Abstract
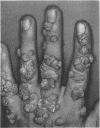
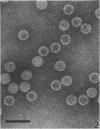
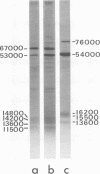
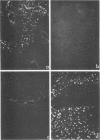
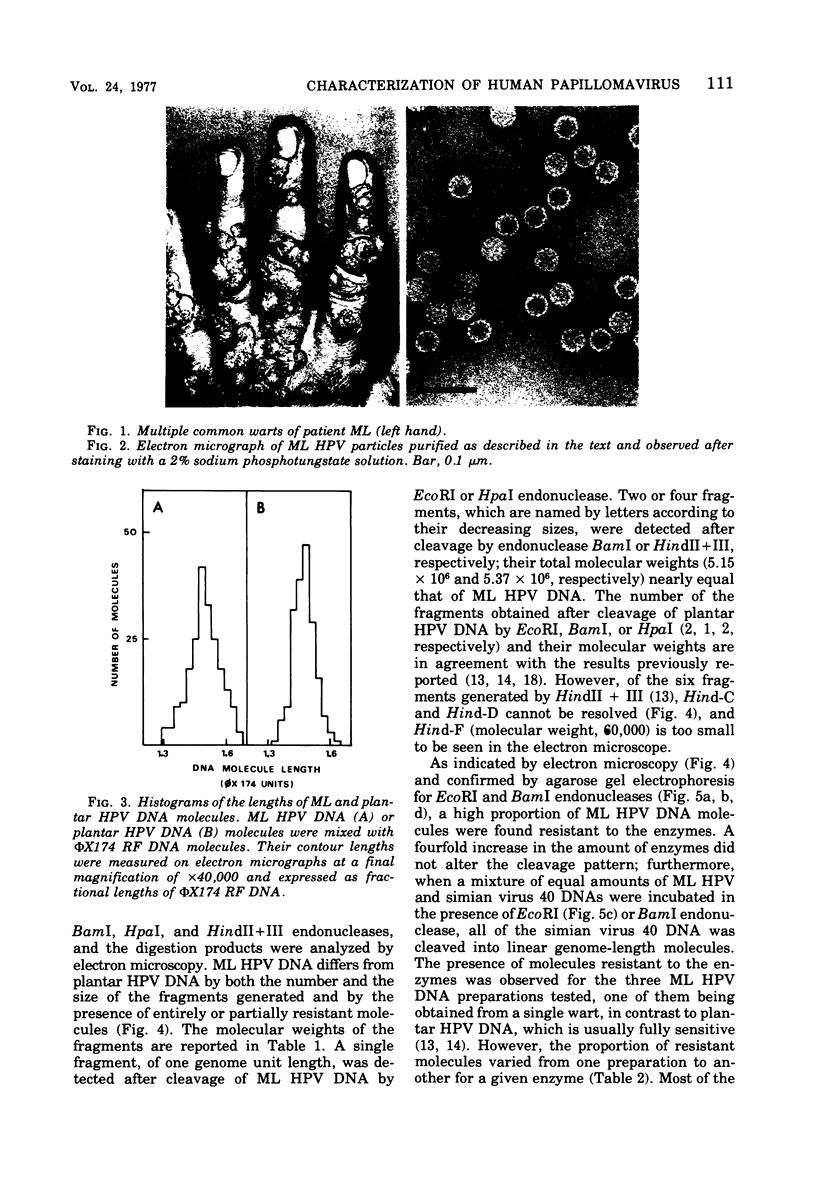
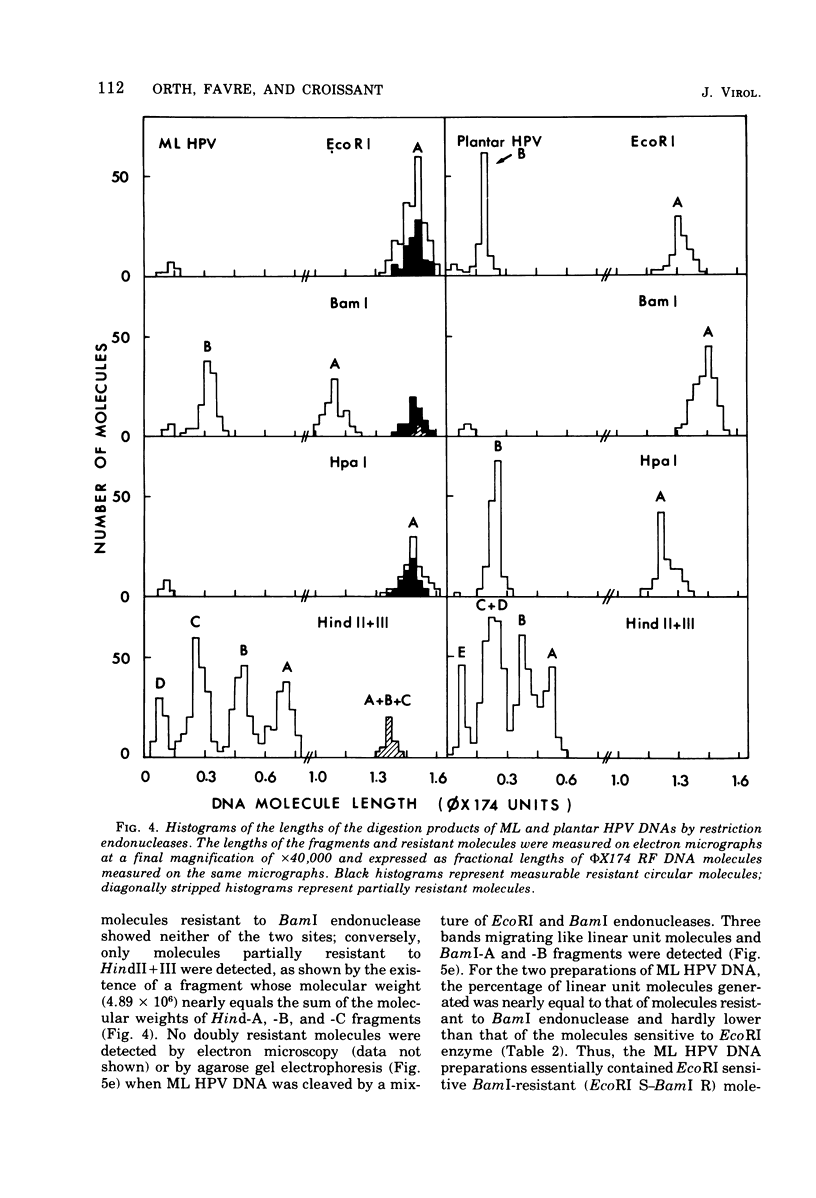
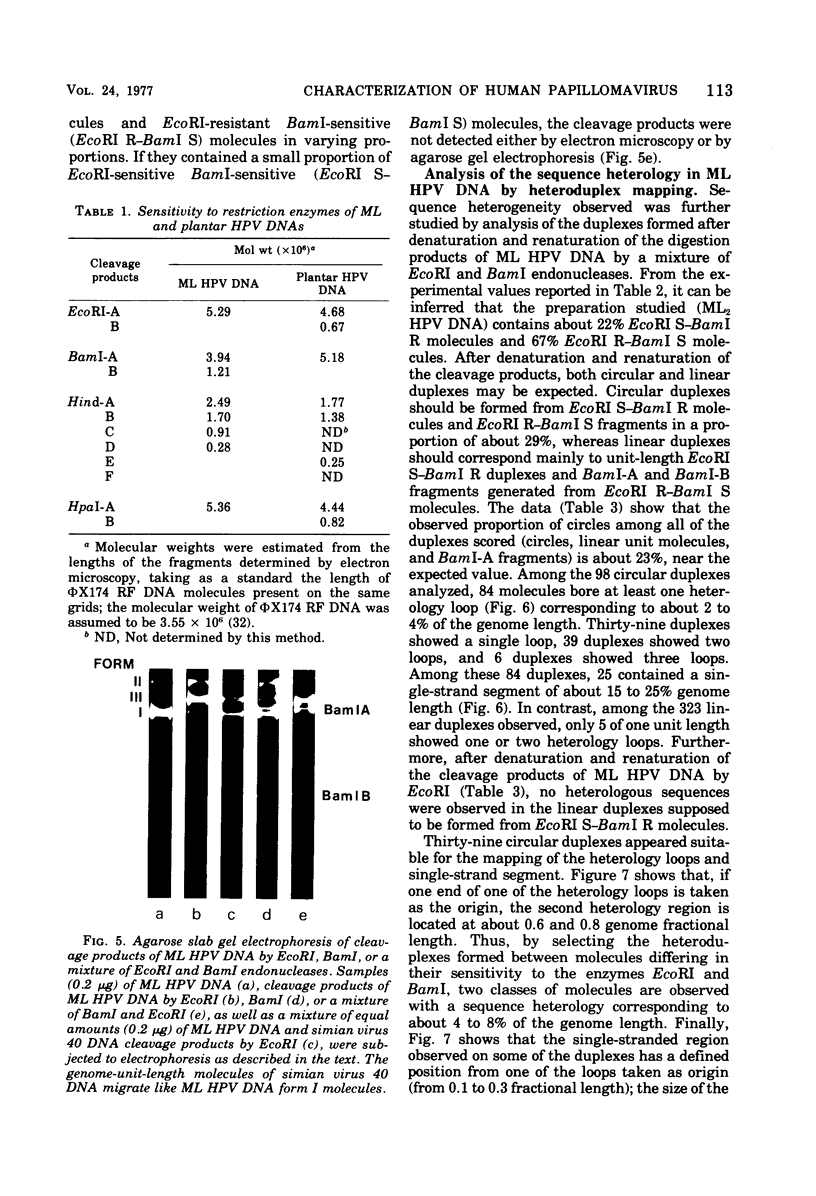
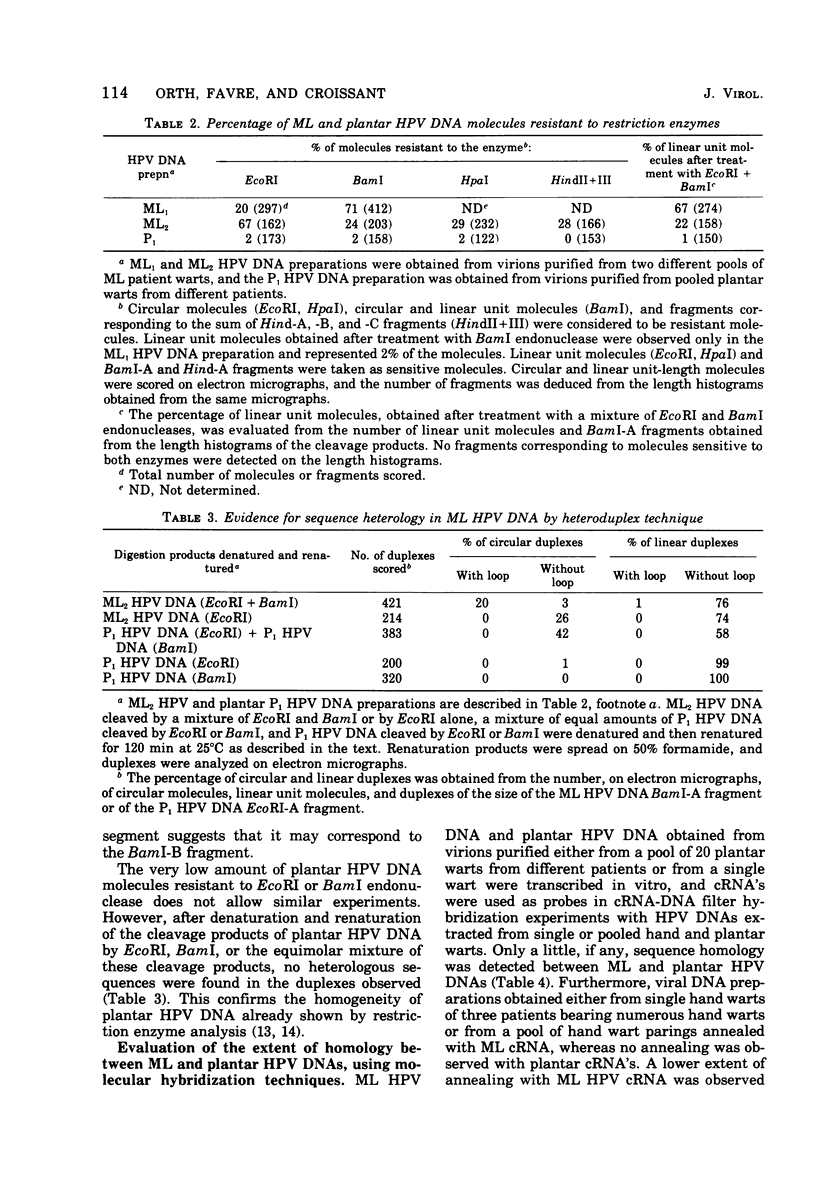
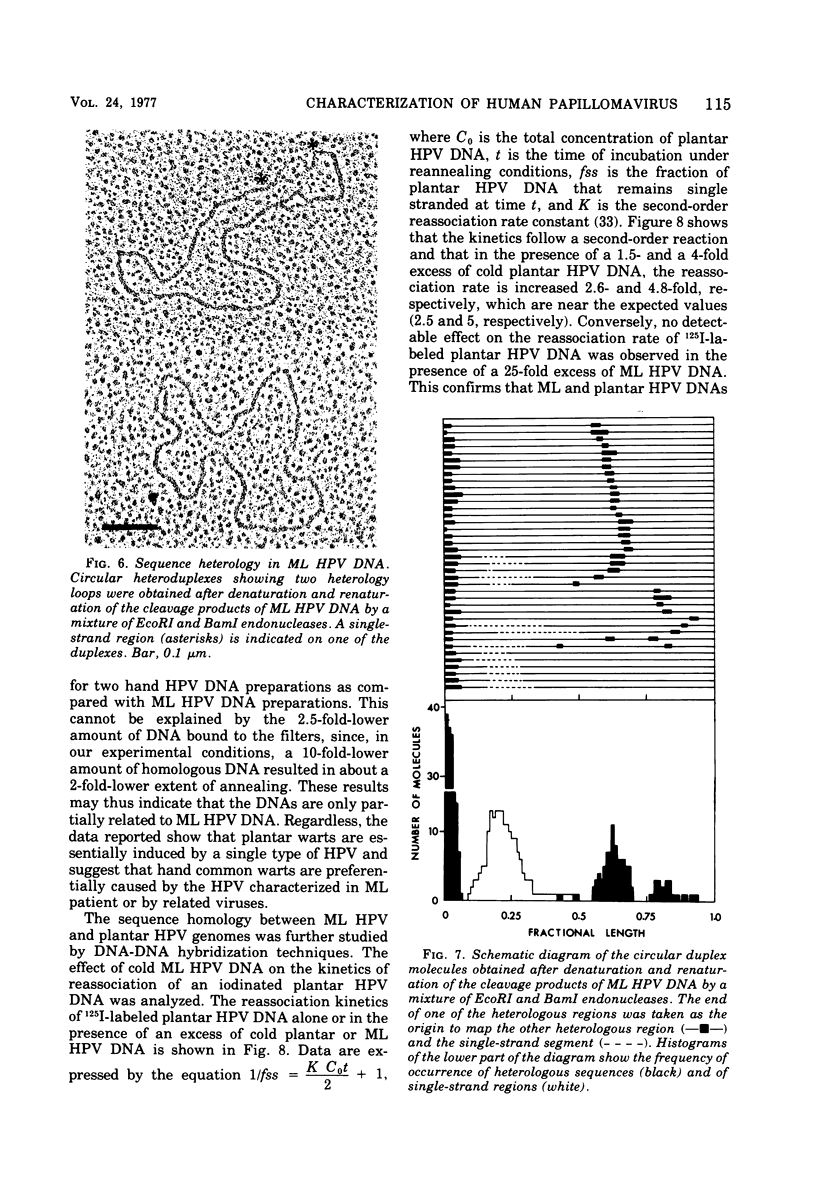
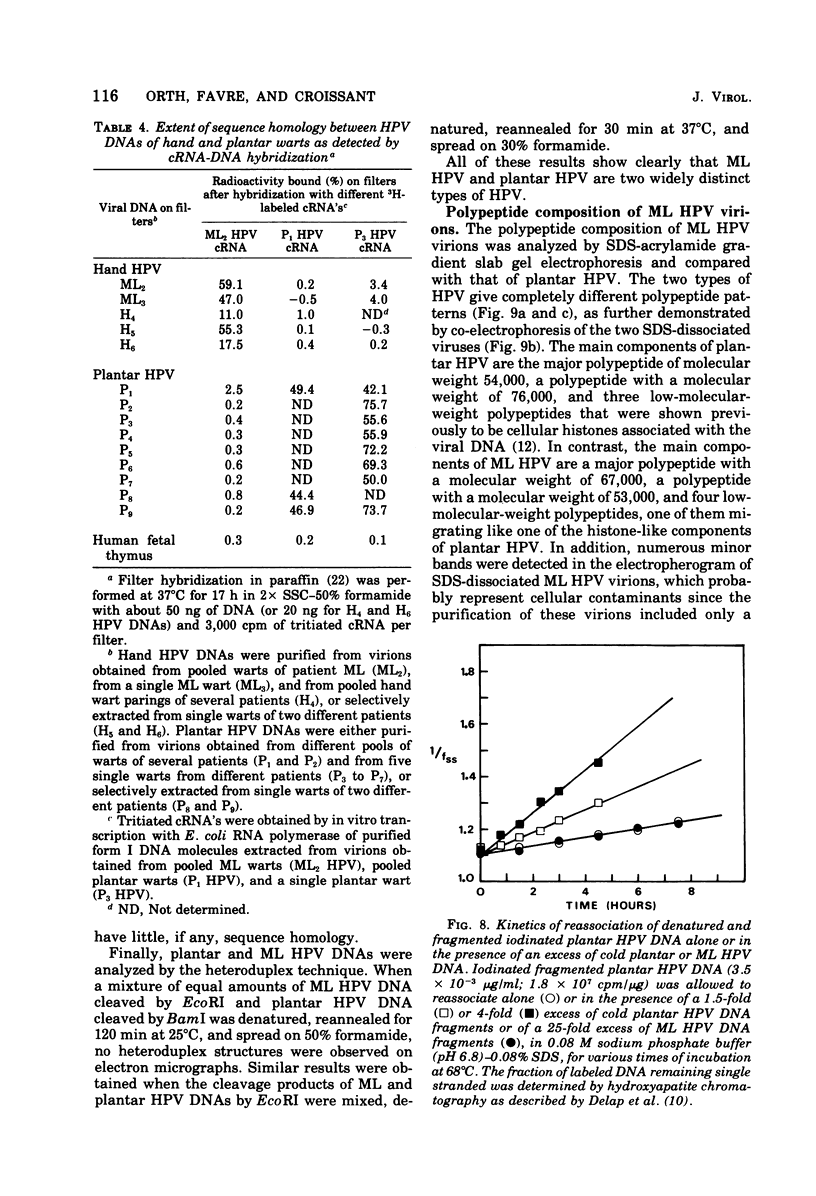
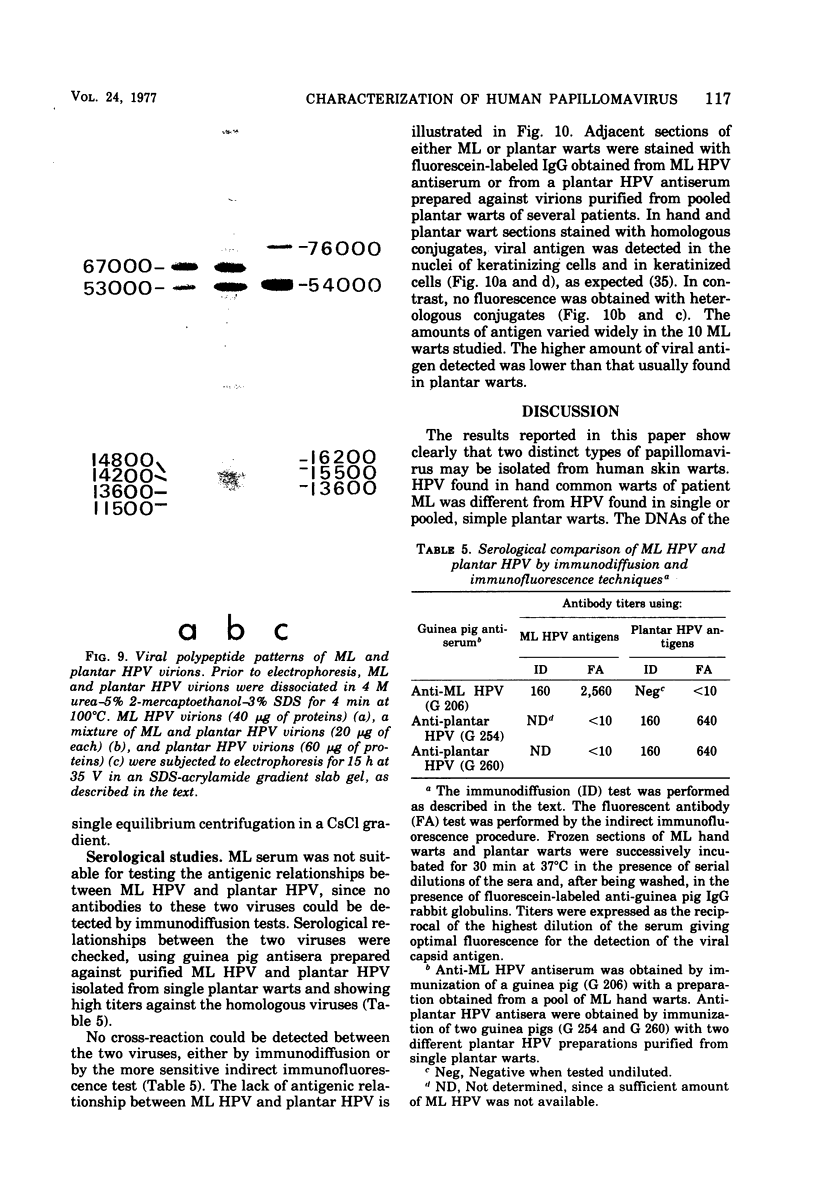
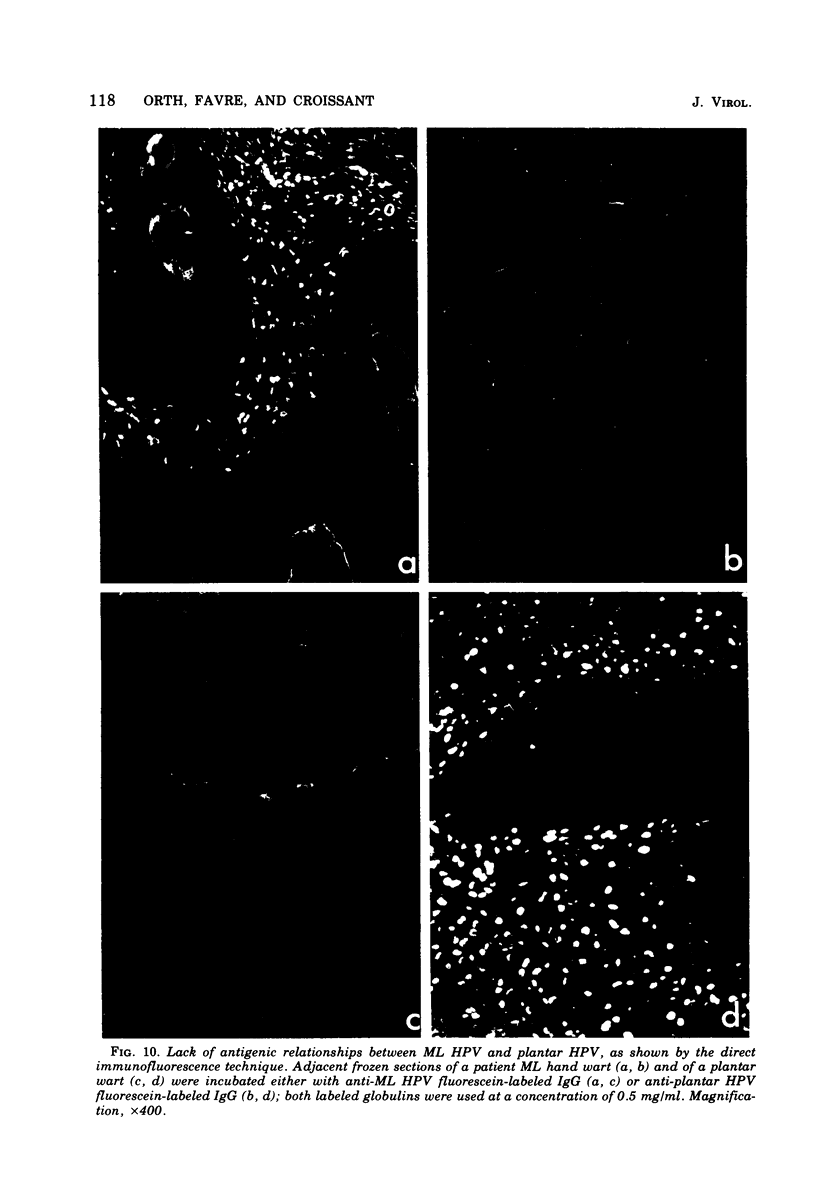
A human papillomavirus (HPV) was isolated from the lesions of a patient (ML) bearing numerous hand common warts. This virus was compared with the well-characterized HPV found in typical plantar warts (plantar HPV). ML and plantar HPV DNAs have similar molecular weights (5.26 × 106 and 5.23 × 106, respectively) but were shown to be different by restriction enzyme analysis. When the cleavage products of both DNAs by endonuclease EcoRI, BamI, HpaI, or Hind were analyzed by electron microscopy, one, two, one, and four fragments were detected for