Abstract
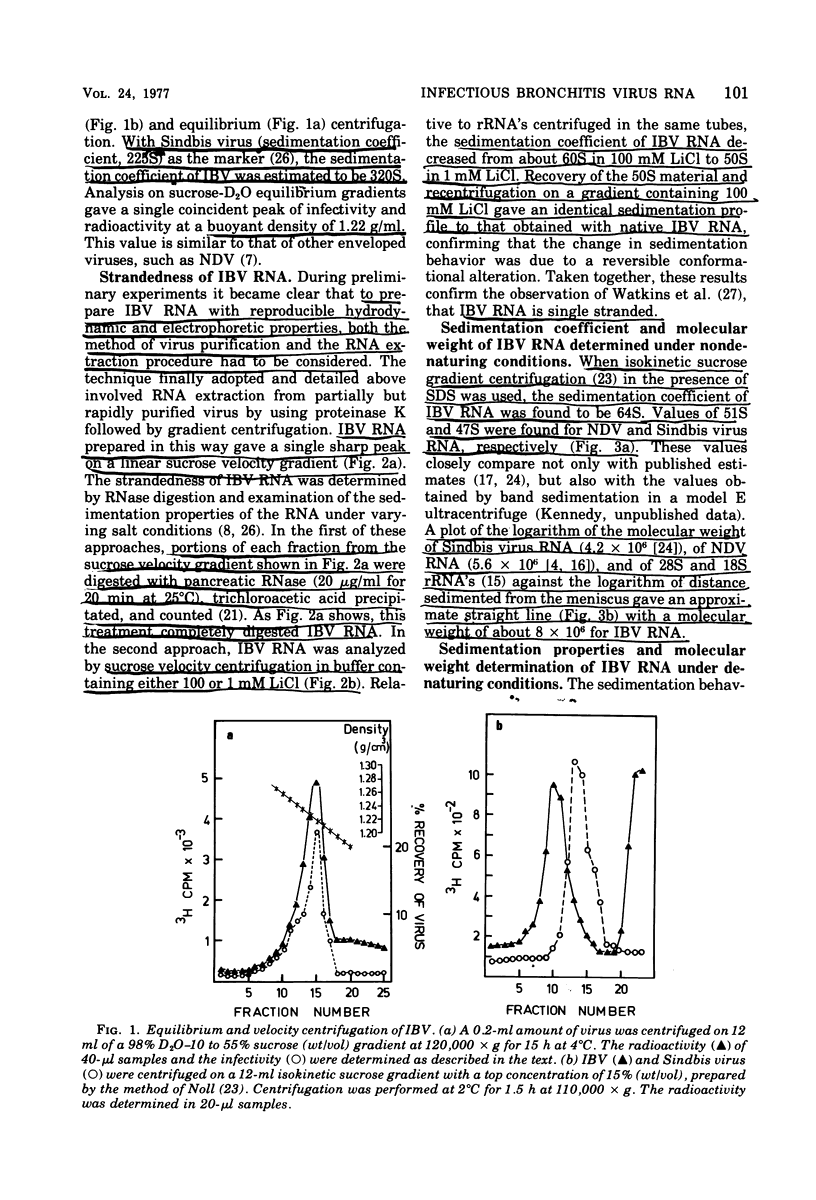
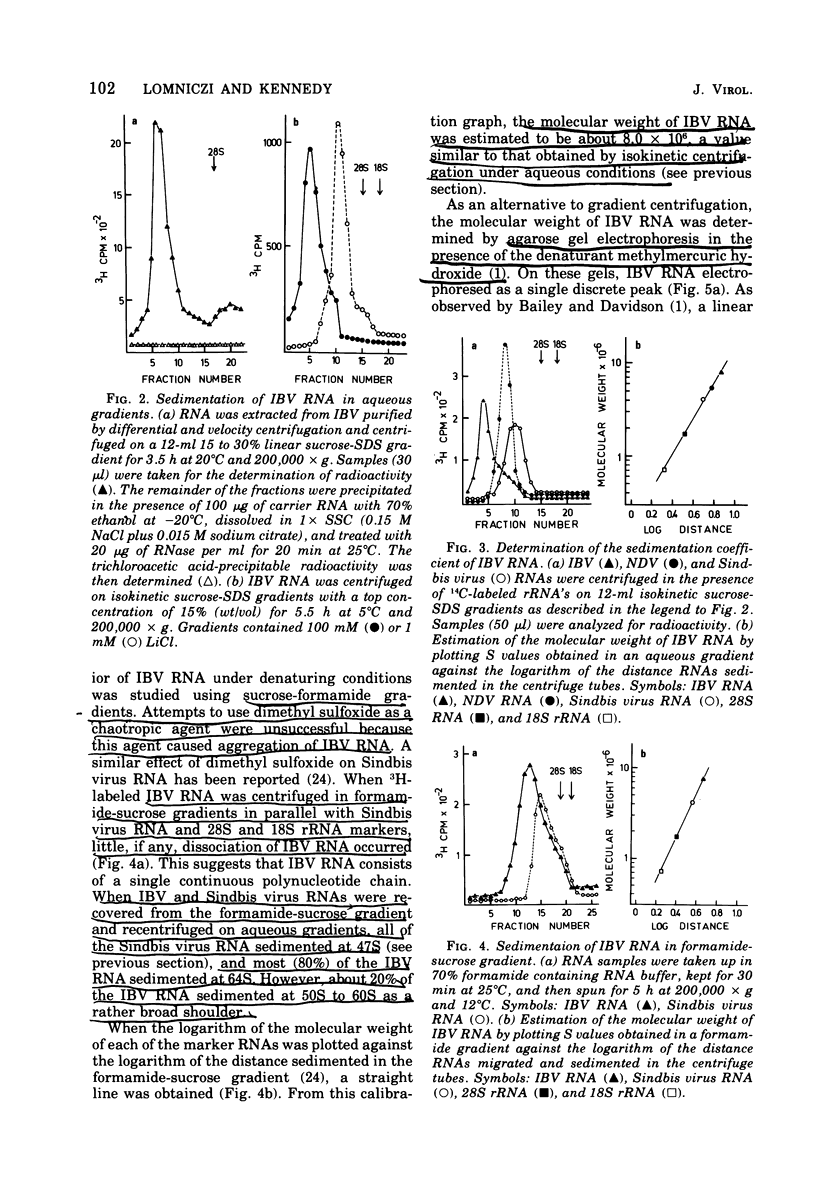
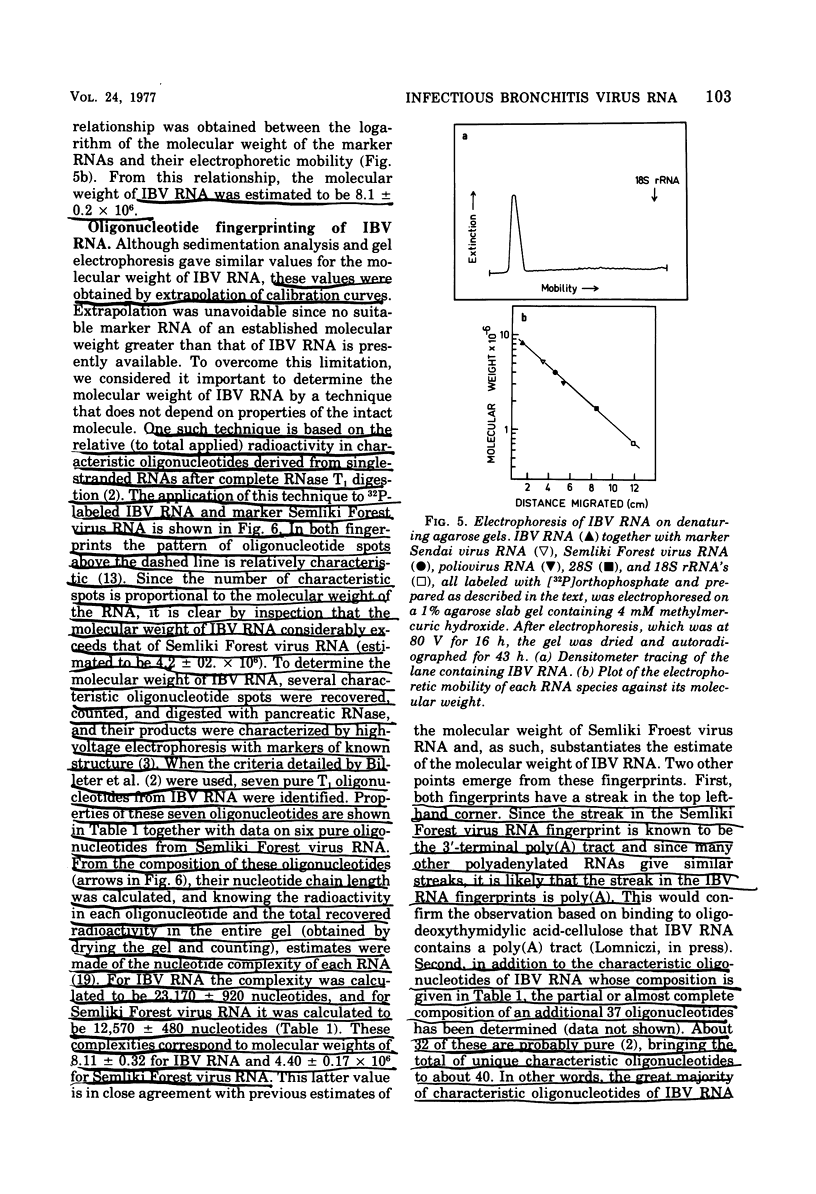
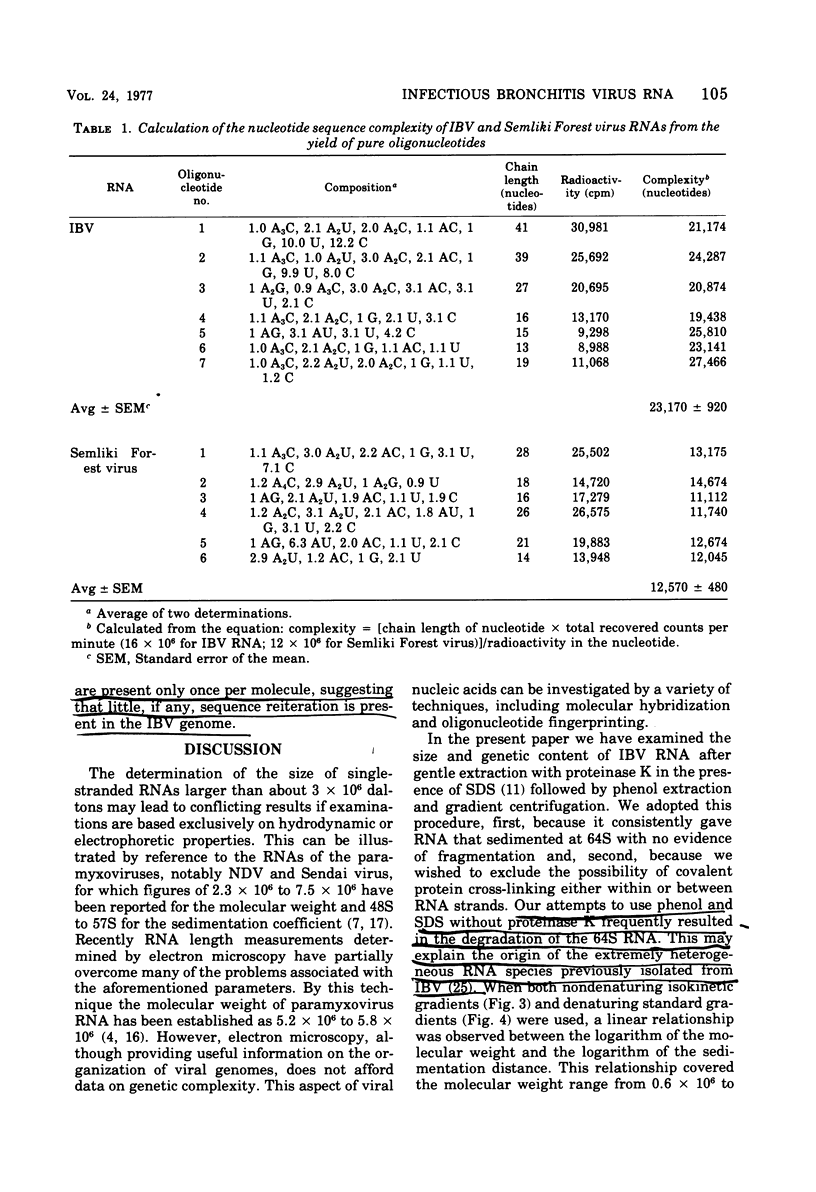
Techniques are described for the growth and rapid purification of the avian coronavirus infectious bronchitis virus (IBV). Purified IBV has a sedimentation coefficient of 320S and a buoyant density of 1.22 g/ml in sucrose-deuterium oxide equilibrium gradients. IBV RNA extracted by proteinase K in the presence of sodium dodecyl sulfate and further purified by phenol extraction and gradient centrifugation is single stranded and has a sedimentation coefficient of 64S, as determined by isokinetic gradient centrifugation. Analysis on sucrose gradients under both aqueous and denaturing conditions together with agarose gel electrophoresis in the presence of the chaotropic agent methylmercuric hydroxide gave a value of 8 X 10(6) for the moleclar weight of IBV RNA. This value was confirmed by RNase T1 fingerprinting, which also indicated that IBV RNA is haploid. No evidence was found of subunit structure in IBV RNA. From these results together with the recently reported observation that IBV RNA is infectious and contains a tract of polyadenylic acid (Lomniczi, J. Gen. Virol., in press), we conclude that the genome of the