Abstract
Background
To send meaningful information to the brain, an inner ear cochlear implant (CI) must become closely coupled to as large and healthy a population of remaining Spiral Ganglion Neurons (SGN) as possible. Inner ear gangliogenesis depends on macrophage migration inhibitory factor (MIF), a directionally attractant neurotrophic cytokine made by both Schwann and supporting cells (Bank et al., 2012). MIF-induced mouse embryonic stem cell (mESC)-derived “neurons” could potentially substitute for lost or damaged SGN. mESC-derived “Schwann cells” produce MIF as do all Schwann cells (Huang et al., 2002; Roth et al., 2007, 2008) and could attract SGN to “ cell coated” implant.
Results
Neuron- and Schwann cell-like cells were produced from a common population of mESC in an ultra-slow flow microfluidic device. As the populations interacted; “neurons” grew over the “Schwann cell” lawn and early events in myelination were documented. Blocking MIF on the Schwann cell side greatly reduced directional neurite outgrowth. MIF-expressing “Schwann cells” were used to “coat” a CI: mouse SGN and MIF-induced “neurons” grew directionally to the CI and to a wild type but not MIF-knock out Organ of Corti explant.
Conclusions
Two novel stem cell-based approaches for treating the problem of sensorineural hearing loss are described.
Keywords: inner ear-like neurons from stem cells, stem cell derived Schwann cells, microfluidics, macrophage migration inhibitory factor, neurotrophic cytokines, hearing
INTRODUCTION
Development of new stem cell-based therapeutic approaches is bringing the era of regenerative medicine into more immediate focus. For example, a current area of intense interest to neuroscientists and bioengineers is the potential replacement of lost or diseased central and peripheral neurons and/or peripheral nervous system Schwann cells with biologically engineered stem cells. Ideally, stem cells would be capable of taking on the mature properties of both neuronal and Schwann cell types and could also participate in the critical crosstalk that leads to myelination of the neurons by Schwann cells.
The inner ear’s spiral ganglion’s myelinated bipolar neurons (SGN) provide the conduit for sensory messages from peripheral sensory receptors of mechanosensory hair cells to the central neurons in auditory brain stem nuclei. When the hair cells in the Organ of Corti are lost or incapacitated, due to injury, ageing or loss of function resulting from genetic disorders, spiral ganglion neurons lose their peripheral targets and can progressively degenerate, so that the remaining neurons are less capable of functional interaction with either the remaining sensory Organ of Corti hair cells or with a cochlear implant/prosthesis (Pfingst et al., 2015, review). Although spiral ganglion neuron loss in humans is less pronounced than in animal models of hair cell loss or Knock out mouse models of hair cell loss (Bermingham-McDonogh and Rubel, 2003; Bodmer, 2008; Breuskin et al., 2008), it appears that even if spiral ganglion neurons remain as long as 40 years post onset of deafness, their functionality can be impaired (Sato et al., 2006; Wise et al., 2010),(Jiang et al., 2006).
In order to interact productively with a cochlear implant, the surviving spiral ganglion neurons must be healthy and sufficiently well-distributed along the cochlea to cover the range of frequency distribution required to deliver the necessary information for speech discrimination (Parkins, 1985). Although conductive hearing loss can be remediated by using hearing aids, middle ear implants and bone anchored hearing aids, a direct stimulation of the auditory nerve is required when sensorineural hearing loss is severe. Such stimulation can be achieved by implanting cochlear prostheses/implants in the scala tympani of the cochlea. However, in the future, direct auditory brain stem implants may well become the standard therapeutic approach (reviewed in Merkus et al, 2014).
Some very promising studies of hearing restoration have recently been done in mammalian animal models. Endogenous Supporting cells of the inner ear have been shown to differentiate and replace hair cell function (e.g. Mellado Lagarde et al., 2014; Wan et al., 2013; Monzack and Cunningham review, 2014). Human inner ear stem cells transplanted into deafened animal models also restored hearing function (Chen, Jongkamonwiwat et al., 2012).
