Abstract
Background
Adjuvant! Online is a tool used for clinical decision making in patients with early stage colon cancer. As details of the tool’s construction are not published, the ability of Adjuvant! Online to accurately predict outcomes for older patients (age 70+) with node positive colon cancer receiving adjuvant chemotherapy is unclear.
Methods
Individual data from older patients with stage III colon cancer who enrolled into multiple trials within the ACCENT database were entered into the Adjuvant! Online program to obtain predicted probabilities of 5-year overall survival (OS) and recurrence-free survival (RFS). Median predictions were compared with known rates. As co-morbidities were not known for ACCENT patients, but required for calculator entry, patients were assumed to have either “minor” or “average for age” co-morbidities.
Results
2,967 older patients from 10 randomized studies were included. When “minor” co-morbidities were assumed, the median predicted 5-year OS rate of 64% nearly matched the actual rate of 65%; when “average for age” co-morbidities were assumed, the median prediction dropped to 58%, outside the CI for the actual rate. On the other hand, assuming “minor” co-morbidities gave a median 5-year RFS prediction of 62%, outside the 95% CI for the actual rate of 58%, while assuming “average for age” co-morbidities yielded a better median prediction of 57%.
Conclusion
Adjuvant! Online is reasonably accurate overall for predicting outcomes in older trial patients with stage III colon cancer, though accuracy may differ between 5-year RFS and 5-year OS predictions when a fixed degree of co-morbidities is assumed.
INTRODUCTION
Colorectal cancer is one of the most commonly diagnosed cancers with a worldwide incidence of > 1,000,000 per year(1), and the probability of developing the disease increasing substantially in the 7th decade of life(2). The majority of patients with stage I or II colon cancer are cured by surgery alone, while patients with stage III disease are normally considered for adjuvant chemotherapy after surgery. At present, oxaliplatin-based combination chemotherapy is considered standard of care for such patients(3–6). However, a recent report from the ACCENT database suggested reduced benefit from combination chemotherapy in the adjuvant setting for patients aged 70 or older compared to the use of single agent fluoropyrimidines(7). Indeed, it may be difficult to distinguish survival gains between adjuvant treatments as patients grow older, mostly due to competing risks of death from other causes.
The Adjuvant! Online colon cancer calculator provides probabilities for recurrence-free survival, relapse, overall survival, death from cancer, and death from other causes by 5 years post-treatment, both with and without chemotherapy(8). Input values include age (in years), sex, co-morbidities (minor, average for age, and three levels of major), tumor stage (T1, T2, T3, T4), number of positive nodes (0, 1–3, 4–10, >10), number of nodes examined (0, 1–3, 4–10, >10), histologic grade (1, 2, 3), and treatment (none vs. 5FU-based vs. oxaliplatin-based). Although reportedly based on a large sample from the population rather than data from clinical trials, this tool does not include additional demographic and disease variables such as race, BMI, performance status, and tumor location; furthermore, the methodology and internal/external validation of this calculator has not been published by its authors. In 2011, Gill et al. compared the performance of Adjuvant! Online and Numeracy(9) web calculators in predicting outcomes for patients with stage II and stage III disease using a population dataset and patient data from two clinical trials(10). In this study, the authors concluded that the calculators showed equivalent accuracy for patients treated with surgery and 5FU, while Adjuvant! Online yielded more accurate predictions for patients treated with surgery alone. The Numeracy calculator was subsequently replaced by an ACCENT-derived prognostic calculator for stage III patients, which showed enhanced predictive ability relative to Numeracy in an external validation(11). Despite lack of transparent model publication and other apparent limitations, the Adjuvant! Online calculator is well known and widely used. It remains unclear whether Adjuvant! Online can accurately predict outcomes for older patients with node positive colon cancer who received adjuvant single agent or combination chemotherapy.
MATERIALS AND METHODS
ACCENT Database
The Adjuvant Colon Cancer Endpoints (ACCENT) database contains patient-level information on more than 30,000 patients enrolled to 25 adjuvant colon cancer trials since 1977(12–15). Endpoints in ACCENT include overall survival (OS), defined as the time from randomization to death due to any cause, and recurrence-free survival (RFS), defined as the time from randomization to disease recurrence or death due to any cause. The present analysis focuses on the subset of patients with stage III disease who were aged 70 years or older (Table 1).
Table 1.
