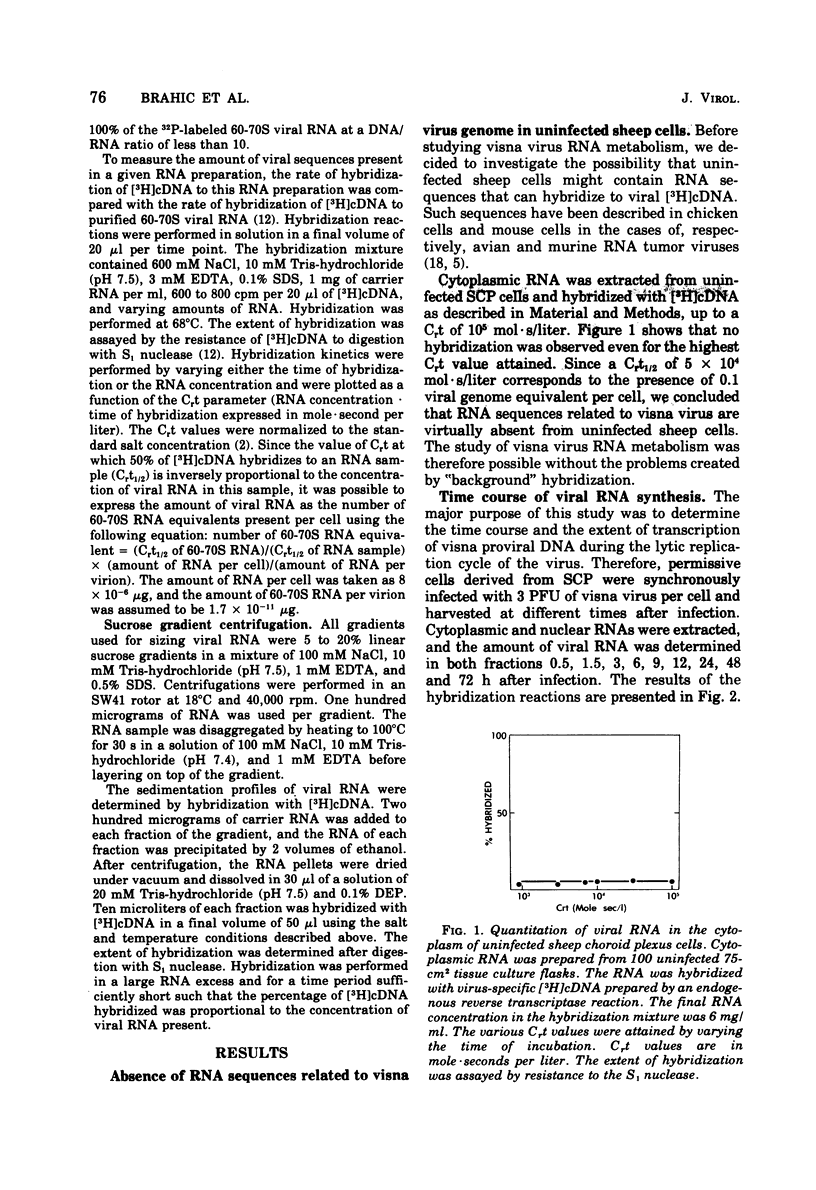
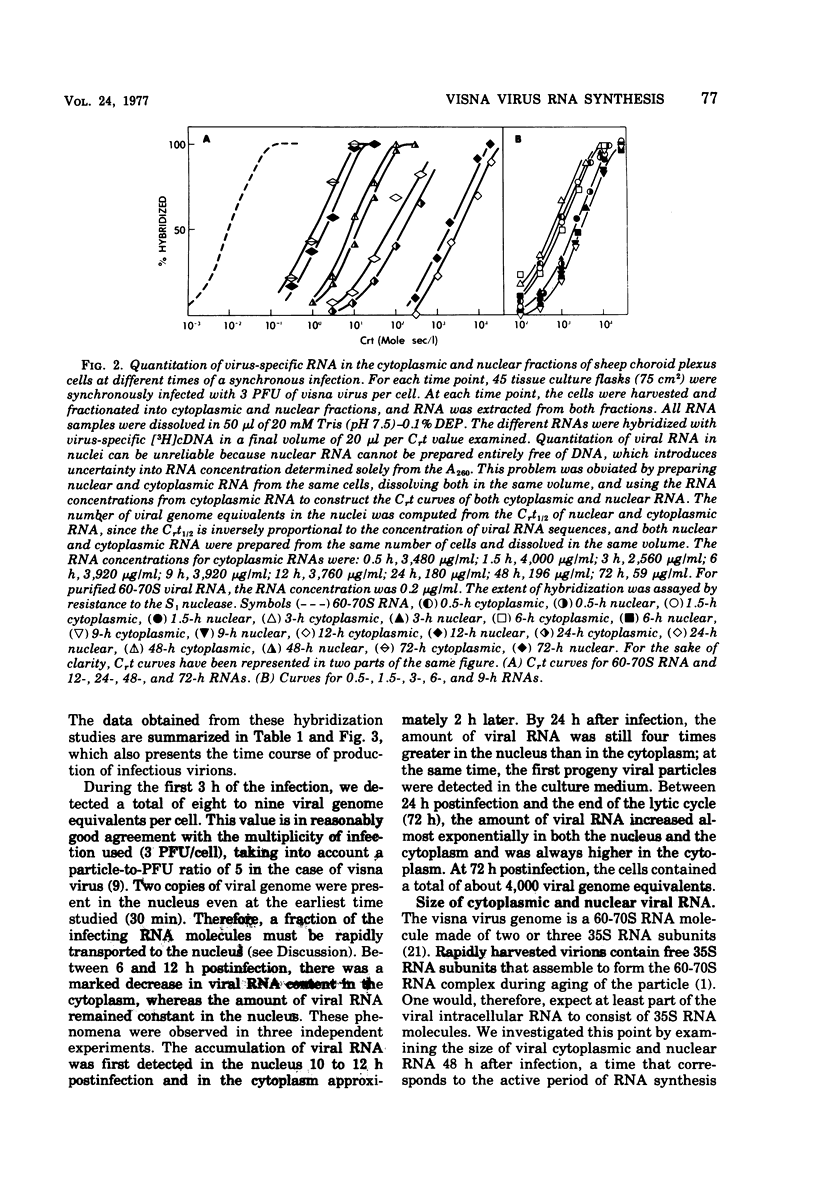
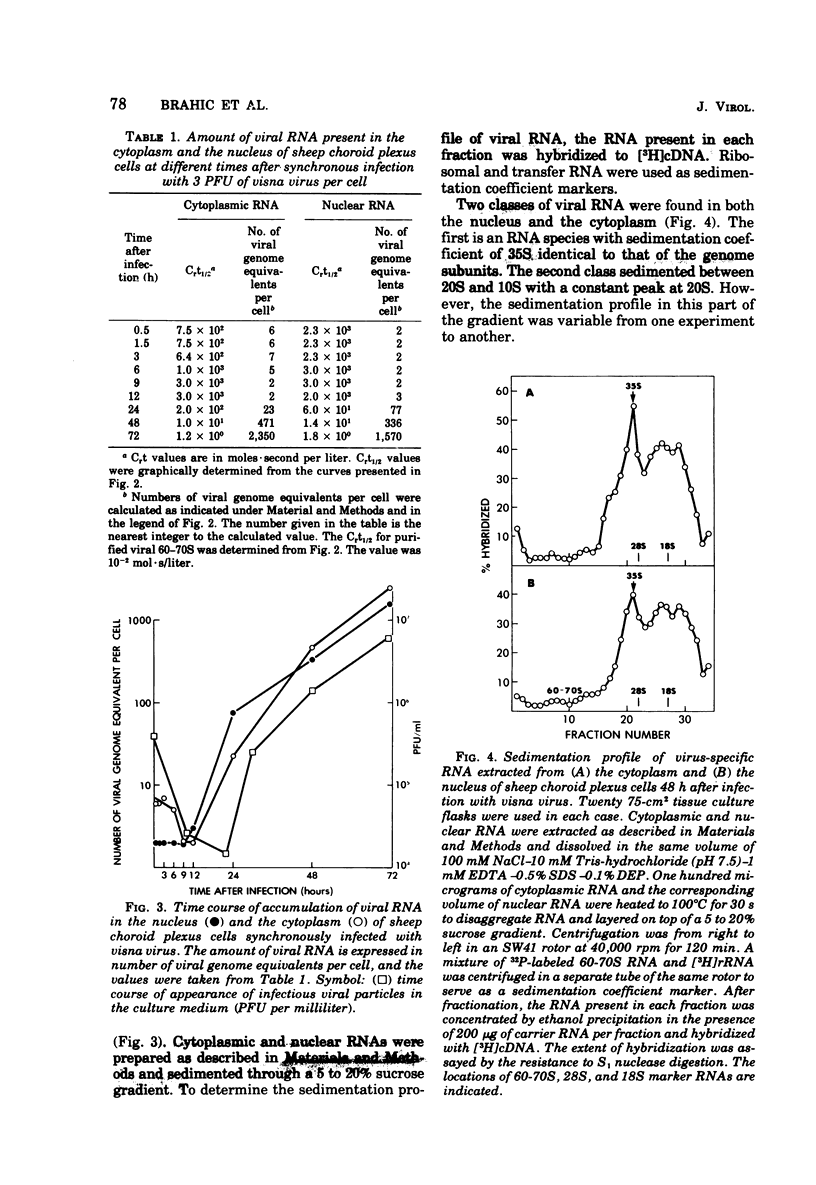
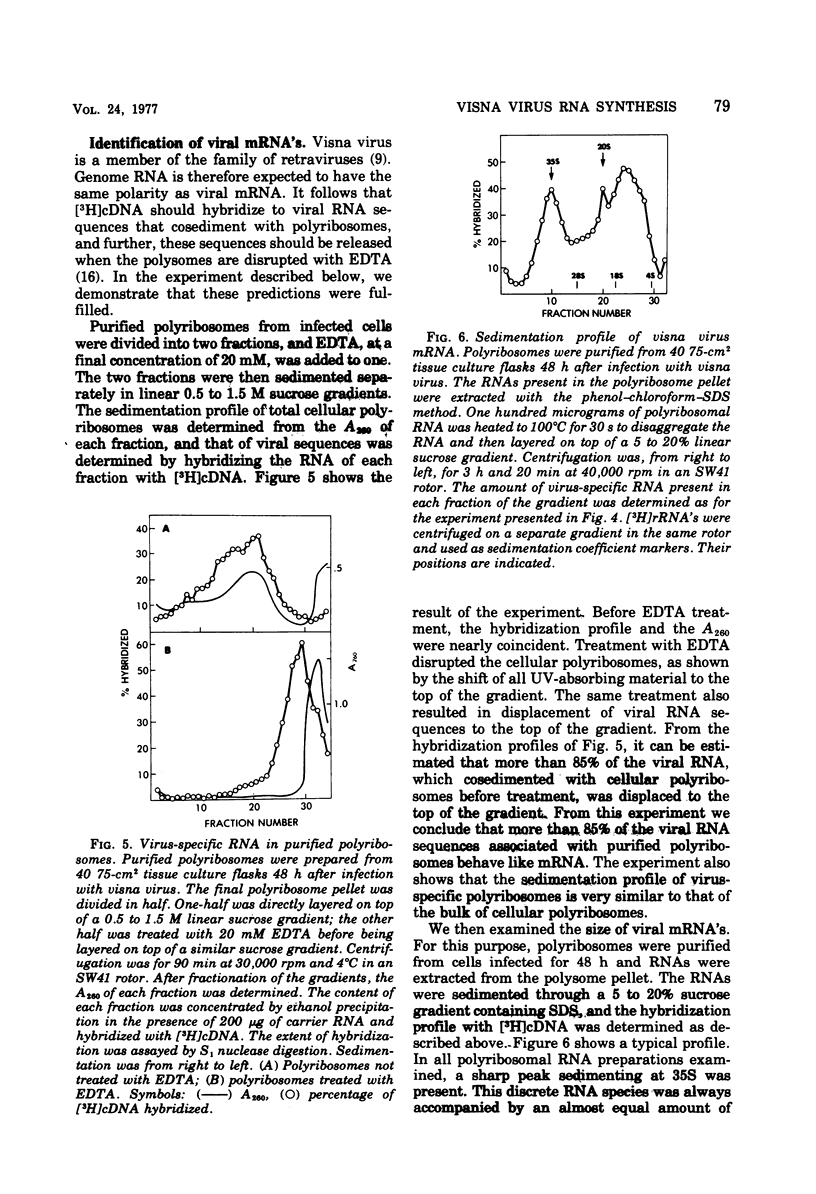
Abstract
Visna is a classical slow infection in which virus characteristically persists in the face of the host immune response. The agent of this disease belongs to the retravirus group. The persistence of infection and the slow spread of virus are at least in part a consequence of restriction of the expression of virus genetic information in tissues of an infected animal (A. T. Haase et al., Science 195:175-177, 1977), but the point at which the virus life cycle is interrupted in vivo and the mechanism of restriction are unknown. We have embarked on a molecular analysis of restriction, focusing first on transcription. In this paper we have established the levels of viral RNA synthesis under permissive conditions, as a base line for subsequent studies in vivo. We show that (i) uninfected cells do not contain RNA sequences related to the visna virus genome, (ii) parental RNA is rapidly transported to the nucleus of the infected