Strategies for prolonging survival or regenerating spiral ganglion neurons, many focused on earlier-identified inner ear neurotrophins as inducing agents (Roehm and Hansen, 2005; Ramekers et al., 2012) have been a focus of research for many years. To restore hearing function and prolong survival of existing spiral ganglion neurons, some researchers have developed paradigms in which a single neurotrophin or a cocktail of neurotrophins was administered to deafened animals using mini osmotic pumps or via cochlear implants coated with various gels/hydrogels that can slowly release such neurotrophins (Winter et al., 2007; Jun et al., 2008; Winter et al., 2008; Jhaveri et al., 2009). However, such treatment options have not yet progressed to clinical or even pre-clinical trials in patients with hearing loss (Miller et al., 2002; Pettingill et al., 2007a, b; O’Leary et al., 2009b; Pfingst et al., 2015).
To improve the performance of cochlear implants, a variety of different strategies to improve hearing perception are being tested; among these are: 1. Advanced engineering of cochlear implant devices, which can communicate well with the brain stem (for a review see Pfingst et al., 2015), 2. Cell replacement therapies, involving various types of stem cells to augment or substitute for lost or malfunctioning neurons (Corrales et al, 2006; Coleman et al., 2007: Reyes et al., 2008; Chen, Jongkamonwiwat et al., 2012) 3. Re-growing spiral ganglion neuronal processes to improve connections with the implant and concomitantly to reduce the distance between them (Altschuler et al., 1999); 4. “Classical” neurotrophin-releasing Schwann cells used to ‘coat’ cochlear implants have been shown to enhance neurite contacts with the devices (O’Leary et al., 2009).
The research described in this report focuses on two stem cell-based strategies to address sensorineural hearing loss: Replacement of damaged or lost spiral ganglion neurons and neurotrophic factor-producing cells that could enhance the attractive properties of a cochlear implant. We used a very-slow-differential-flow microfluidic device (Park et al., 2009), to differentiate a common population of embryonic stem cells into two different types of cells—neuron-like cells and Schwann cell-like cells, using differential flow to deliver inducing agents for neurons and Schwann cells simultaneously in two streams of fluid, which, although side by side move at different flow rates.
When macrophage migration inhibitory factor (MIF)—and not nerve growth factor (NGF) or ciliary neurotrophic factor (CNTF)-- is the neuron-inducing agent, we show that the neuron-like cells bear some significant resemblance to statoacoustic ganglion or spiral ganglion neurons of the inner ear. NGF and CNTF also induce neuronal phenotypes; we have shown in other studies that NGF produces dorsal root ganglion-like neurons and CNTF induced motor neuron-like neurons (Roth et al., 2007, 2008; Bank et al., 2012). We have previously shown that MIF is the inner ear’s first developmentally important neurotrophin (Holmes et al., 2011; Shen et al., 2011; Shen et al., 2012; Bank et al., 2012, cited in Faculty of 1000) and that receptors for MIF remain on spiral ganglion neurons into adulthood (Bank et al, 2012). These earlier studies were done in conventional tissue culture devices/dishes.
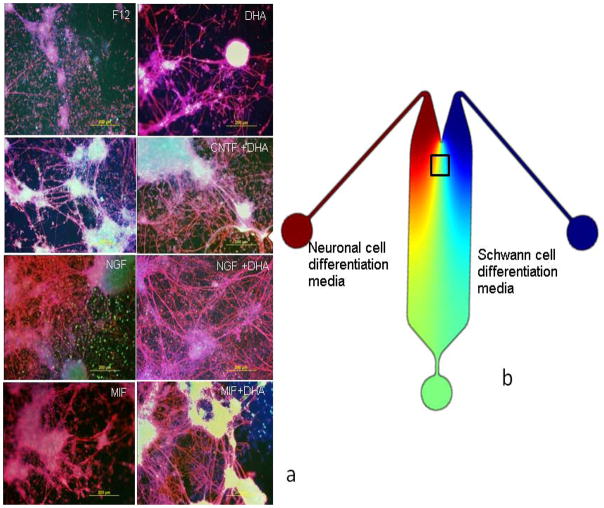
In this study, the MIF-induced neuron-like cells produced on the “neuronal” differentiation side of the slow-flow microfluidic devices were characterized for electrophysiological functional maturation by patch clamping and for transporters, neurotransmitters and appropriate ion channel expression by immunocytochemistry and RTqPCR. The MIF-induced neuron-like cells’ properties were compared to the neuron-like cells induced with Nerve Growth Factor (NGF) or Ciliary Neurotrophic Factor (CNTF) as we had done previously in our conventional tissue culture studies (Roth et al., 2007, 2008; Bank et al., 2012). The neuron-like cells’ maturation is enhanced by exposure to docosahexaenoic acid (DHA), which is capable of enhancing both electrophysiological functional maturation (Uauy et al., 2001; Khedr et al., 2004) and myelination in the microfluidic device (Fig. 4).