coronaviruses is a single continuous chain of about 23,000 mononucleotides that is of messenger polarity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Porter A., Kennedy S. I. Defective-interfering particles of Semliki Forest virus: intracellular events during interference. J Gen Virol. 1976 Jun;31(3):397–416. doi: 10.1099/0022-1317-31-3-397. [DOI] [PubMed] [Google Scholar]
- Chi Y. Y., Bassel A. R. Electron microscopy of viral RNA: molecular weight determination of bacterial and animal virus RNAs. J Virol. 1974 Jun;13(6):1194–1199. doi: 10.1128/jvi.13.6.1194-1199.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. H. Avian infectious bronchitis. Adv Vet Sci Comp Med. 1970;14:105–148. [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Isolation of the nucleic acid of Newcastle disease virus (NDV). Proc Natl Acad Sci U S A. 1965 Sep;54(3):794–800. doi: 10.1073/pnas.54.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Replication of bacteriophage ribonucleic acid: some physical properties of single-stranded, double-stranded, and branched viral ribonucleic acid. J Virol. 1967 Feb;1(1):64–75. doi: 10.1128/jvi.1.1.64-75.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwes D. J., Pocock D. H., Wijaszka T. M. Identification of heat-dissociable RNA complexes in two porcine coronaviruses. Nature. 