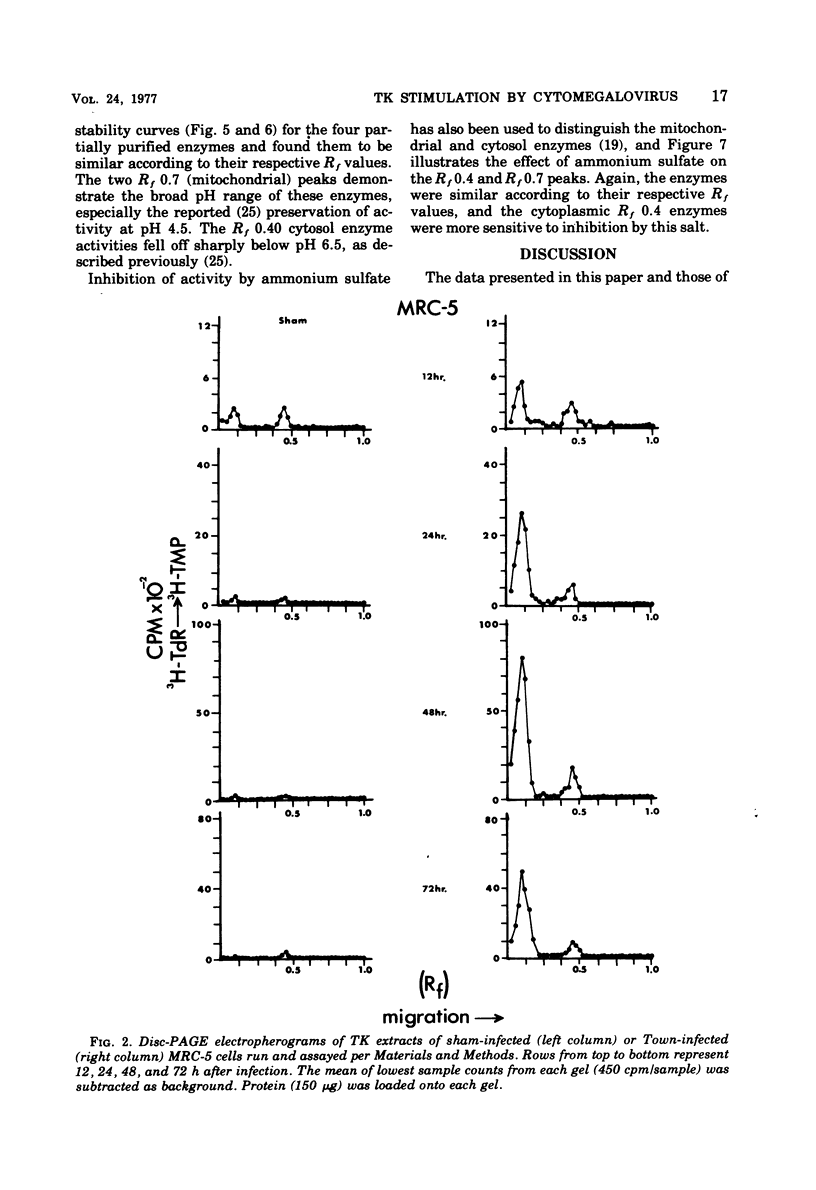
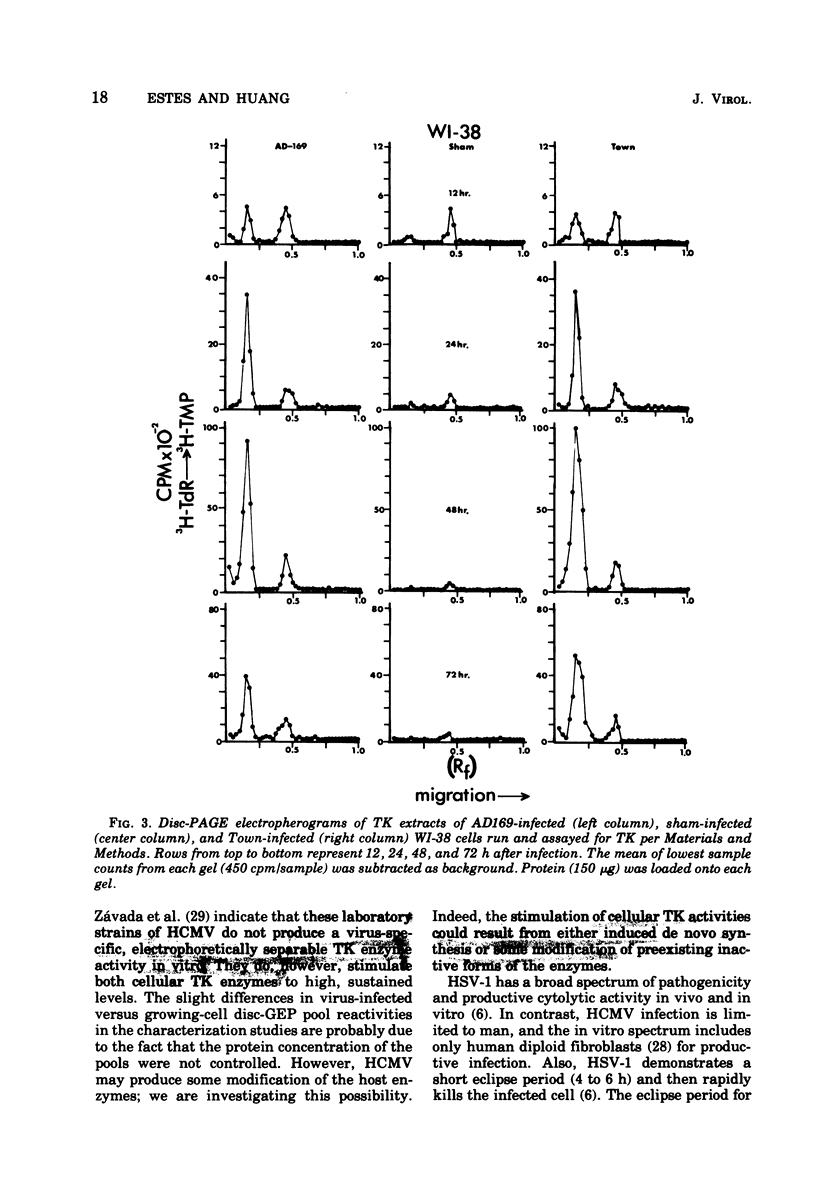
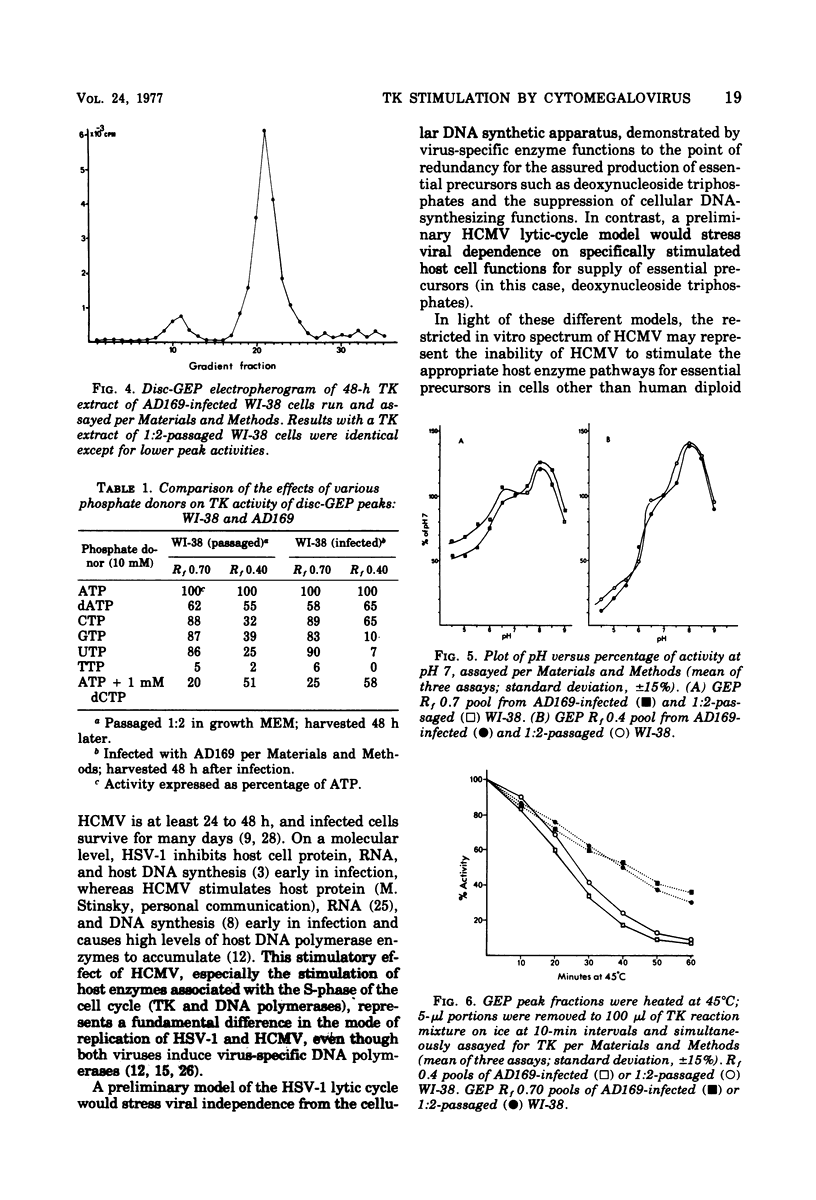
Abstract
Thymidine kinase (TK) activity in WI-38 and MRC-5 human fibroblasts was analyzed by discontinuous polyacrylamide gel electrophoresis (disc-PAGE) and discontinuous glycerol gradient electrophoresis (disc-GEP) after subculture or human cytomegalovirus (HCMV) infection. Two peaks of TK activity with different relative fraction-of-migration (Rf) values were resolved by disc-PAGE or disc-GEP in extracts from log-phase and infected cells. Growing WI-38 cells expressed a slowly migrating (Rf = 0.14 PAGE, Rf = 0.4 GEP) peak of TK activity, which was partially inhibited by 1.0 mM dCTP, but which retained little activity at pH 4.5. Growing MRC cells also displayed a slowly migrating peak (Rf = 0.10 PAGE) with similar properties. Both cell types expressed a faster-migrating TK activity (Rf = 0.45 PAGE, Rf = 0.7 GEP) in the growing and resting state that was strongly inhibited by 1 mM dCTP but retained 50% activity at pH 4.5. When either cell type was infected with HCMV, there was a rapid and high-level stimulation of the slowly migrating form of TK and a slight stimulation of the faster-migrating form. Two strains of HCMV (AD169 and Town) failed to produce an electrophoretically distinct virus TK in either cell type after infection. TK enzymes were partially purified by disc-GEP from extracts of log-phase WI-38 or AD169-infected WI-38 cells. Characterization of these enzymes with respect to phosphate donor specificity, pH optima, thermostability, and salt inhibition showed the HCMV-stimulated TKs to be of cellular origin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., McAuslan B. R. Expression of thymidine kinase variants is a function of the replicative state of cells. Cell. 1974 Jun;2(2):113–117. doi: 10.1016/0092-8674(74)90100-7. [DOI] [PubMed] [Google Scholar]