Figure 4.
Observations of myelination onset as neuron-like cells and Schwann cell-like cells interact in the mid-section of the Microfluidic device Row II. The cultures were stained for neurofilament Heavy 200 kDa (NF-H) (red), Myelin Basic Protein (blue) and VgluT1 (green). F12=the basal medium, CNTF=ciliary neurotrophic factor; NGF=nerve growth factor; MIF=macrophage migration inhibitory factor; DHA=docosahexaenoic acid (b) Merged images of multi-labelled devices (c) Cartoon showing the location of Row II, which is also shown Fig. 1.
Neuregulin (Gambarotta et al., 2013) was used to induce Schwann cell-like cells as in our previous studies (Roth et al., 2007, 2008) in the other fluid stream of the device. Our laboratory made the first embryonic stem cell-derived Schwann cells almost a decade ago (Roth et al., 2007). We demonstrated previously that these engineered Schwann cells have all the properties of myelinating Schwann cells (Roth et al., 2007), acquire Schwann cell-like properties in the expected order during maturation and that they produce MIF as do all vertebrate Schwann cells (Huang et al., 2002).
Such stem cell-derived neuron-like and Schwann cell-like cells could be used independently or together to provide therapeutic approaches to alleviate a variety of neurological disorders, including multiple sclerosis (Taupin, 2011; Zhang et al., 2011) spinal cord injury (Paino et al., 1994; Goodman et al., 2007; Zhang et al., 2007;Boomkamp et al., 2012); Parkinsonism (Mena and Garcia de Yebenes, 2008); peripheral nerve regeneration (Namqung et al., 2015) and, most importantly for these studies, sensorineural hearing loss/deafness (Pettingill et al., 2008). Replacement of the sensory hair cell population from stem cells endogenous to the inner ear is also under investigation (Li et al., 2003a,b; Liu et al, 2014) although not in these studies.
It has been difficult to study the stages of neuron-Schwann cell interactions that result in myelination in conventional tissue cultures of primary peripheral system neurons and Schwann cells (Gingras et al., 2008; Heinen et al., 2008; Callizot et al., 2011; Jarjour et al., 2012) for several reasons: 1) the inability to distinguish living primary neurons from Schwann cells in conventional cell co-cultures during the differentiation and interaction process (Paivalainen et al., 2008), unless one population or the other is tagged with a live cell label; 2) the difficulty in obtaining immature cultures of both primary neurons and Schwann cells in order to observe and document the key EARLY steps in their interactions that lead to myelination and 3) sufficiently specific stage-specific markers of myelination progress, as our earlier studies documented (Roth et al., 2007, 2008).
Tissue engineered co-culture systems have been somewhat successful in identifying key molecular steps and markers in studies of myelination (Hsu and Ni, 2009; Liazoghli et al., 2012). A number of such co-culture systems have involved variously derived stem cells (Yang et al., 2008: Wei et al., 2010; Xiong et al., 2012; Yang et al., 2012) mostly to provide the “neuronal” component. However, none of these engineered stem cell based systems has been sufficient to observe the continuum of cell-cell interactions that result in early events in myelination.
The very slow-flow devices used in these studies allows us to study neuronal/Schwann cell interactions that result in onset of myelination during the course of the experiment, which can be carried out for up to 6 weeks. An osmotic pump is essential to achieving sufficiently slow flow with low shear that does not cause cell detachment and that allows us to culture cells in devices with nutrient replenishment and eliminate wastes for up to 6 weeks.
The device was designed to generate overlapping gradients of one of the neurotrophic factors (MIF, NGF or CNTF; Bank et al., 2012) as well as Schwann cell-inducing factors (Neuregulin; Roth et al., 2007) from two different inlets (see cartoon in Fig. 1). By stably maintaining flow over a 3–6 week period, we were able to correlate cell differentiation and neuronal outgrowth to computed concentrations of the different factors and to document many aspects of the interactions between the two cell types in the device—particularly the very early events in myelination. The microfluidic device allows us to study the cell-cell interactions between the “neurons” and the “Schwann cells” with time-lapse digital cinematography and also permits molecular, immunohistological and electrophysiological analyses of the cells in the device itself.