cell, (iii) virus RNA is synthesized in the nucleus and then transported to the cytoplasm (iv) synthesis of RNA proceeds mostly exponentially to reach levels of about 4,000 copies per cell at the end of the growth cycle, (v) nuclear and cytoplasmic RNA sediment in two size classes, 35S and 10-20S, (vi) viral mRNA has the same polarity as genome RNA and also sediments in two size classes of 35S and 10-20S.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahic M., Vigne R. Properties of visna virus particles harvested at short time intervals: RNA content, infectivity, and ultrastructure. J Virol. 1975 May;15(5):1222–1230. doi: 10.1128/jvi.15.5.1222-1230.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Hanafusa H. Penetration and intracellular release of the genomes of avian RNA tumor viruses. Virology. 1972 Nov;50(2):440–458. doi: 10.1016/0042-6822(72)90396-0. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Gielkens A. L., Salden M. H., Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Garapin A. C., Faras A. J., Varmus H. E., Bishop J. M. Characterization of the nucleic acid product of the visna virus RNA dependent DNA polymerase. Virology. 1974 Jan;57(1):251–258. doi: 10.1016/0042-6822(74)90125-1. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Varmus H. E. Demonstration of a DNA provirus in the lytic growth of visna virus. Nat New Biol. 1973 Oct 24;245(147):237–239. doi: 10.1038/newbio245237a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Baltimore D. Size of murine RNA tumor virus-specific nuclear RNA molecules. J Virol. 1976 Aug;19(2):331–337. doi: 10.1128/jvi.19.2.331-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Fan H. Monospecific immunoprecipitation of murine leukemia virus polyribosomes: identification of p30 protein-specific messenger RNA. Cell. 1976 Dec;9(4 Pt 1):579–588. doi: 10.1016/0092-8674(76)90040-4. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Coffin J. M., Haroz R. K., Bromley P. A., Weissmann C. Quantitative determination and location of newly synthesized virus-specific ribonucleic acid in chicken cells infected with Rous sarcoma virus. J Virol. 1973 May;11(5):761–774. doi: 10.1128/jvi.11.5.761-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Robin M. S., Green M. A possible requirement for protein synthesis early in the infectious cycle of the murine sarcoma-leukemia virus. Virology. 1977 Jan;76(1):341–351. doi: 10.1016/0042-6822(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Shanmugam G., Bhaduri S., Green M. The virus-specific RNA species in free and membrane-bound polyribosomes of transformed cells replicating murine sarcoma-leukemia viruses. Biochem Biophys Res Commun. 1974 Feb 4;56(3):697–702. doi: 10.1016/0006-291x(74)90661-5. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Fan W. J., Heasley S., Bishop J. M. Synthesis of viral DNA in the cytoplasm of duck embryo fibroblasts and in enucleated cells after infection by avian sarcoma virus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3874–3878. doi: 10.1073/pnas.71.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne R., Brahic M., Filippi P., Tamalet J. Complexity and polyadenylic acid content of visna virus 60-70S RNA. J Virol. 1977 Jan;21(1):386–395. doi: 10.1128/jvi.21.1.386-395.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