ACCENT trials and number of elderly stage III patients per trial used for the Adjuvant! Online evaluation analyses.
| Trial | Years | Treatment Arms | N |
|---|---|---|---|
| CALGB 89803(18) | 1999–2001 | FU/LV vs. FU/LV + IFL | 141 |
| INT-0089(19) | 1990–1992 | FU/LEV vs. FU/LV (HD or LD) vs. FU/LV/LEV | 721 |
| MOSAIC(3) | 1998–2001 | FU/LV vs. FOLFOX | 178 |
| N0147(15) | 2004–2009 | mFOLFOX6 vs. mFOLFOX6 + cetuximab | 445 |
| NCCTG-89-46-51(20) | 1989–1991 | FU/LV +/− LEV for 6 or 12 months | 211 |
| NSABP C07(6) | 2000–2002 | FU/LV vs. FOLFOX | 291 |
| NSABP C08(14) | 2004–2006 | mFOLFOX6 vs. mFOLFOX6 + bevacizumab | 311 |
| PETACC-3(21) | 1999–2002 | FU/LV (AIO or LVFU2) vs. FOLFIRI | 152 |
| SWOG 9415(22) | 1995–1999 | Bolus vs. infusional FU/LEV/LV | 157 |
| XELOXA(5) | 2003–2004 | FU/LV vs. XELOX | 360 |
| TOTAL ACCENT | 1989–2009 | 2,967 |
Abbreviations: CALGB, Cancer and Leukemia Group B; FU, fluorouracil; LV, leucovorin; IFL, irinotecan; INT, Intergroup; LEV, levamisole; HD, high dose; LD, low dose; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; mFOLFOX6, modified FOLFOX 6 as infusional/bolus fluorouracil, leucovorin, and oxaliplatin; NSABP, National Surgical Adjuvant Breast and Bowel Project; AIO, folic acid, fluorouracil, and irinotecan; LVFU2, semi-monthly fluorouracil and leucovorin; FOLFIRI, fluorouracil, leucovorin, and irinotecan; SWOG, Southwest Oncology Group; XELOX, intravenous oxaliplatin plus oral capecitabine.
Adjuvant! Online Predictions
For each older patient in ACCENT with stage III disease, the following characteristics were entered into the Adjuvant! Online calculator: age (in years), sex (male, female), tumor stage (T1, T2, T3, T4), tumor grade (1, 2, 3), number of examined lymph nodes (1–3, 4–10, > 10), number of positive lymph nodes (1–3, 4–10, > 10), and chemotherapy (none, 5FU/LV, or FOLFOX). From these entries, estimated probabilities of 5-year OS and 5-year RFS were obtained and recorded. Because degree of co-morbidities (minor, average for age, or major) is a required calculator entry, but not recorded with the ACCENT database, both “average for age” and “minor” co-morbidities were considered in two separate analyses. This was felt appropriate, considering that these were patients who were entered into clinical trials, and unlikely to have major co-morbidities. No definition is provided by the Adjuvant! Online calculator for the different degrees of co-morbidity. Also, data collection procedures for the N0147(15) and C08(14) trials did not distinguish between grade 1 versus grade 2 tumors; to address this, these sub-categorizations were randomly imputed for patients with tumors described as “low” grade at rates equal to those otherwise observed in ACCENT.
Statistical Methods
Distributions of patient and disease characteristics and treatment were summarized descriptively within the analysis set of elderly ACCENT patients. Concordance between predicted and actual 5-year OS and 5-year RFS status from Adjuvant! Online and actual patient outcomes were assessed as follows. First, for each endpoint, the median 5-year predicted probability was computed across ACCENT patients and compared with the associated 5-year Kaplan-Meier estimate from the observed patient data and its 95% confidence interval. If the median Adjuvant! Online 5-year prediction for a given endpoint fell within the Kaplan-Meier interval, the performance of Adjuvant! Online was deemed satisfactory. Next, rates of correct prediction were computed as the percentage agreement between observed outcomes (event, no event) and predicted outcomes (using 50% predicted probability as a dichotomizing threshold) across patients. Finally, sensitivity and specificity of the Adjuvant Online! calculator predictions were calculated for each endpoint, where sensitivity is the “true positive” rate (percentage of patients who survive to 5 years who are correctly identified as likely survivors) and specificity is the true negative rate (percentage of patients who do not survive to 5 years who are correctly identified as unlikely to survive). All concordance metrics described above were calculated overall and by subgroups defined by variables included in the calculator (e.g., tumor stage and type of chemotherapy). For the purpose of examining a possible time trend in the accuracy of Adjuvant! Online predictions, evaluations were also performed within decades of ACCENT trial initiation (e.g., 1990–1999 vs. 2000–2009). All analyses were repeated according to the two different assumed levels of co-morbidities, while ACCENT patients with other missing calculator-required or outcome data were excluded from analyses.