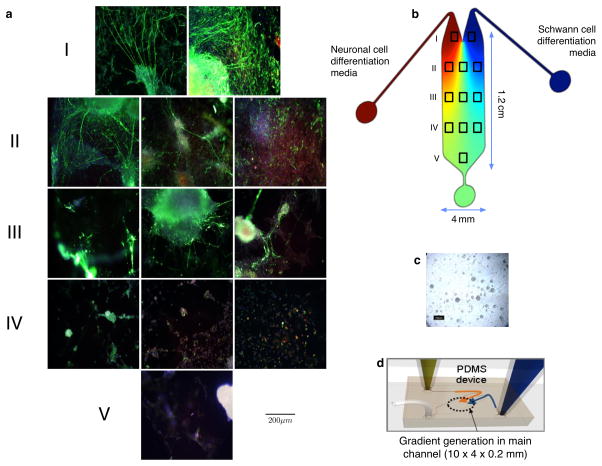
Figure 1.
The mouse embryonic stem cells (mESC) seeded on the device differentiated into both neuron-like and Schwann cell-like cells.. (a) Photomigrographs of the areas of the device depicted as boxes in the cartoon in (b) were taken after the whole device was stained for neurofilament 150 kDa – neurites (green); Dapi-nucleus (blue); Myelin Basic Protein – evidence of Schwann cell myelination (red). After 3 weeks, the top three rows (I, II, III) show significant neuronal differentiation as well as directional outgrowth of neurites (green) towards the “Schwann cell” sectors (those on the right). In the bottom two rows (those with the most media intermixing and least preserved gradients), the cells apparently did not differentiate into any specific lineage (IV and V). The white arrows in (a) indicate the directional outgrowth of neurites. (c) Undifferentiated mES cells in the microfluidic device. (d) A cartoon of the microfluidic device. The gradient flow illustration (b) and microfluidic chip channel dimensions depicted in (d) are adapted from (Park et al., 2009).
The role of MIF produced by the “Schwann cells” in promoting directional outgrowth of processes from the “neurons” towards the Schwann cell side of the device was tested in two ways. After the embryonic stem cells had differentiated into “Schwann cells” or “neurons” we blocked MIF production on the Schwann cell side of the device with the biochemical MIF inhibitor, 4-iodo-6-phenylpyrimidine (4-IPP; Specs, Delft, Netherlands) at 0.1 μM 4-IPP for 7 days, as we have done previously (Shen et al., 2012). In another set of experiments, we blocked MIF downstream pathways with a monoclonal antibody to MIF using conditions we previously established for studies of both mouse and chick cultured primary neurons and stem cell derived neurons (Bank et al., 2012). Blocking MIF resulted in a significant reduction in directional neurite outgrowth towards the “Schwann cell” lawn in the microfluidic devices (Fig. 2), just as we previously demonstrated in conventional tissue culture experiments (Bank et al., 2012).
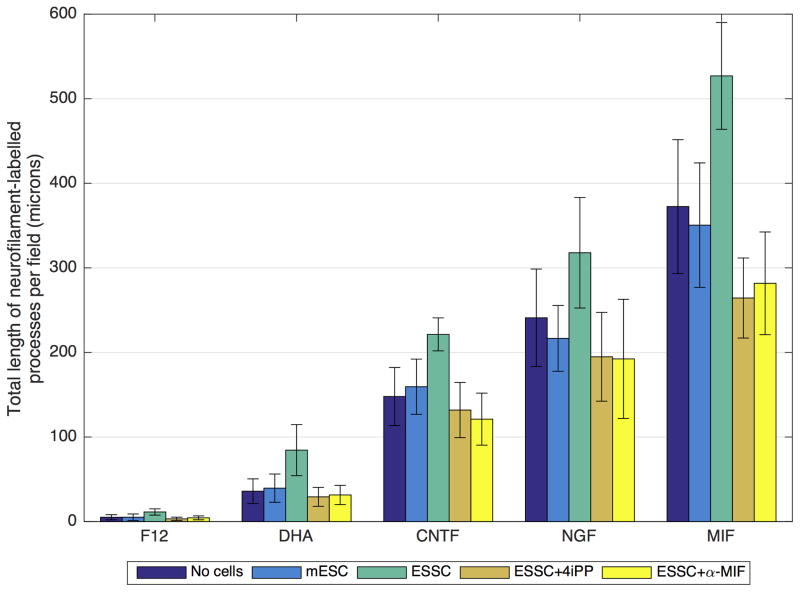
Figure 2.