ML HPV DNA instead of the two, one, two, and six fragments, respectively, detected for plantar HPV DNA. In contrast to plantar HPV DNA, a high proportion of ML HPV DNA molecules were resistant to these restriction enzymes. Most, if not all, of the molecules were either resistant to BamI and sensitive to EcoRI or sensitive to BamI and resistant to EcoRI. After denaturation and renaturation of the cleavage products of ML HPV DNA by a mixture of the two enzymes, the circular “heteroduplexes” formed showed one to three heterology loops corresponding to about 4 to 8% of the genome length. No sequence homology was detected between ML and plantar HPV DNAs by cRNA-DNA filter hybridization, by measuring the reassociation kinetics of an iodinated plantar HPV DNA in the presence of a 25-fold excess of ML HPV DNA, or by the heteroduplex technique. The two viruses had distinct electrophoretic polypeptide patterns and showed no antigenic cross-reaction by immunodiffusion or immunofluorescence techniques. Preliminary cRNA-DNA hybridization experiments, using viral DNAs from single or pooled plantar or hand warts, suggest that hand common warts are associated with viruses similar or related to ML HPV. The existence of at least two distinct types of HPVs that cause skin warts was demonstrated; they were provisionally called HPV type 1 and HPV type 2, with plantar HPV and ML HPV as prototypical viruses, respectively.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Goffe A. P. Antibody to wart virus in human sera demonstrated by electron microscopy and precipitin tests. Lancet. 1965 Dec 11;2(7424):1205–1207. doi: 10.1016/s0140-6736(65)90633-1. [DOI] [PubMed] [Google Scholar]
- BARRERA-ORO J. G., SMITH K. O., MELNICK J. L. Quantitation of papova virus particles in human warts. J Natl Cancer Inst. 1962 Sep;29:583–595. [PubMed] [Google Scholar]
- Butel J. S. Studies with human papilloma virus modeled after known papovavirus systems. J Natl Cancer Inst. 1972 Feb;48(2):285–299. [PubMed] [Google Scholar]
