Abstract
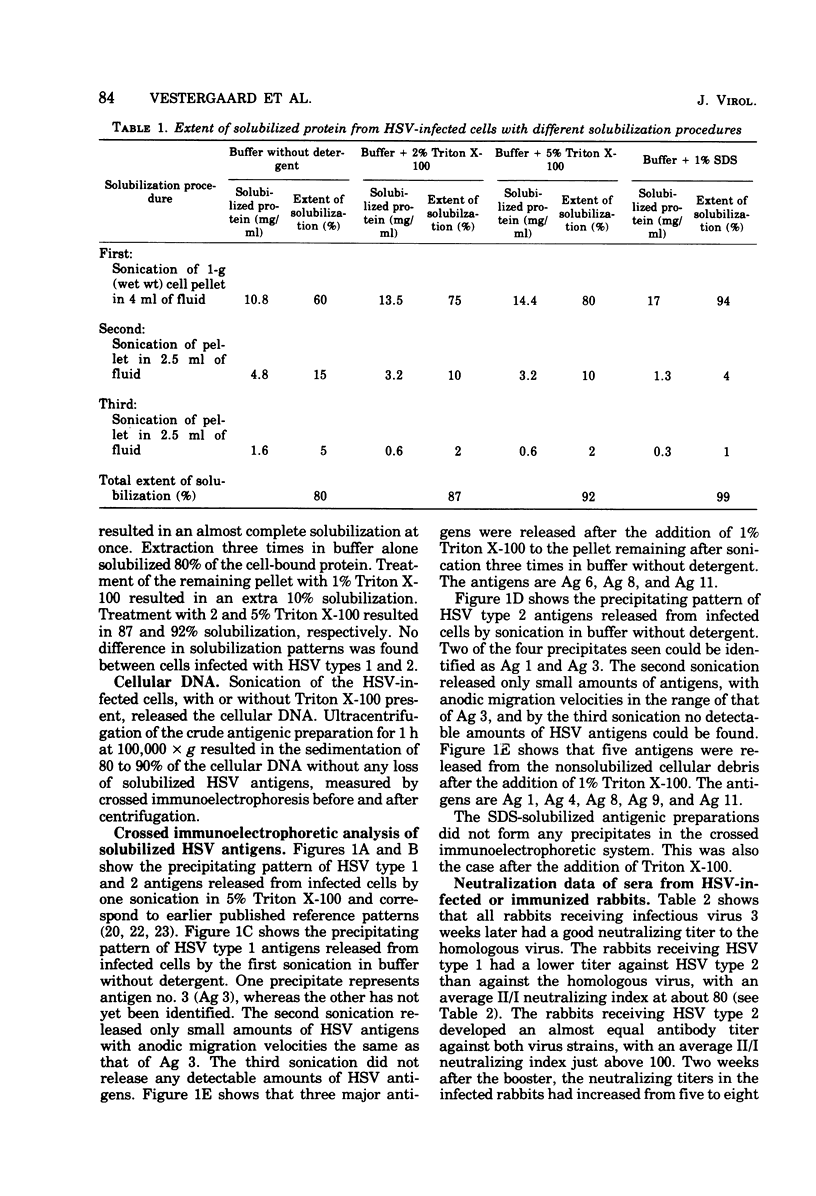
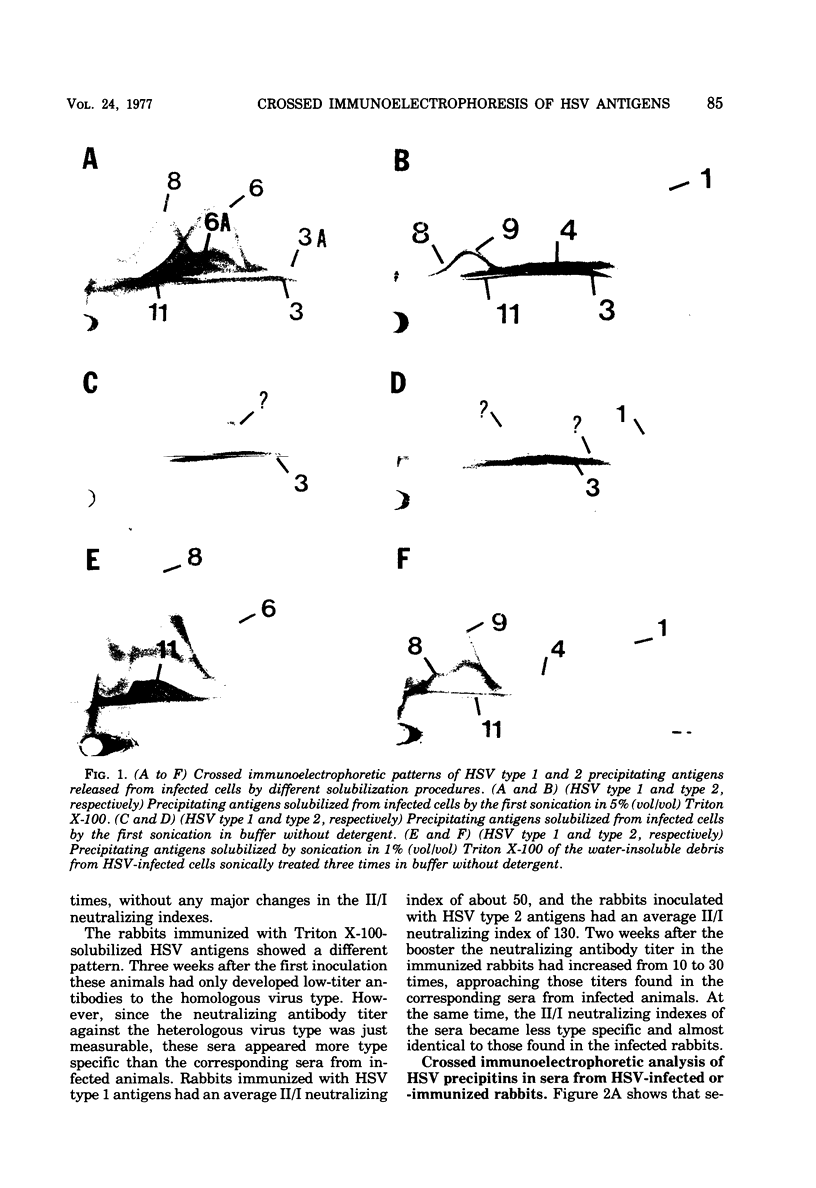
The nonionic detergent Triton X-100 was capable of solubilizing 90% of the protein content in herpes simplex virus (HSV)-infected rabbit cornea cells. The solubilized HSV antigens formed well-characterized precipitates by crossed immunoelectrophoresis in Triton X-100-containing agarose gel, allowing both identification and relative quantitation. Water-soluble and detergent-requiring HSV antigens were identified by different solubilization procedures in buffer with and without detergent. Five glycoprotein antigens were solubilized only in the presence of detergent, indicating their membrane-bound state. One non-glycosylated antigen was present in both a water-soluble and a membrane-bound form. Based upon the crossed immunoelectrophoretic precipitating patterns of Triton X-100-solubilized HSV antigens, it has been estimated that infected cells yield an amount of virus-specific protein equivalent to 2,000 enveloped virions per cell. Rabbits inoculated intracutaneously with Triton X-100-solubilized HSV antigens developed neutralizing antibodies against HSV almost as effectively as rabbits with an active HSV infection. Precipitins against individual HSV antigens in sera from rabbits infected with HSV and immunized with the Triton X-100-solubilized HSV antigens were assayed by the crossed immunoelectrophoretic technique. Sera from infected rabbits reacted more strongly and with a higher number of HSV antigens than sera from immunized rabbits.
