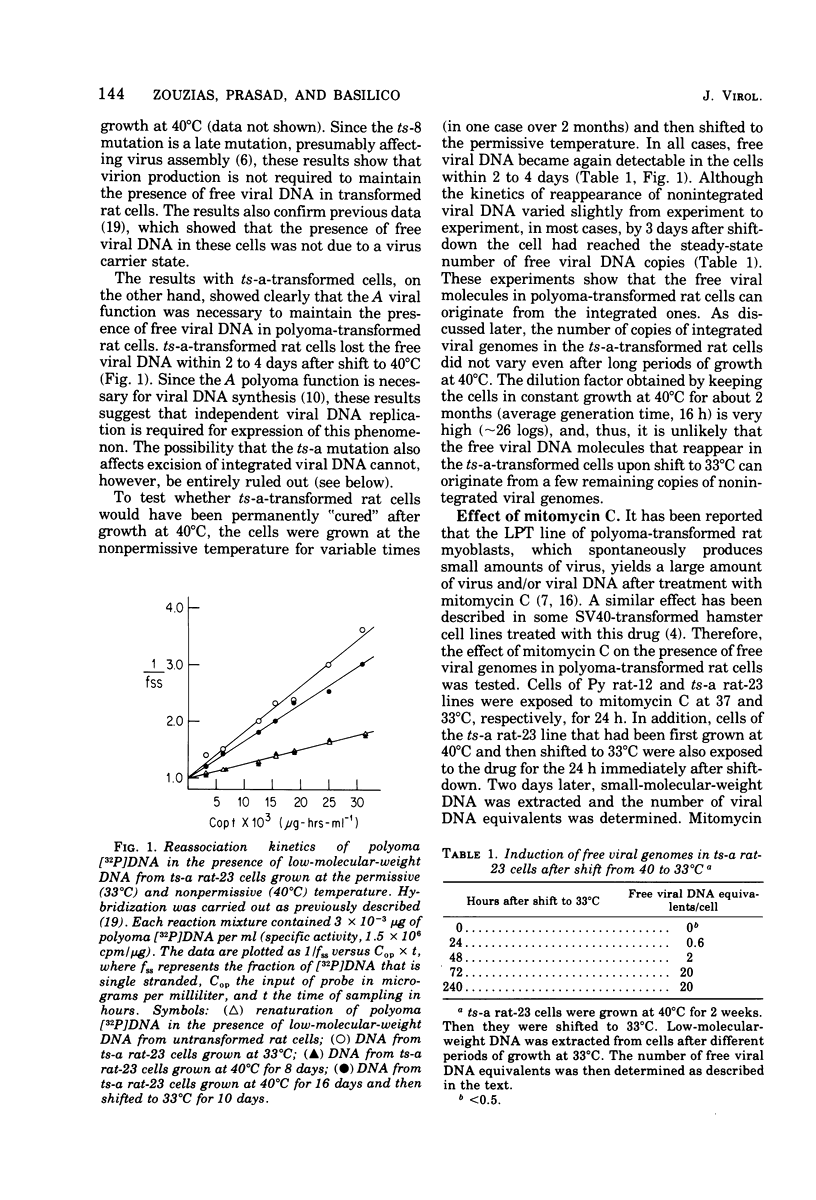
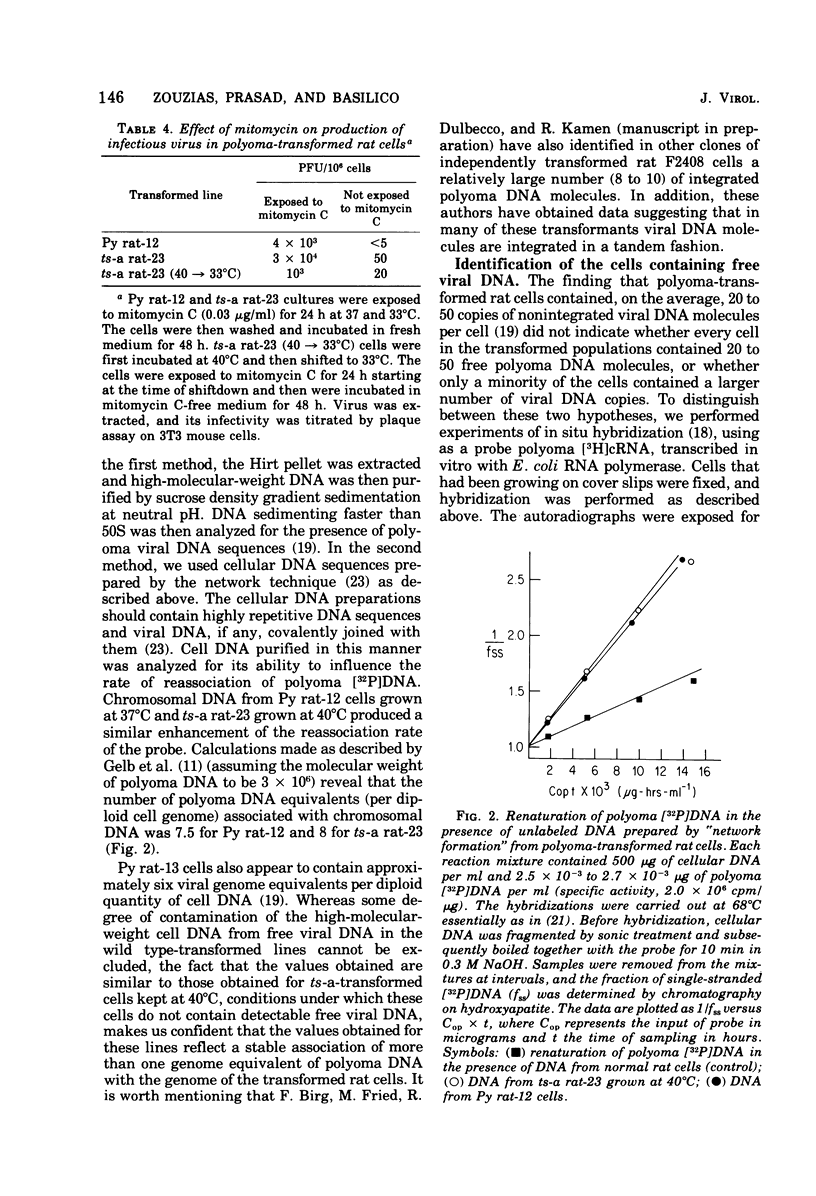
Abstract
F2408 rat cells transformed by polyoma virus contained integrated and nonintegrated viral DNA. The presence of nonintegrated viral DNA is under control of the A early viral function. Polyoma ts-a-transformed rat cells lose the free viral DNA when growth at the nonpermissive temperature (40 degrees C), but they reexpress it 1 to 3 days after they are shifted back to the permissive temperature. In contrast, rat cells transformed by a late viral mutant, ts-8, contain free viral DNA at both permissive and nonpermissive temperatures. Treatment of the transformed rat cells with mitomycin C produces a large increase in the quantity of free viral DNA and some production of infectious virus. Experiments of in situ hybridization, with 3H-labeled polyoma complementary RNA as a probe, show that only a minority (approximately 0.1%) of the transformed cells contain nonintegrated viral DNA at any given time. These results suggest that the presence of free viral DNA in polyoma-transformed rat cells is caused by a spontaneous induction of viral DNA replication, occurring with low but constant probability in the transformed cell population, and that the free viral DNA molecules originate from the integrated ones, probably through a phenomenon of excision and limited replication.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Burstin S. J. Multiplication of polyoma virus in mouse-hamster somatic hybrids: a hybrid cell line which produces viral particles containing predominantly host deoxyribonucleic acid. J Virol. 1971 Jun;7(6):802–812. doi: 10.1128/jvi.7.6.802-812.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., DiMayorca G. Mutant of polyoma virus with impaired adsorption to BHK cells. J Virol. 1974 Apr;13(4):931–934. doi: 10.1128/jvi.13.4.931-934.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Burns W. H., Black P. H. Analysis of simian virus 40-induced transformation of hamster kidney tissue in vitro. V. Variability of virus recovery from cell clones inducible with mitomycin C and cell fusion. J Virol. 1968 Jun;2(6):606–609. doi: 10.1128/jvi.2.6.606-609.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L., CRAWFORD L. V. SOME CHARACTERISTICS OF LARGE-PLAQUE AND SMALL-PLAQUE LINES OF POLYOMA VIRUS. Virology. 1964 Feb;22:235–244. doi: 10.1016/0042-6822(64)90008-x. [DOI] [PubMed] [Google Scholar]