- Cook T. A., Brunschwig J. P., Butel J. S., Cohn A. M., Goepfert H., Rawls W. E. Laryngeal papilloma: etiologic and therapeutic considerations. Ann Otol Rhinol Laryngol. 1973 Sep-Oct;82(5):649–655. doi: 10.1177/000348947308200507. [DOI] [PubMed] [Google Scholar]
- Crawford L. V. Nucleic acids of tumor viruses. Adv Virus Res. 1969;14:89–152. doi: 10.1016/s0065-3527(08)60558-8. [DOI] [PubMed] [Google Scholar]
- Cubie H. A. Serological studies in a student population prone to infection with human papilloma virus. J Hyg (Lond) 1972 Dec;70(4):677–690. doi: 10.1017/s0022172400022531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delap R. D., Friedman-klen A., Rush M. G. The absence of human papilloma viral DNA sequences in condylomata acuminata. Virology. 1976 Oct 1;74(1):268–272. doi: 10.1016/0042-6822(76)90155-0. [DOI] [PubMed] [Google Scholar]
- Delap R. J., Zouzias D., Rush M. G. Preparation of radioiodinated simian virus 40 DNA for use in DNA - DNA reassociation kinetics experiments. Biochim Biophys Acta. 1976 Feb 5;418(3):257–265. doi: 10.1016/0005-2787(76)90288-4. [DOI] [PubMed] [Google Scholar]