RESULTS
Descriptive Statistics
A total of 2,967 older patients with stage III disease from 10 randomized trials in ACCENT (Table 1) contributed to the assessment of Adjuvant! Online. Trials containing surgery-only arms were excluded due to relevance to current clinical practice, as were trials for which one or more key Adjuvant! Online variables were not available. Among the remaining trials, patient and disease characteristics and treatment were distributed as shown in Table 2. Included elderly patients were predominantly between 70 and 74 years of age (66%), had tumor stage T3 (69%) or grade 2 tumors (66%), between 1–3 positive nodes (64%), more than 10 nodes examined (63%), were treated with 5FU/LV without oxaliplatin (61%), and were enrolled since the 2000s (47%). Male and female older patients were approximately equally represented.
Table 2.
Demographics and disease characteristics of patients used for the elderly Adjuvant! Online evaluation analyses.
| Variable | Number | Percentage |
|---|---|---|
| Age (years) | ||
| 70–74 | 1944 | 66 |
| 75+ | 1023 | 34 |
| Sex | ||
| Male | 1569 | 53 |
| Female | 1398 | 47 |
| Tumor Stage | ||
| T1 | 83 | 3 |
| T2 | 441 | 15 |
| T3 | 2062 | 69 |
| T4 | 381 | 13 |
| Tumor Grade | ||
| Grade 1 | 278 | 9 |
| Grade 2 | 1950 | 66 |
| Grade 3+ | 739 | 25 |
| Positive Nodes | ||
| 1–3 | 1899 | 64 |
| 4–10 | 937 | 32 |
| >10 | 131 | 4 |
| Nodes Examined | ||
| 1–3 | 127 | 4 |
| 4–10 | 985 | 33 |
| >10 | 1855 | 63 |
| Adjuvant Therapy | ||
| 5-FU/LV | 1814 | 61 |
| + Oxaliplatin | 1153 | 39 |
| Trial Initiation Decade | ||
| 1980s | 211 | 7 |
| 1990s | 1349 | 45 |
| 2000s | 1407 | 47 |
| Total | 2,967 | 100 |
Kaplan-Meier plots for RFS and OS among the older stage III patients are shown in Figure 1. Median RFS among elderly patients was 7.5 years; median OS was not reached. The 5-year recurrence-free survival rate among older patients was 58% (95% CI: 56% to 60%), while the 5-year overall survival rate was 65% (95% CI: 63% to 66%). Median follow-up among surviving patients was 7.0 years.
Figure 1.
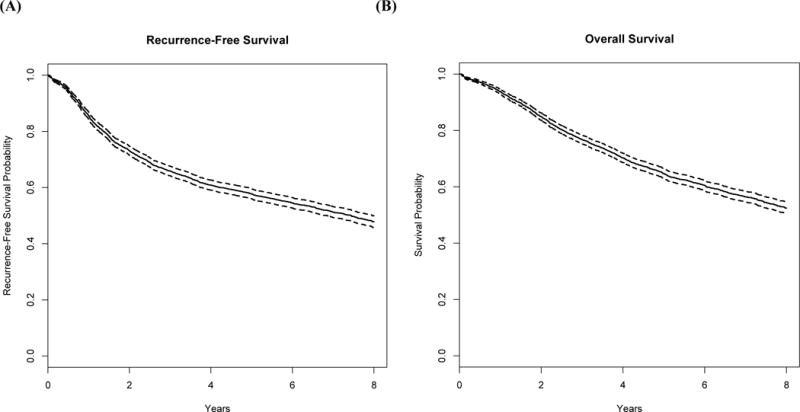
Kaplan-Meier curves with dashed 95% pointwise confidence intervals for the 2,656 ACCENT elderly patients with stage III colon cancer used in the evaluation of Adjuvant! Online: (A) recurrence-free survival (B) overall survival.
Concordance of Adjuvant! Online Predictions and Patient Outcomes
Concordance measures between Adjuvant! Online predictions and actual older stage III patient outcomes, overall and within subgroups of interest, are shown in Table 3 (overall survival) and Table 4 (recurrence-free survival).
Table 3.