Neurite measurements of the neuron-like cells differentiated with different neurotrophic factors with and without DHA in the microfluidic device. The “neurites” of neuron-like cells cocultured with Schwann cell-like cells in the microfluidic device grew directionally towards and over the “Schwann cell” lawn. Total process lengths were measured using MetaMorph® or ImageJ software for this directional outgrowth; 8 experiments using a minimum of 10 photographed fields of view in each were used. mESC = undifferentiated mouse embryonic (D3) stem cells on the “Schwann cell” side of the device. ESSC = Embryonic Stem cell-derived Schwann cells; these are Neuregulin-induced “Schwann cells” derived from the mESC. ESSC +4iPP: under these conditions, the differentiated “Schwann cell lawns on the Schwann cell side of the device were treated with the biochemical MIF inhibitor 4iPP (as described in Methods and in Shen et al., 2012). ESSC+ α-MIF: Downstream MIF effects were blocked by addition of a function-blocking anti-MIF antibody (α-MIF) as we had done in previous studies (Bank et al., 2012).
Significance Calculations for these data
Differences between treatment groups and statistical significance were analyzed using Mann-Whitney U tests performed in Matlab. There were statistically significant differences found amongst these treatment groups with p values as indicated below:
DHA alone: (mESC vs ESSC, p =0.0023; ESSC+4iPP vs ESSC, p = 3.1080 ×10−4; ESSC+ α-MIF vs ESSC, p = 5.4390 ×10−4)
CNTF alone: (mESC vs ESSC, p =1.5540 ×10−4; ESSC+4iPP vs ESSC, p =7.7700 ×10−5; ESSC+ α-MIF vs ESSC, p = 7.7700 ×10−5)
NGF alone: (mESC vs ESSC, p =0.0050; ESSC+4iPP vs ESSC, p =0.0015; ESSC+ α-MIF vs ESSC, p = 0.0052)
MIF alone: (mESC vs ESSC, p =5.4390 ×10−4; ESSC+4iPP vs ESSC, p = 7.7700 ×10−5; ESSC+ α-MIF vs ESSC, p = 7.7700 ×10−5)
Once we had convincing data confirming our earlier studies that MIF appears to be involved in directional neurite outgrowth either through its direct effects (see fig. 1 in Bank et al., 2012) or through its production by an mESC-derived “Schwann cell” lawn (see fig. 5B in Roth et al., 2007), we used the MIF-producing mouse embryonic stem cell-derived Schwann cells, encapsulated in sodium algenate hydrogels, to coat two types of cochlear implants: the multi-electrode type commonly used in human patients and the simple ball type used in animal studies.
Directional outgrowth of neurites from primary mouse statoacoustic ganglion (which is the embryonic precursor of both the spiral ganglion and vestibular ganglion in the vertebrates that develop both SG and VG) and spiral ganglion explants extending neurites toward the devices was observed by time-lapse cinematography. Contacts with the implants were visualized by immunocytochemistry using specific markers.
MIF-induced neuron-like cells were also co-cultured with mouse Organ of Corti explants and directional outgrowth of neurites towards the target was documented towards wild type but not the MIF-knock out Organ of Corti explants. We had already demonstrated that this could be done with primary neurons in conventional tissue culture (Bank et al., 2012).
This study could eventually benefit two types of patients—1) those with low spiral ganglion neuron counts who are not good candidates for cochlear implant therapy, for whom a replacement population of stem cell derived neuron-like exogenous cells could enhance functional contacts with their own native Organ of Corti sensory hair cells and 2) patients for whom cochlear implants coated with MIF-producing Schwann cell-like cells could markedly improve directional outgrowth of their remaining spiral ganglion neurites to the cochlear implant.
RESULTS
Concomitant differentiation of mouse embryonic stem cells (mESC) into neuron-like cells and Schwann cell-like cells in a slow flow microfluidic device
We differentiated mouse embryonic stem cells into both neuron-like cells and Schwann cell-like cells in the same microfluidic device to study cell differentiation and maturation (neuronal or Schwann cell) as well as any cell-cell interactions between the Schwann cells and the neurites emerging from the neuron-like cells; e.g. those that might result in evidence of myelination upon contact with the Schwann cell-like cells.