- Favre M., Breitburd F., Croissant O., Orth G. Chromatin-like structures obtained after alkaline disruption of bovine and human papillomaviruses. J Virol. 1977 Mar;21(3):1205–1209. doi: 10.1128/jvi.21.3.1205-1209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M., Orth G., Croissant O., Yaniv M. Human papillomavirus DNA: physical map. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4810–4814. doi: 10.1073/pnas.72.12.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M., Orth G., Croissant O., Yaniv M. Human papillomavirus DNA: physical mapping of the cleavage sites of Bacillus amyloliquefaciens (BamI) and Haemophilus parainfluenzae (HpaII) endonucleases and evidence for partial heterogeneity. J Virol. 1977 Mar;21(3):1210–1214. doi: 10.1128/jvi.21.3.1210-1214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol. 1975 May;15(5):1239–1247. doi: 10.1128/jvi.15.5.1239-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genner J. Verrucae vulgares. II. Demonstration of a complement fixation reaction. Acta Derm Venereol. 1971;51(5):365–373. [PubMed] [Google Scholar]
- Gissmann L., Pfister H., Zur Hausen H. Human papilloma viruses (HPV): characterization of four different isolates. Virology. 1977 Feb;76(2):569–580. doi: 10.1016/0042-6822(77)90239-2. [DOI] [PubMed] [Google Scholar]
- Gissmann L., zur Hausen H. Human papilloma virus DNA: physical mapping and genetic heterogeneity. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1310–1313. doi: 10.1073/pnas.73.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jablonska S., Dabrowski J., Jakubowicz K. Epidermodysplasia verruciformis as a model in studies on the role of papovaviruses in oncogenesis. Cancer Res. 1972 Mar;32(3):583–589. [PubMed] [Google Scholar]
- KLUG A., FINCH J. T. STRUCTURE OF VIRUSES OF THE PAPILLOMA-POLYOMA TYPE. I. HUMAN WART VIRUS. J Mol Biol. 1965 Feb;11:403–423. doi: 10.1016/s0022-2836(65)80066-3. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Mercereau O., Gros D., Tremblay G. Hybridization of filters with competitor DNA in the liquid phase in a standard and a micro-assay. Biochimie. 