ACCENT elderly 5-year survival rates (%) and Adjuvant! Online predictions assuming either “average for age” or “minor” comorbidities. Median predictions within corresponding Kaplan-Meier 95% confidence intervals and absolute differences less than 5% are shown in bold.
| N | K-M | 95% CI | Average for Age Comorbidities Assumed | Minor Comorbidities Assumed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A!O Pred | Delta (O–E) | % Agree | Sens | Spec | A!O Pred | Delta (O–E) | % Agree | Sens | Spec | ||||
| Overall | 2967 | 65 | (63, 66) | 58 | 7 | 65 | 82 | 40 | 64 | 1 | 65 | 89 | 29 |
| Age | |||||||||||||
| 70–74 | 1944 | 67 | (65, 69) | 61 | 6 | 67 | 88 | 31 | 65 | 2 | 67 | 91 | 25 |
| 75+ | 1023 | 61 | (58, 64) | 53 | 8 | 63 | 70 | 54 | 59 | 2 | 61 | 84 | 34 |
| Sex | |||||||||||||
| Male | 1569 | 63 | (61, 66) | 56 | 7 | 63 | 77 | 43 | 62 | 1 | 63 | 87 | 30 |
| Female | 1398 | 66 | (64, 69) | 62 | 4 | 68 | 88 | 36 | 66 | 0 | 67 | 91 | 27 |
| TStage | |||||||||||||
| T1 | 83 | 84 | (77, 92) | 74 | 10 | 83 | 100 | 8 | 79 | 5 | 81 | 100 | 0 |
| T2 | 441 | 67 | (63, 72) | 68 | −1 | 63 | 98 | 9 | 74 | −7 | 63 | 99 | 5 |
| T3 | 2062 | 66 | (64, 68) | 57 | 9 | 65 | 82 | 38 | 63 | 3 | 65 | 90 | 26 |
| T4 | 381 | 49 | (45, 55) | 46 | 3 | 66 | 57 | 72 | 51 | −2 | 64 | 66 | 61 |
| Grade | |||||||||||||
| 1 | 278 | 68 | (63, 74) | 61 | 7 | 65 | 86 | 29 | 65 | 3 | 66 | 93 | 20 |
| 2 | 1950 | 66 | (64, 68) | 60 | 6 | 65 | 86 | 31 | 65 | 1 | 65 | 92 | 21 |
| 3+ | 739 | 59 | (56, 63) | 53 | 6 | 66 | 70 | 63 | 57 | 2 | 65 | 79 | 48 |
| + Node | |||||||||||||
| 1–3 | 1899 | 72 | (70, 74) | 63 | 9 | 69 | 94 | 15 | 68 | 4 | 69 | 99 | 6 |
| 4–10 | 937 | 54 | (51, 58) | 50 | 4 | 57 | 56 | 59 | 54 | 0 | 56 | 69 | 43 |
| > 10 | 131 | 31 | (24, 40) | 31 | 0 | 70 | 0 | 98 | 32 | −1 | 79 | 0 | 100 |
| # Node | |||||||||||||
| 1–3 | 127 | 72 | (64, 80) | 53 | 19 | 65 | 74 | 48 | 58 | 14 | 73 | 91 | 38 |
| 4–10 | 985 | 61 | (58, 64) | 58 | 3 | 64 | 79 | 44 | 63 | −2 | 63 | 85 | 34 |
| > 10 | 1855 | 66 | (64, 69) | 59 | 7 | 66 | 85 | 37 | 64 | 2 | 66 | 91 | 25 |
| Adj Rx | |||||||||||||
| 5FU/LV | 1814 | 62 | (60, 64) | 58 | 4 | 63 | 81 | 40 | 63 | −1 | 63 | 88 | 30 |
| + Ox | 1153 | 69 | (67, 72) | 60 | 9 | 69 | 85 | 39 | 65 | 4 | 69 | 92 | 26 |
| Decade | |||||||||||||
| 1980s | 211 | 51 | (45, 59) | 66 | −15 | 49 | 95 | 14 | 72 | −21 | 46 | 95 | 9 |
| 1990s | 1349 | 62 | (60, 65) | 57 | 5 | 64 | 80 | 44 | 62 | 0 | 64 | 87 | 34 |
| 2000s | 1407 | 69 | (67, 72) | 59 | 10 | 69 | 84 | 41 | 64 | 5 | 69 | 91 | 27 |
Abbreviations: TStage, tumor stage; +Node, positive nodes; # Node, nodes examined; Adj Rx, adjuvant chemotherapy; K-M, Kaplan-Meier; CI, confidence interval, Pred, median prediction; Diff, difference; O, observed; E, expected; Agree, agreement; Sens, sensitivity; Spec, specificity.
Table 4.