The microfluidic system was designed to establish a gradient flow to nourish the stem cells with nutrients and growth factors or neurotrophic cytokines in order to facilitate the differentiation of two different cell populations from a single undifferentiated stem cell population seeded on the device. Figure 1c is a bright-field image of undifferentiated mouse embryonic stem cells (ESC; Doetschman et al., 1985) seeded over the entire surface area of the device after coating the device with sterile porcine gel (see Experimental Procedures). A cartoon of the device, with the “neuron” side depicted in red and the “Schwann cell” side depicted in blue is seen in Fig. 1b. Then, using differential flow, Schwann cell-like cells were induced to form on the right half of the device (blue outflow source seen in the cartoon in Figs. 1b and 1d) using concentrations of Neuregulin that we had previously determined produced myelinating Schwann cells (Roth, Ramamurthy et al., 2007, 2008).
One of 3 different inducing agents was used to produce the neuron-like cells in the left (red Fig. 1b) half of the device: NGF, CNTF or MIF at concentrations we had previously optimized (Roth et al., 2007, 2008; Bank et al., 2012; see Experimental Methods). The “neuron”-inducing agent was delivered form the reservoir indicated in red in the cartoon in Figs. 1b and 1d. DHA enhancement was also tested in the microfluidic devices in some cases and it was delivered through the same portal. DHA enhancement had proven to be very effective in our previous studies, even at the lowest concentration we tested (Bank et al., 2012). Three to 8 duplicate devices for each inducing agent or condition were prepared in parallel, incubated, fixed, labelled (e.g. with antibodies to Neurofilament or TUJ1), photographed and analyzed for each set of conditions using MetaMorph© software.
After one week in the microfluidic devices, we saw both morphological and molecular evidence of neuronal differentiation on the “neuronal” side of the device under all neuron-inducing conditions at the concentrations tested (see experimental methods), including DHA alone. Neurofilament-positive cell bodies with extensive processes were documented photographically and analyzed by MetaMorph© or NIH ImageJ software. By contrast, little if any neuronal differentiation was seen at 1 week in the presence of F12 medium alone (control) (Fig. 2), as we had also previously documented (Roth, Ramamurthy et al., 2007, 2008; Bank et al., 2012).
After 2–3 weeks, we observed that more than 75% of the mES cells exhibited neuronal processes in response to NGF, CNTF or MIF as well as to any of these inducing agents enhanced with DHA. However, because the “neuron-like” cells were clumped together and the processes were found in fascicular bundles (e.g. Bank et al., 2012, Fig. 2), it was often difficult to determine the actual numbers of neuron-like cell bodies in the clumps or how many processes were present in the fascicles. Fasciculation was enhanced markedly by the addition of DHA.
Directional Neurite Outgrowth is observed from the Neuron-like cells towards the “Schwann cell” sectors in Microfluidic Devices
To determine if there was preferential neurite outgrowth from the neuron-like cells toward the Schwann cell-like cells in the microfluidic device (Fig. 1a), we used time-lapse videomicrography and end-stage photomicrography to identify immuno-labelled (neurofilament or TUJ1) cells and processes on the “Schwann cell” side of the devices. Photographs of the immuno-labelled cells were analyzed by MetaMorph© or NIH ImageJ software. For these analyses, we virtually sectioned the device into 5 rows (I, II, III, IV, V; Figs. 1a and 1b). Rows II, III and IV were divided into three columns for taking the photomicrographs used in these analyses (see the cartoon in Fig. 1b). The mouse embryonic stem cells showed concentration-dependent differentiation from the channel inlet to the outflow port (depicted in the cartoon in (Fig. 1b) as a round green area at the bottom. ES cells took on neuronal morphologies in areas closest to the left inlet (Fig 1a: I, II). The cells that became neuron-like in sectors I, II and any found in III extended neurites toward the Schwann-cell like cell lawns (e.g. Fig. 1a Row I right image), since Schwann cell-like cells produce MIF, which is both a neurite outgrowth and survival factor for many different neuronal subtypes, including those in the inner ear (Bank et al., 2012) and eye (Ito et al., 2008). We tested the role of MIF directly in additional studies (Fig. 2 below). The extension of ramifying processes of the neuron-like cells over the Schwann cell lawn