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- BESSMAN M. J., LEHMAN I. R., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. II. General properties of the reaction. J Biol Chem. 1958 Jul;233(1):171–177. [PubMed] [Google Scholar]
- Bjursell G., Reichard P. Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J Biol Chem. 1973 Jun 10;248(11):3904–3909. [PubMed] [Google Scholar]
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Kaplan A. S. Replication of human cytomegalovirus DNA: lack of dependence on cell DNA synthesis. J Virol. 1976 Jun;18(3):1063–1070. doi: 10.1128/jvi.18.3.1063-1070.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geder K. M., Lausch R., O'Neill F., Rapp F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science. 1976 Jun 11;192(4244):1134–1137. doi: 10.1126/science.179143. [DOI] [PubMed] [Google Scholar]
- Geder L. Evidence for early nuclear antigens in cytomegalovirus-infected cells. J Gen Virol. 1976 Aug;32(2):315–319. doi: 10.1099/0022-1317-32-2-315. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Keir H. M., Subak-Sharpe H., Shedden W. I., Watson D. H., Wildy P. Immunological evidence for a specific DNA polymerase produced after infection by herpes simplex virus. Virology. 1966 Sep;30(1):154–157. doi: 10.1016/s0042-6822(66)81022-x. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Anken M. Altered properties of thymidine kinase after infection of mouse fibroblast cells with herpes simplex virus. J Virol. 