ACCENT elderly 5-year recurrence-free survival rates (%) and Adjuvant! Online predictions assuming either “average for age” or “minor” comorbidities. Median predictions within corresponding Kaplan-Meier 95% confidence intervals and absolute differences less than 5% are shown in bold.
| N | K-M | 95% CI | Average for Age Comorbidities Assumed | Minor Comorbidities Assumed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A!O Pred | Delta (O–E) | % Agree | Sens | Spec | A!O Pred | Delta (O–E) | % Agree | Sens | Spec | ||||
| Overall | 2967 | 58 | (56, 60) | 57 | 1 | 61 | 78 | 41 | 62 | −4 | 61 | 86 | 31 |
| Age | |||||||||||||
| 70–74 | 1944 | 59 | (57, 62) | 60 | −1 | 61 | 83 | 34 | 64 | −5 | 62 | 90 | 26 |
| 75+ | 1023 | 55 | (52, 58) | 52 | 3 | 61 | 68 | 54 | 58 | −3 | 59 | 79 | 38 |
| Sex | |||||||||||||
| Male | 1569 | 56 | (53, 58) | 55 | 1 | 58 | 71 | 45 | 61 | −5 | 58 | 84 | 31 |
| Female | 1398 | 60 | (58, 63) | 61 | −1 | 65 | 86 | 37 | 65 | −5 | 63 | 89 | 30 |
| TStage | |||||||||||||
| T1 | 83 | 77 | (68, 86) | 73 | 4 | 76 | 100 | 5 | 78 | −1 | 73 | 100 | 0 |
| T2 | 441 | 62 | (57, 66) | 67 | −5 | 61 | 97 | 10 | 73 | −11 | 60 | 99 | 6 |
| T3 | 2062 | 59 | (57, 61) | 57 | 2 | 60 | 77 | 40 | 61 | −2 | 60 | 86 | 28 |
| T4 | 381 | 43 | (38, 48) | 45 | −2 | 64 | 48 | 73 | 49 | −6 | 65 | 63 | 66 |
| Grade | |||||||||||||
| 1 | 278 | 60 | (54, 66) | 60 | 0 | 58 | 82 | 29 | 64 | −4 | 60 | 93 | 21 |
| 2 | 1950 | 59 | (57, 62) | 58 | 1 | 61 | 82 | 33 | 64 | −5 | 60 | 90 | 22 |
| 3+ | 739 | 53 | (50, 57) | 51 | 2 | 64 | 65 | 63 | 56 | −3 | 62 | 72 | 53 |
| + Node | |||||||||||||
| 1–3 | 1899 | 65 | (63, 67) | 62 | 3 | 62 | 91 | 17 | 66 | −1 | 63 | 97 | 8 |
| 4–10 | 937 | 48 | (44, 51) | 48 | 0 | 57 | 50 | 63 | 52 | −4 | 54 | 61 | 48 |
| > 10 | 131 | 27 | (20, 36) | 29 | −2 | 77 | 0 | 100 | 32 | −5 | 79 | 0 | 100 |
| # Node | |||||||||||||
| 1–3 | 127 | 58 | (50, 68) | 52 | 6 | 57 | 61 | 51 | 56 | 2 | 63 | 85 | 33 |
| 4–10 | 985 | 54 | (51, 58) | 56 | −2 | 60 | 74 | 45 | 61 | −7 | 59 | 84 | 34 |
| > 10 | 1855 | 60 | (57, 62) | 58 | 2 | 63 | 82 | 39 | 63 | −3 | 61 | 88 | 28 |
| Adj Rx | |||||||||||||
| 5FU/LV | 1814 | 55 | (53, 57) | 55 | 0 | 60 | 73 | 46 | 60 | −5 | 59 | 83 | 34 |
| + Ox | 1153 | 62 | (60, 65) | 61 | 1 | 63 | 87 | 33 | 65 | −3 | 63 | 93 | 23 |
| Decade | |||||||||||||
| 1980s | 211 | 45 | (38, 52) | 64 | −19 | 49 | 95 | 16 | 70 | −25 | 47 | 95 | 11 |
| 1990s | 1349 | 56 | (53, 59) | 54 | 2 | 61 | 71 | 51 | 59 | −3 | 61 | 81 | 39 |
| 2000s | 1407 | 62 | (59, 64) | 58 | 4 | 63 | 84 | 37 | 64 | −2 | 63 | 91 | 26 |
Abbreviations: TStage, tumor stage; +Node, positive nodes; # Node, nodes examined; Adj Rx, adjuvant chemotherapy; K-M, Kaplan-Meier; CI, confidence interval, Pred, median prediction; Diff, difference; O, observed; E, expected; Agree, agreement; Sens, sensitivity; Spec, specificity.