Full text
PDF


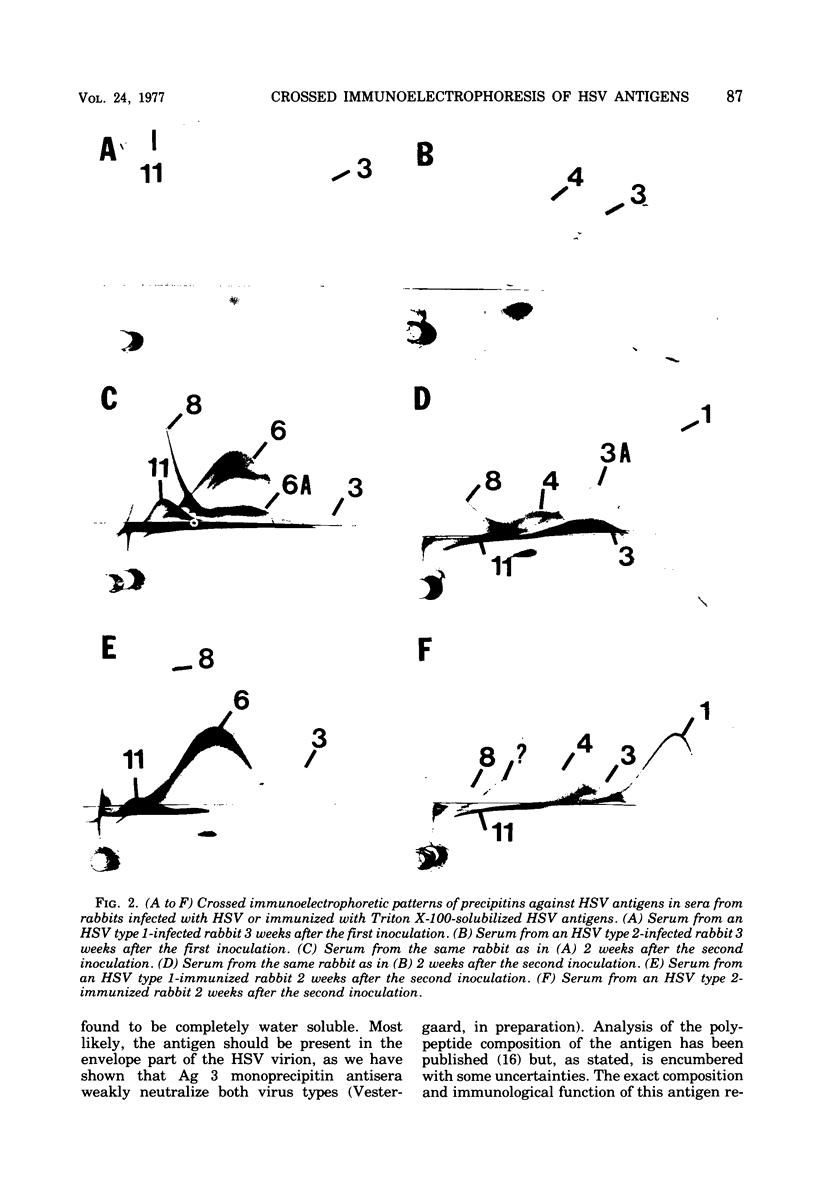
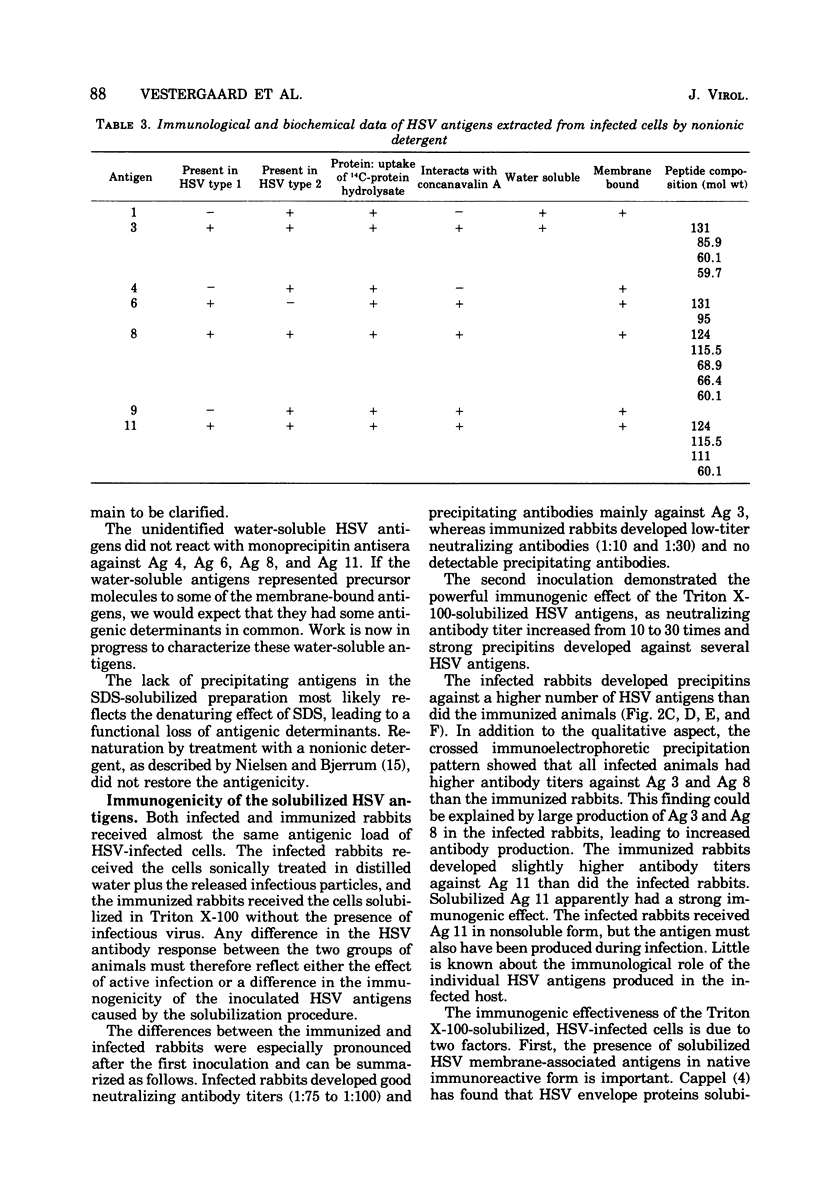


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H. Intermediate gel in crossed and in fused rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:71–77. doi: 10.1111/j.1365-3083.1973.tb03782.x. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J., Bog-Hansen T. C. The immunochemical approach to the characterization of membrane proteins. Human erythrocyte membrane proteins analysed as a model system. Biochim Biophys Acta. 1976 Nov 11;455(1):66–89. doi: 10.1016/0005-2736(76)90154-1. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J., Lundahl P. Crossed immunoelectrophoresis of human erythrocyte membrane proteins. Immunoprecipitation patterns for fresh and stored samples of membranes extensively solubilized with non-ionic detergents. Biochim Biophys Acta. 1974 Mar 14;342(1):69–80. [PubMed] [Google Scholar]
- Cappel R. Comparison of the humoral and cellular immune response after immunization with live, UV inactivated herpes simplex virus and a subunit vaccine and efficacy of these immunizations. Arch Virol. 1976;52(1-2):29–35. doi: 10.1007/BF01317862. [DOI] [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- Courtney R. J., Powell K. L. Immunological and biochemical characterization of polypeptides induced by herpes simplex virus types 1 and 2. IARC Sci Publ. 1975;(11 Pt 1):63–73. [PubMed] [Google Scholar]
- Elsayed S., Bennich H. The primary structure of allergen M from cod. Scand J Immunol. 1975;4(2):203–208. doi: 10.1111/j.1365-3083.1975.tb02618.x. [DOI] [PubMed] [Google Scholar]
- Grauballe P. C., Vestergaard B. F., Hornsleth A., Leerhoy J., Johnsson T. Demonstration by immunoelectro-osmophoresis of precipitating antibodies to a purified rubella virus antigen. Infect Immun. 1975 Jul;12(1):55–61. doi: 10.1128/iai.12.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Helenius A., Söderlund H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim Biophys Acta. 1973 May 11;307(2):287–300. doi: 10.1016/0005-2736(73)90096-5. [DOI] [PubMed] [Google Scholar]