is also typical of the behaviour of primary neurons on such engineered Schwann cell lawns (see Roth et al., 2007 fig. 5b). Neurofilament positive neuron-like cells that differentiated in Row III (Fig. 1 a: III middle) had minimal numbers of neuronal processes; however, the few processes that were present grew directionally towards the Schwann cell lawn (blue side of the device Figs. 1a,b). Neuron-like cells in row IV also could be labelled for neurofilament but no processes developed in this region (Fig. 1a IV). There were cells in row IV that were not stained for neurofilament (Fig. 1a) but could be stained for myelin basic protein MBP, indicating that they were “Schwann cells” (see also Fig. 4) (as in our previous work, Roth et al., 2008). Cells in region V did not express markers for either Schwann cells or neurons; nor was there evidence of myelination, indicating the undifferentiated ES status of these cells, many of which still expressed Oct4 (Roth et al., 2007 2008; Bank et al., 2012), a marker for undifferentiated stem cells (Pan et al., 2002). The gradient profile created in the device, in which the highest concentrations of neuron-inducing and Schwann cell-inducing media predominated near the inlets facilitates ES cells’ ‘differentiation into neuron-like cells and Schwann cell-like cells with a much higher expression level of their cell type specific protein markers (Fig. 1a I, II, III). Neurites extending over the Schwann cell lawn in row I (Fig. 1a I right) also exhibited the most neurite fasciculation compared with the other sections of the device. We observed cell differentiation by both time-lapse cinematography and end-stage photomicrography. We measured the total neurite outgrowth of the processes using confocal microscopy and light photomicrography, analyzed by MetaMorph© software or NIH ImageJ software (Experimental Procedures).
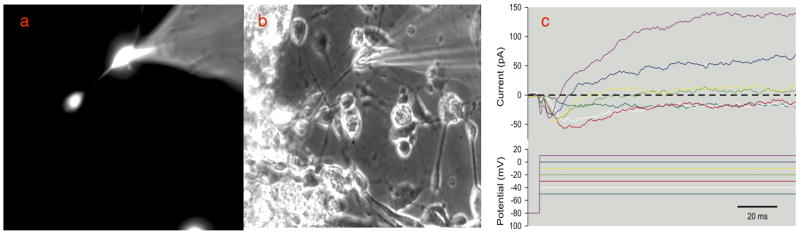
Figure 5.
Patch clamp studies performed on the neuron-like cells induced by MIF. a. Electrodes were filled with ionic fluorescent rhodamine dye and used to patch clamp the cell membrane of cells identified as having a neuronal morphology. b. Phase contrast image of a group of differentiated neuron-like cells; the cells that were identified as having a bipolar neuronal morphology were patch-clamped using a fire polished glass pipette electrode. MIF- and DHA-treated ES cells showed voltage dependent inward and outward current with long active response times, demonstrating voltage-dependent currents in a neuron-like bipolar cell. Voltage dependent changes were seen in response to step depolarization from a holding potential of −80 mV. Both transient inward currents and maintained outward currents were observed. The linear leak currents and most of the linear capacity currents were removed during data acquisition with a P/8 protocol (although a small residual capacity transient is visible immediately after the voltage steps). The ionic conditions were set so that ENa and EK were at normal levels and neither sodium nor potassium channels were blocked, so the responses to small voltage steps are dominated by transient inward sodium currents that got faster as the cell was depolarized, and the response to large steps was dominated by a maintained outward current.
MIF production by Schwann cell-like cells appears to play a role in directional process outgrowth
When devices were stained with an antibody to Neurofilament and appropriate secondary antibodies, little or no directional outgrowth towards the “Schwann cell” side of the device was seen under any of the “neuron-inducing” conditions (Fig. 2) if there were no cells on the “Schwann cell” side of the device. This was also the case if there were undifferentiated mESC (not exposed to Neuregulin) on the “Schwann cell” side. However, if Neuregulin had been used to induce a Schwann cell-like phenotype, there was a significant directional process outgrowth towards the Schwann cell lawns (Fig. 2). Compare the process outgrowth towards the undifferentiated mouse embryonic stem cell lawns [mESC] with outgrowth towards mESC induced to become Schwann cell like with Neuregulin [ESSC] (Fig. 2). mES Schwann cell differentiation resulted in ramification of “neuron-like” processes over the Schwann cell lawns, no matter which neuronal inducing agent was used on the “neuron” side (see also Fig. 5B Roth et al., 2007). However, more extensive process outgrowth and more extensive fasciculation were seen if the inducing agent was MIF (Fig. 2), and the difference was significant (compare the statistics for the various neurotrophic inducing agents included in the caption to Fig. 2).