Adjuvant! Online’s predictions for 5-year overall survival were broadly accurate when “minor” co-morbidities were assumed for the older ACCENT patients, both overall and within most of the patient subgroups examined, while predictions were less accurate when assuming the patients had “average for age” co-morbidities. On the other hand, 5-year RFS predictions were considerably more accurate when “average for age” rather than “minor” co-morbidities were assumed for the older ACCENT subjects.
Overall survival prediction accuracy
Among the older patients with stage III disease, Adjuvant Online! predicted probabilities of 5-year OS were well-calibrated when “minor” co-morbidities were assumed, with the median prediction across patients of 64% nearly matching the actual rate of 65%, and a rate of agreement of 65% across individual patients (Table 3). However, when “average for age” co-morbidities were assumed, the predicted 5-year survival rate dropped to 58%, or 7% below the actual rate. Median Adjuvant! Online predicted 5-year survival rates assuming “minor” co-morbidities were also equal to or within the 95% Kaplan-Meier intervals of the actual rates for most patient and disease subgroups, including both age subgroups and sexes, all tumor grades and categories of node positivity, both treatment groups, and older patients enrolled to ACCENT trials during the 1990s. Notably, among elderly ACCENT patients enrolled since 2000, the median Adjuvant! Online 5-year survival prediction of 64% was 5 percentage points below the actual rate of 69% (95% CI: 67% to 72%), suggesting the calculator’s survival calculations are perhaps less accurate for more recently diagnosed older patients than in past decades where the (unpublished) data used to develop the calculator might have been more current.
Across ACCENT older patient subgroups, percentage agreement between predicted (using 50% as a dichotomizing threshold) and actual 5-year survival ranged from 46% to 81% when “minor” co-morbidities were assumed, similar to 49% to 83% when “average for age” co-morbidities were assumed, though it is worth noting that a high degree of concordance across individual patients did not generally correspond to accurate median predictions within individual groups of interest. Sensitivity of the Adjuvant! Online calculator for predicting 5-year survival status among older patients was generally high: 89% overall and at least 65% in all but one subgroup (patients with more than 10 positive nodes) assuming “minor” co-morbidities; sensitivity was lower overall (82%) and within most subgroups when “average for age” co-morbidities were assumed. Specificity of the calculator was generally low: 29% overall when “minor” co-morbidities were assumed, 40% overall when “average for age” co-morbidities were assumed, and < 50% in most subgroups under either assumption.
Recurrence-free survival prediction accuracy
In contrast to the predictions for 5-year OS, the Adjuvant Online! calculator’s predicted probabilities of 5-year RFS were better calibrated when “average for age” rather than “minor” co-morbidities were assumed, with a median prediction across older patients of 57% (versus 62%) nearly matching the actual rate of 58% (95% CI: 56% to 60%; Table 4). The overall percentage agreement between observed and predicted outcomes among older ACCENT patients was identical between assumptions for co-morbidities (61%). Median Adjuvant! Online predicted 5-year recurrence-free survival assuming “average for age” co-morbidities was also within 95% Kaplan-Meier intervals for actual rates for all older patient subgroups except patients with T2 tumors or 1–3 positive lymph nodes, or patients enrolled in the 1980s or 2000s. In contrast, when “minor co-morbidities” were assumed for the older ACCENT patients, neither the overall nor most subgroup-specific Adjuvant! Online median predictions of 5-year RFS fell within the respective Kaplan-Meier 95% confidence intervals for actual rates. Notably, among older patients enrolled in the 1990s or 2000s, median predictions were reasonably accurate (though 5–6 percentage points apart) under either assumption for co-morbidities, contrasting with our observation that Adjuvant! Online estimates of 5-year survival seemed less accurate for more recently enrolled older patients.
Across ACCENT older patient subgroups, percentage agreement between predicted (using 50% probability as a dichotomizing threshold) and actual recurrence-free patient survival status at 5 years ranged from 49% to 77% when “average for age” co-morbidities were assumed, similar to a range of 47% to 79% for “minor” co-morbidities. Sensitivity of the Adjuvant! Online calculator for predicting 5-year survival status was generally high: 78% overall and more than 65% in most older patient subgroups assuming “average for age” co-morbidities; sensitivity was slightly higher overall (86%) and within most subgroups when “minor” co-morbidities were assumed. Specificity of the calculator was generally low: 41% overall when “average for age” co-morbidities were assumed, 31% overall when “minor” co-morbidities were assumed, and < 50% in most subgroups under either assumption.