1974;56(9):1215–1221. doi: 10.1016/s0300-9084(74)80014-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ogilvie M. M. Serological studies with human papova (wart) virus. J Hyg (Lond) 1970 Sep;68(3):479–490. doi: 10.1017/s0022172400042388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriel J. D., Almeida J. D. Demonstration of virus particles in human genital warts. Br J Vener Dis. 1970 Feb;46(1):37–42. doi: 10.1136/sti.46.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriel J. D. Natural history of genital warts. Br J Vener Dis. 1971 Feb;47(1):1–13. doi: 10.1136/sti.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G., Jeanteur P., Croissant O. Evidence for and localization of vegetative viral DNA replication by autoradiographic detection of RNA-DNA hybrids in sections of tumors induced by Shope papilloma virus. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1876–1880. doi: 10.1073/pnas.68.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. New human papovaviruses. Prog Med Virol. 1976;22:1–35. [PubMed] [Google Scholar]
- Pyrhönen S., Johansson E. Regression of warts. An immunological study. Lancet. 1975 Mar 15;1(7907):592–596. doi: 10.1016/s0140-6736(75)91880-2. [DOI] [PubMed] [Google Scholar]
- Rowson K. E., Mahy B. W. Human papova (wart) virus. Bacteriol Rev. 1967 Jun;31(2):110–131. doi: 10.1128/br.31.2.110-131.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Shirodaria P. V., Matthews R. S. An immunofluorescence study of warts. Clin Exp Immunol. 1975 Aug;21(2):329–338. [PMC free article] [PubMed] [Google Scholar]
- WALTER E. L., Jr, WALKER D. L., COOPER G. A. LOCALIZATION OF SPECIFIC ANTIGEN IN HUMAN WARTS. Arch Pathol. 1965 Apr;79:419–423. [PubMed] [Google Scholar]
- Yoshike K., Defendi V. Presence of deletion molecules in human wart virus DNA. J Virol. 1977 Jan;21(1):415–418. doi: 10.1128/jvi.21.1.415-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Meinhof W., Scheiber W., Bornkamm G. W. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974 May 15;13(5):650–656. doi: 10.1002/ijc.2910130509. [DOI] [PubMed] [Google Scholar]