Abstract
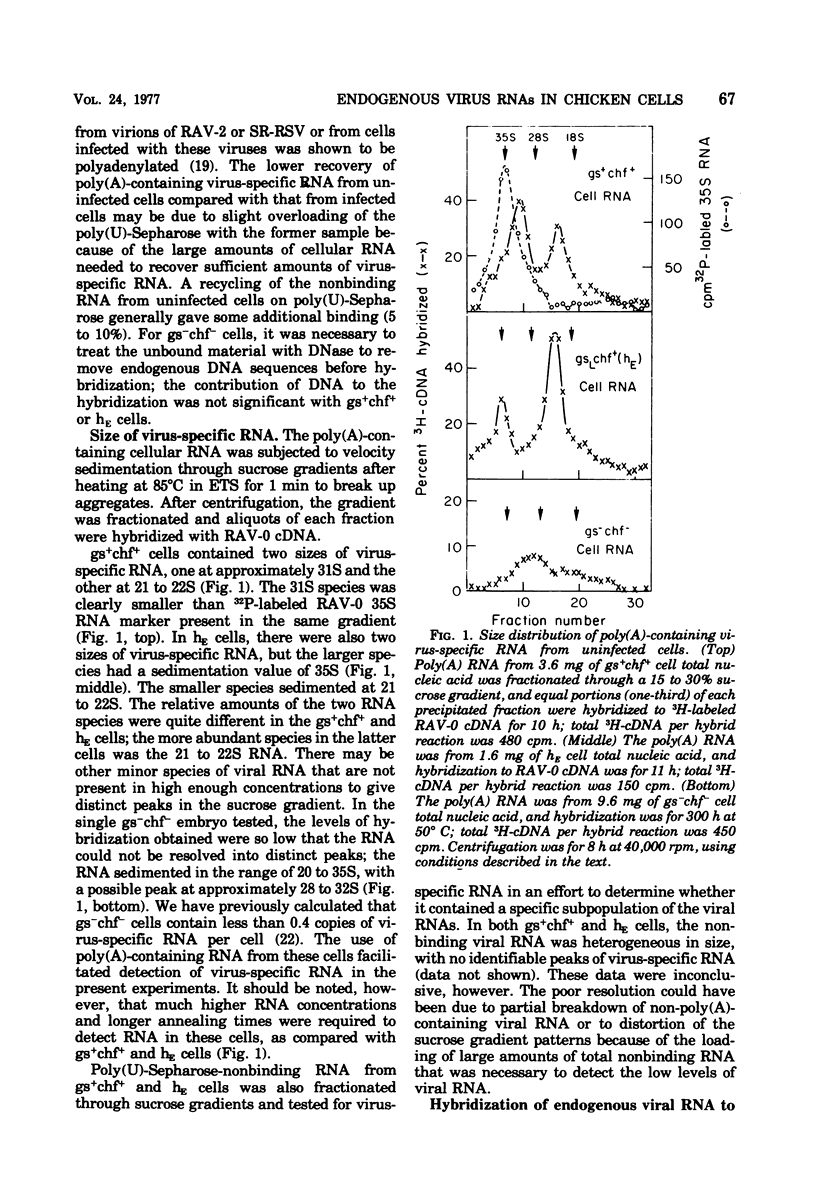
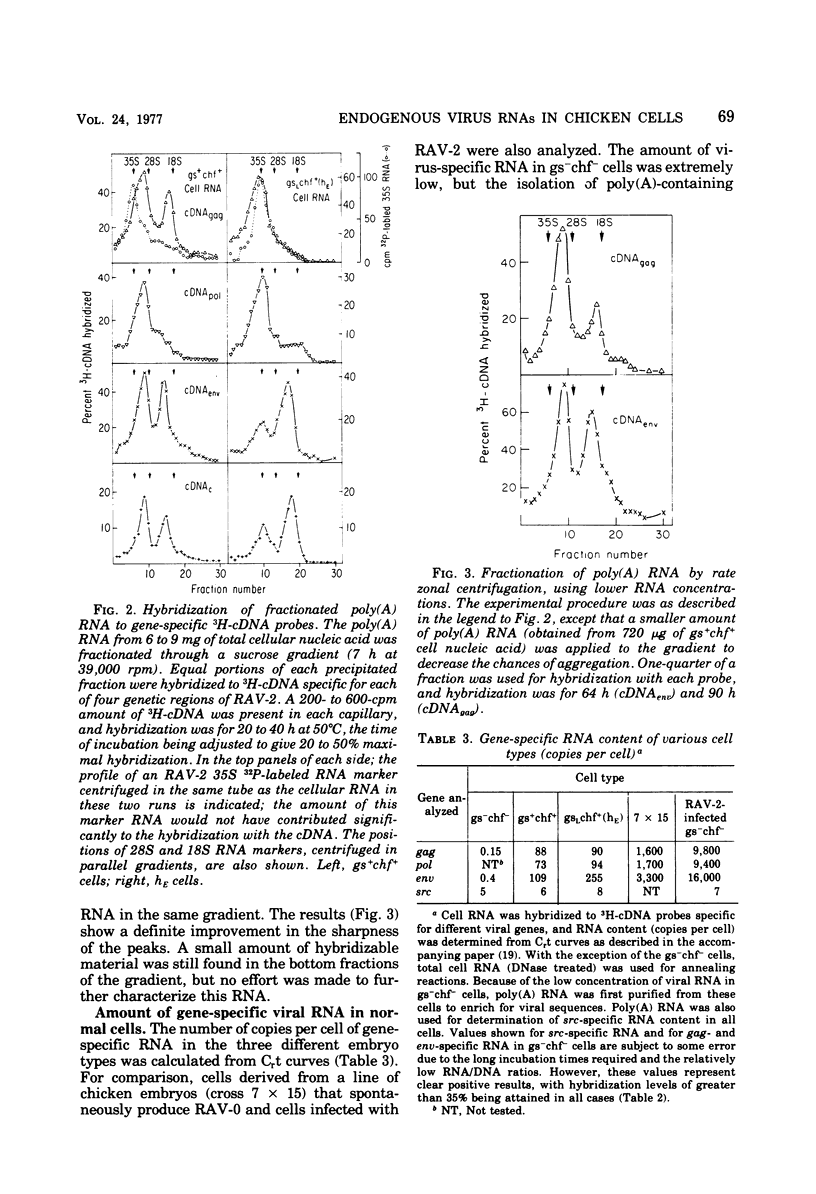
Uninfected cells from two different phenotypes of chicken embryos express significant amounts of endogenous viral information, though they do not produce virus particles. Cells of the phenotype gs+chf+ are positive for both group-specific (gs) antigens and chicken helper factor (chf) activity, whereas cells of a second phenotype, gsLchf+(hE), demonstrate noncoordinate expression of these two viral activities (very low amounts of gs antigens, but extremely high helper activity). RNA from these cells was analyzed to determine the size, genetic content, and relative abundance of virus-specific RNAs in cells of each phenotype. Two major size classes of polyadenylic acid-containing RNA, homologous to the avian leukosis virus genome, were detectable in cells of both types. The larger RNA, which contained most of the sequences of the leukosis virus genome, was of different sizes in the two phenotypes, 31S in gs+chf+ cells but 35S in the noncoordinate cell type. Analysis of the viral RNA with gene-specific complementary DNA probes revealed the following characteristics. (i) The 31S RNA appeared to lack portions of the gag and pol genes. (ii) A smaller RNA species, which sedimented at 21S in both cell types, was a transcript of the 3′-proximal portion of the viral genome, consisting of the env gene and the “common” sequences. (iii) The amount of env-specific RNA in the 21S region was more than six times higher in the noncoordinate cell type than in the gs+chf+ cells; this difference was concordant with the 5- to 10-fold higher chf activity in the noncoordinate cells. (iv) The endogenous viral RNA in uninfected cells and the RNA from Rous-associated virus-0 virions hybridized only partially with DNA complementary to the common region of the Rous-associated virus-2 genome, whereas the RNA of all exogenous viruses tested hybridized almost completely to this complementary DNA. Small amounts of src-specific polyadenylated RNA were also present in uninfected chicken cells. This RNA sedimented as a single peak at 26S and was not covalently linked to any other identifiable virus-specific RNA sequences. The amount of src RNA was the same in the above two types of expression-positive cells and also in cells that were gs−chf−, indicating that the transcription of the cellular sequences homologous to the src gene is independent of the transcription of the other endogenous viral genes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T., Toyoshima K. Genetic control of chick helper factor in cells which lack natural group-specific antigen of avian leukosis. Virology. 1976 Sep;73(2):521–527. doi: 10.1016/0042-6822(76)90413-x. [DOI] [PubMed] [Google Scholar]