DISCUSSION
To our knowledge, this is the first study looking at the validity of predictions based on the Adjuvant! Online program in older patients with colon cancer. A similar study looking at older patients with breast cancer was recently published; however, in that study the patients included came from a population-based cohort rather than clinical trials(16). The conclusion from that study was that Adjuvant! Online did not accurately predict overall survival and recurrence in older patients with early breast cancer. It is clear that a similar study looking at a population-based cohort of older colon cancer patients including information on co-morbidities would be of great interest.
A limitation of our study relates to the fact that co-morbidities are not collected in the ACCENT database. It was considered reasonable to make the assumption that these were fit patients with a good performance status, since they were entered into clinical trials. Therefore, two datasets were created, where patients were assumed to have either “minimal” or “average for age” co-morbidities. Adjuvant! Online does not provide a definition for the categories of co-morbidity used and this may lead to differences in how clinicians define and enter this parameter into the calculator. It can be argued that in everyday clinical practice, at least some patients over 70 years may have reduced treatment tolerance, limited life expectancy due to co-existing medical problems, and be more frail. Therefore, the results of our study which included clinical trial patients need to be interpreted with some caution. In addition, the limited inclusion of patients over the age of 75 in the clinical trials evaluated makes the application of our findings to this patient group even more difficult. Ideally, data on nutrition, physical functioning, social support, and polypharmacy should also be available when investigating the impact of adjuvant therapy as they can all influence survival in older patients.
In the context of adjuvant treatment for colorectal cancer in older patients, the impact of adjuvant chemotherapy is more likely to be reflected on RFS rather than OS due to competing deaths from other causes(4, 17). This would be consistent with our findings in terms of OS for the dataset of patients where “minimal” co-morbidities were assumed. These patients would be expected to have both an RFS and OS benefit conferred by adjuvant chemotherapy. However, the discrepancy in terms of RFS prediction for this group is unexplained. When “average for age” co-morbidities were assumed for the ACCENT patients, the predictions were more accurate for RFS, but not for OS, the latter of which was underestimated. Patients with increased co-morbidities would be expected to have a benefit from adjuvant therapy in terms of RFS. However, with time, co-morbidities can potentially impact on OS due to non-cancer related deaths.
Interestingly, among older ACCENT patients enrolled since 2000, the median Adjuvant! Online 5-year survival prediction of 64% was still 5 percentage points below the actual rate of 69% when “minor” comorbidities were assumed. The last 15 years have produced an overall improvement in patient outcomes due to a number of different factors including optimization of surgery, increased utilization of adjuvant chemotherapy and more aggressive management relapse. At the same time, it is possible that the data used to develop the calculator may reflect older practices.
Overall, our findings show that the Adjuvant! Online colon cancer prognostic calculator is reasonably accurate overall for predicting outcomes in older patients with stage III colon cancer who entered clinical trials, though accuracy may differ between 5-year RFS and 5-year OS predictions when a fixed degree of co-morbidities is assumed.
Acknowledgments
The ACCENT (Adjuvant Colon Cancer Endpoints) Group consists of: D.J. Sargent, E. Green, A. Grothey, S.R. Alberts, Q. Shi, L.A. Renfro (Mayo Clinic, Rochester, MN); G. Yothers, M.J. O’Connell, N. Wolmark (NSABP [National Surgical Adjuvant Breast and Bowel Project] Biostatistical and Operations Centers, Pittsburgh, PA); A. de Gramont (CTD-INCa GERCOR, Assistance Publique des Hôpitaux de Paris, UPMC Paris VI, Paris, France); R. Gray, D. Kerr (QUASAR [Quick and Simple and Reliable] Collaborative Group, Birmingham and Oxford, United Kingdom); D.G. Haller (Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA); K. Guthrie (SWOG [Southwest Oncology Group] Statistical Center, Seattle, WA); M. Buyse (IDDI, [International Drug Development Institute], Louvain-la-Neuve, Belgium); R. Labianca (Ospedali Riuniti, Bergamo, Italy); J.F. Seitz (University of the Mediterranean, Marseilles, France); C.J. O’Callaghan (NCIC-CTG [National Cancer Institute of Canada Clinical Trials Group], Queens University, Kingston, Ontario, Canada); G. Francini (University of Siena, Siena, Italy); P.J. Catalano (ECOG [Eastern Cooperative Oncology Group] Statistical Center, Boston, MA); C.D. Blanke (Oregon Health Sciences University, Portland, OR); T. Andre (Hôpital Saint Antoine, Paris, France); R.M. Goldberg (Ohio State University Comprehensive Cancer Center, Columbus, OH); A. Benson (Northwestern University, Chicago, IL); C. Twelves (University of Bradford, West Yorkshire, United Kingdom); F. Sirzen (Roche, Basel, Switzerland); E. Van Cutsem (University Hospital Gasthuisberg, Gasthuisberg, Belgium); and L. Saltz (Memorial Sloan-Kettering Cancer Center, New York, NY).