1967 Feb;1(1):238–240. doi: 10.1128/jvi.1.1.238-240.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Trkula D. Properties of mitochondrial thymidine kinases of parental and enzyme-deficient HeLa cells. Arch Biochem Biophys. 1973 Oct;158(2):503–513. doi: 10.1016/0003-9861(73)90542-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence W. C. Evidence for a relationship between equine abortion (herpes) virus deoxyribonucleic acid synthesis and the S phase of the KB cell mitotic cycle. J Virol. 1971 Jun;7(6):736–748. doi: 10.1128/jvi.7.6.736-748.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- RAYMOND S. ACRYLAMIDE GEL ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:350–365. doi: 10.1111/j.1749-6632.1964.tb14208.x. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- Roller B., Cohen G. H. Deoxyribonucleoside triphosphate pools in synchronized human cells infected with herpes simplex virus types 1 and 2. J Virol. 1976 Apr;18(1):58–64. doi: 10.1128/jvi.18.1.58-64.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Furukawa T., Plotkin S. A. Human cytomegalovirus stimulates host cell RNA synthesis. J Virol. 1975 Feb;15(2):297–304. doi: 10.1128/jvi.15.2.297-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. T., Stafford M. A., Jones O. W. Properties of thymidine kinase partially purified from human fetal and adult tissue. J Biol Chem. 1972 Mar 25;247(6):1930–1935. [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Weissbach A., Schlabach A., Fridlender B., Bolden A. DNA polymerases from human cells. Nat New Biol. 1971 Jun 9;231(23):167–170. doi: 10.1038/newbio231167a0. [DOI] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]