- Bachenheimer S., Darnell J. E. Adenovirus-2 mRNA is transcribed as part of a high-molecular-weight precursor RNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4445–4449. doi: 10.1073/pnas.72.11.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Hanafusa H. Detection of a protein of avian leukoviruses in uninfected chick cells by radioimmunoassay. J Virol. 1974 Feb;13(2):340–346. doi: 10.1128/jvi.13.2.340-346.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Hayward W. S., Hanafusa H. Avian tumor virus proteins and RNA in uninfected chicken embryo cells. J Virol. 1974 Dec;14(6):1419–1429. doi: 10.1128/jvi.14.6.1419-1429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden L. B., Motta J. V., Smith E. J. Genetic control of RAV-O production in chickens. Virology. 1977 Jan;76(1):90–97. doi: 10.1016/0042-6822(77)90285-9. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Derman E., Darnell J. E. Relationship of chain transcription to poly(A) addition and processing of hnRNA in HeLa cells. Cell. 1974 Nov;3(3):255–264. doi: 10.1016/0092-8674(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S. Lack of relationship between infection with avian leukosis virus and the presence of COFAL antigen in chick embryos. Virology. 1966 Aug;29(4):586–595. doi: 10.1016/0042-6822(66)90282-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Besmer P. RNA metabolism of murine leukemia virus II. Endogenous virus-specific RNA in the uninfected BALB/c cell line JLS-V9. J Virol. 1975 Apr;15(4):836–842. doi: 10.1128/jvi.15.4.836-842.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Mueller-Lantzsch N. RNA metabolism of murine leukemia virus. III. Identification and quantitation of endogenous virus-specific mRNA in the uninfected BALB/c cell line JLS-V9. J Virol. 1976 May;18(2):401–410. doi: 10.1128/jvi.18.2.401-410.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T., Kawai S. Genetic control of expression of endogenous virus genes in chicken cells. Virology. 1974 Apr;58(2):439–448. doi: 10.1016/0042-6822(74)90078-6. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Miyamoto T., Hanafusa T. A cell-associated factor essential for formation of an infectious form of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):314–321. doi: 10.1073/pnas.66.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T., Fleissner E. Existence and expression of tumor virus genes in chick embryo cells. Virology. 1972 Feb;47(2):475–482. doi: 10.1016/0042-6822(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Independent regulation of endogenous and exogenous avian RNA tumor virus genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2259–2263. doi: 10.1073/pnas.73.7.