FUNDING
This work was supported by the National Cancer Institute at the National Institutes of Health [grant number CA 25224].
Footnotes
DISCLOSURES AND CONFLICT OF INTEREST STATEMENTS
The authors have declared no conflicts of interest.
AUTHOR CONTRIBUTIONS
Study Concepts: D Papamichael, L Renfro, D Sargent
Study Design: D Papamichael, L Renfro, D Sargent
Data Acquisition: L Renfro, D Sargent
Quality Control of Data and Algorithms: L Renfro, D Sargent
Data Analysis and Interpretation: D Papamichael, L Renfro, D Sargent
Statistical Analysis: L Renfro, D Sargent
Manuscript Preparation: D Papamichael, L Renfro, D Sargent, C Matthaiou
Manuscript Editing: D Papamichael, L Renfro, C Matthaiou, G Yothers, L Saltz, K Guthrie, E Van Cutsem, HJ Schmoll, R Labianca, T André, M O’Connell, SR Alberts, DG Haller1, P Kountourakis, D Sargent
Manuscript Review: D Papamichael, L Renfro, C Matthaiou, G Yothers, L Saltz, K Guthrie, E Van Cutsem, HJ Schmoll, R Labianca, T André, M O’Connell, SR Alberts, DG Haller1, P Kountourakis, D Sargent
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26(3):463–76. doi: 10.1093/annonc/mdu253. [DOI] [PubMed] [Google Scholar]
- 3.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 4.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–16. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 5.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465–71. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 6.Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768–74. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCleary Nadine J, Meyerhardt Jeffrey A, Green Erin, Yothers Greg, de Gramont Aimery, Van Cutsem Eric, et al. Impact of Age on the Efficacy of Newer Adjuvant Therapies in Patients With Stage II/III Colon Cancer: Findings From the ACCENT Database. J Clin Oncol. 2013;31(20):2600–6. doi: 10.1200/JCO.2013.49.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adjuvant! Online program. http://www.adjuvantonline.com/index.jsp.
- 9.Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22(10):1797–806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 10.Gill S, Loprinzi C, Kennecke H, Grothey A, Nelson G, Woods R, et al. Prognostic web-based models for stage II and III colon cancer: A population and clinical trials-based validation of numeracy and adjuvant! online. Cancer. 2011;117(18):4155–65. doi: 10.1002/cncr.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renfro LA, Grothey A, Xue Y, Saltz LB, André T, Twelves C, et al. ACCENT-based web calculators to predict recurrence and overall survival in stage III colon cancer. J Natl Cancer Inst. 2014;106(12) doi: 10.1093/jnci/dju333. pii: dju333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664–70. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 13.Sargent D, Shi Q, Yothers G, Van Cutsem E, Cassidy J, Saltz L, et al. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer. 2011;47(7):990–6. doi: 10.1016/j.ejca.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29(1):11–6. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307(13):1383–93. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Glas NA, van de Water W, Engelhardt EG, Bastiaannet E, de Craen AJ, Kroep JR, et al. Validity of Adjuvant! Online program in older patients with breast cancer: a population-based study. Lancet Oncol. 2014;15(7):722–9. doi: 10.1016/S1470-2045(14)70200-1. [DOI] [PubMed] [Google Scholar]
- 17.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 18.Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25(23):3456–61. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 19.Haller DG, Catalano PJ, Macdonald JS, O’Rourke MA, Frontiera MS, Jackson DV, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23(34):8671–8. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 20.O’Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ, Jr, Erlichman C, Shepherd L, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16(1):295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 21.Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27(19):3117–25. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 22.Poplin EA, Benedetti JK, Estes NC, Haller DG, Mayer RJ, Goldberg RM, et al. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol. 2005;23(9):1819–25. doi: 10.1200/JCO.2005.04.169. [DOI] [PubMed] [Google Scholar]