- Johansson B. G., Malmquist J. Quantitative immunochemical determination of lysoqyme (muramidase) in serum and urine. Scand J Clin Lab Invest. 1971 May;27(3):255–261. doi: 10.3109/00365517109080216. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lesso J., Hána L., Matis J. Reactions of immune sera against the nucleocapsid, envelope and whole herpes simplex virus type 1. Acta Virol. 1976 Feb;20(1):48–52. [PubMed] [Google Scholar]
- Norrild B., Vestergaard B. F. Polyacrylamide gel electrophoretic analysis of herpes simplex virus type 1 immunoprecipitates obtained by quantitative immunoelectrophoresis in antibody-containing agarose gel. J Virol. 1977 Apr;22(1):113–117. doi: 10.1128/jvi.22.1.113-117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Watson D. H. Some structural antigens of herpes simplex virus type 1. J Gen Virol. 1975 Nov;29(2):167–178. doi: 10.1099/0022-1317-29-2-167. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Iwamoto K., Adam E., Melnick J. L. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J Immunol. 1970 Mar;104(3):599–606. [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard B. F., Hornsleth A., Pedersen S. N. Occurrence of herpes- and adenovirus antibodies in patients with carcinoma of the cervix uteri. Measurement of antibodies to herpesvirus hominis (types 1 and 2), cytomegalovirus, EB-virus, and adenovirus. Cancer. 1972 Jul;30(1):68–74. doi: 10.1002/1097-0142(197207)30:1<68::aid-cncr2820300111>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Wildy P. The preparation of 'monoprecipitin' antisera to herpes virus specific antigens. J Gen Virol. 1969 Mar;4(2):163–168. doi: 10.1099/0022-1317-4-2-163. [DOI] [PubMed] [Google Scholar]