- Di Mayorca G., Callender J., Marin G., Giordano R. Temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):126–133. doi: 10.1016/0042-6822(69)90134-2. [DOI] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M., Sachs L. Induction of virus synthesis in polyoma transformed cells by ultraviolet light and mitomycin C. Virology. 1970 Jan;40(1):174–177. doi: 10.1016/0042-6822(70)90391-0. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Bancuk J. E. Polyoma genome in hamster BHK-21-C13 cells: integration into cellular DNA and induction of the viral replication. J Virol. 1976 Oct;20(1):133–141. doi: 10.1128/jvi.20.1.133-141.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Griffin B. E. Organization of the genomes of polyoma virus and SV40. Adv Cancer Res. 1977;24:67–113. doi: 10.1016/s0065-230x(08)61013-1. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Effect of mitomycin C on interactions between temperate phages and bacteria. Virology. 1961 Apr;13:493–499. doi: 10.1016/0042-6822(61)90280-x. [DOI] [PubMed] [Google Scholar]
- Manor H., Neer A. Effects of cycloheximide on virus RNA replication in an inducible line of polyoma-transformed rat cells. Cell. 1975 Jul;5(3):311–318. doi: 10.1016/0092-8674(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Khoury G. Integration of DNA tumor virus genomes. Curr Top Microbiol Immunol. 1976;73:35–65. doi: 10.1007/978-3-642-66306-2_2. [DOI] [PubMed] [Google Scholar]
- McDougall J. K., Dunn A. R., Jones K. W. In situ hybridization of adenovirus RNA and DNA. Nature. 1972 Apr 14;236(5346):346–348. doi: 10.1038/236346a0. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. State of the viral DNA in rat cells transformed by polyoma virus. I. Virus rescue and the presence of nonintergrated viral DNA molecules. J Virol. 1976 May;18(2):436–444. doi: 10.1128/jvi.18.2.436-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusanova T., Kaplan J. C., Smales W. P., Black P. H. Excision of viral DNA from host cell DNA after induction of simian virus 40-transformed hamster cells. J Virol. 1976 Jul;19(1):279–285. doi: 10.1128/jvi.19.1.279-285.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. F. Studies on a virogenic clone of SV 40-transformed rabbit cells using cell fusion and in situ hybridization. J Gen Virol. 1973 Oct;21:69–81. doi: 10.1099/0022-1317-21-1-69. [DOI] [PubMed] [Google Scholar]