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Coffin J. M. Rate of virus-specific RNA synthesis in synchronized chicken embryo fibroblasts infected with avian leukosis virus. J Virol. 1976 Feb;17(2):393–401. doi: 10.1128/jvi.17.2.393-401.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Messenger RNA isolation with poly(U) agarose. Methods Enzymol. 1974;34:496–499. doi: 10.1016/s0076-6879(74)34061-x. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Padgett T. G., Stubbledield E., Varmus H. E. Chicken macrochromosomes contain an endogenous provirus and microchromosomes contain sequences related to the transforming gene of ASV. Cell. 1977 Apr;10(4):649–657. doi: 10.1016/0092-8674(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Chubb R. C. Studies on the nature and genetic control of an antigen in normal chick embryos which reacts in the COFAL test. J Gen Virol. 1968 Dec;3(3):379–391. doi: 10.1099/0022-1317-3-3-379. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Lamoreux W. F. Expression of endogenous ALV antigens and susceptibility to subgroup E ALV in three strains of chickens (endogenous avian C-type virus). Virology. 1976 Jan;69(1):50–62. doi: 10.1016/0042-6822(76)90193-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Dastoor M. N., Baluda M. A. Evidence for tandem integration of avian myeloblastosis virus DNA with endogenous provirus in leukemic chicken cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1749–1753. doi: 10.1073/pnas.73.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. J., Stephenson J. R., Crittenden L. B., Aaronson S. A. Avian leukosis-sarcoma virus gene expression. Noncoordinate control of group-specific antigens in virus-negative avian cells. Virology. 1976 Apr;70(2):493–501. doi: 10.1016/0042-6822(76)90290-7. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Gilden R. V., Hatanaka M. Sarcoma-virus-related RNA sequences in normal rat cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4503–4507. doi: 10.1073/pnas.71.11.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Payne L. N. The heritable nature of the factor in chicken cells which acts as a helper virus for Rous sarcoma virus. Virology. 1971 Aug;45(2):508–515. doi: 10.1016/0042-6822(71)90351-5. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Bolden A., Muller R., Hanafusa H., Hanafusa T. Deoxyribonucleic acid polymerase activities in normal and leukovirus-infected chicken embryo cells. J Virol. 1972 Sep;10(3):321–327. doi: 10.1128/jvi.10.3.321-327.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegers U., Hilz H. A new method using 'proteinase K' to prevent mRNA degradation during isolation from HeLa cells. Biochem Biophys Res Commun. 1971 Jul 16;44(2):513–519. doi: 10.1016/0006-291x(71)90632-2. [DOI] [PubMed] [Google Scholar]