1975 Oct 9;257(5526):508–510. doi: 10.1038/257508a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette K. G. Plaque formation by infectious bronchitis virus in chicken embryo kidney cell cultures. Avian Dis. 1973 Apr-Jun;17(2):369–378. [PubMed] [Google Scholar]
- Hilz H., Wiegers U., Adamietz P. Stimulation of proteinase K action by denaturing agents: application to the isolation of nucleic acids and the degradation of 'masked' proteins. Eur J Biochem. 1975 Aug 1;56(1):103–108. doi: 10.1111/j.1432-1033.1975.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Burke D. C. Studies on the structural proteins of Semliki Forest virus. J Gen Virol. 1972 Jan;14(1):87–98. doi: 10.1099/0022-1317-14-1-87. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. The effect of enzymes on structural and biological properties of Semliki forest virus. J Gen Virol. 1974 May;23(2):129–143. doi: 10.1099/0022-1317-23-2-129. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Bruschi A. Molecular weight determination of Sendai RNA by dimethyl sulfoxide gradient sedimentation. J Virol. 1973 May;11(5):615–620. doi: 10.1128/jvi.11.5.615-620.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D. Transfer ribonucleic acid nucleotidyltransferase and transfer ribonucleic acid in Sendai virions. J Virol. 1972 Sep;10(3):555–559. doi: 10.1128/jvi.10.3.555-559.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B. Studies on interferon production and interferon sensitivity of different strains of Newcastle disease virus. J Gen Virol. 1973 Nov;21(2):305–313. doi: 10.1099/0022-1317-21-2-305. [DOI] [PubMed] [Google Scholar]
- Martin B. A., Burke D. C. The replication of Semliki Forest virus. J Gen Virol. 1974 Jul;24(1):45–66. doi: 10.1099/0022-1317-24-1-45. [DOI] [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morser M. J., Kennedy S. I., Burke D. C. Virus-specified polypeptides in cells infected with Semliki Forest virus. J Gen Virol. 1973 Oct;21:19–29. doi: 10.1099/0022-1317-21-1-19. [DOI] [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Tannock G. A. The nucleic acid of infectious bronchitis virus. Arch Gesamte Virusforsch. 1973;43(3):259–271. doi: 10.1007/BF01250421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H., Reeve P., Alexander D. J. The ribonucleic acid of infectious bronchitis virus. Arch Virol. 1975;47(3):279–286. doi: 10.1007/BF01317815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zeijst B. A., Horzinek M. C. The genome of equine arteritis virus. Virology. 1975 Dec;68(2):418–425. doi: 10.1016/0042-6822(75)90283-4. [DOI] [PubMed] [Google Scholar]