As we had demonstrated in our previous studies (Roth, Ramamurthy et al., 2007; Bank et al., 2012), MIF production by the differentiated Schwann cell like cells induced by Neuregulin appears to provide a major impetus for this process extension, whether by primary neurons (Fig. 5B, Roth et al., 2007) or stem cell-derived “neurons” (Fig. 2). We examined this directly by blocking MIF downstream effects in one of two ways: 1) The biochemical MIF inhibitor, 4-iodo-6-phenylpyrimidine (4-IPP; Specs, Delft, Netherlands), at a final concentration of 0.1 μM, was used as described previously (Shen et al., 2012) or 2) a MIF function-blocking monoclonal antibody, also used as described previously (Bank et al., 2012). These MIF blocking strategies were used ONLY on the Schwann cell side of the device (seen in Blue in the cartoon in Fig. 1) once the both cell types (“neuron-like and “Schwann cell-like”) had phenotypically differentiated. Use of either means of inhibiting MIF downstream effects reduced the extent of “neurite” outgrowth over the “Schwann cell” lawns to levels seen when either no cells or undifferentiated mESC (no Neuregulin) were plated on the “Schwann cell” side of the device. This difference was particularly significant for the MIF-induced “neurons” (Fig. 2; significance calculations below in the caption).
In summary, for “neurons” induced by DHA alone and by each neurotrophin (CNTF, NGF and MIF) the ES SC condition has a significantly higher mean value for directional neurite outgrowth than the mESC, ES SC+4iPP, and α-MIF conditions. This is not surprising since all Schwann cells are known to produce MIF (Huang et al., 2002a,b; Nishio et al., 2002; Bank et al., 2002) as well as another neurotrophic cytokine, monocyte chemoattractant protein 1 (Bianchi et al., 2005; Roth et al., 2007, 2008; Bank et al., 2012). Furthermore, vertebrate statoacoustic ganglion neurons, spiral ganglion neurons and MIF-induced stem cell derived neurons are known to express MIF receptors (Shen et al., 2012; Bank et al., 2012) and to continue to express these receptors into adulthood (Bank et al., 2012). We have shown that MIF is a directional neurite outgrowth factor for many neuronal subtypes, particularly those in the inner ear (Roth, Ramamurthy et al., 2007). We have also shown that it is a neuronal survival factor (Bank et al., 2012).
Neuron-like cells in the microfluidic devices develop neuronal characteristics as assessed by appearance of neuronal markers measured with RTqPCR
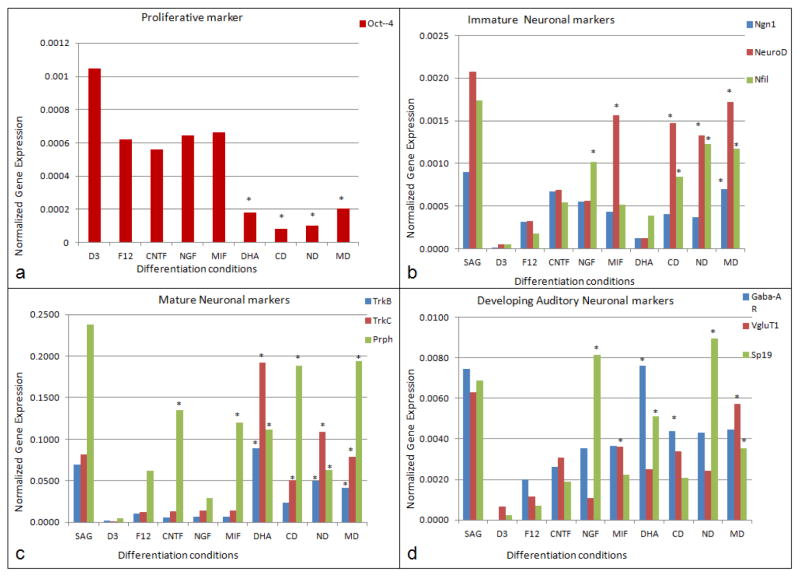
Analysis of neuronal differentiation using primer pairs (Table 1) for neuronal markers and for undifferentiated ES cells (Oct4 expression; Pan et al., 2002) was done by RTqPCR as in our previous studies (Roth et al., 2007; Roth et al., 2008; Bank et al., 2012). Oct4 remained at high expression levels under proliferative conditions (no neuronal differentiation media added) and was down-regulated under differentiation conditions (Fig. 3a). Cells treated with cytokines or neurotrophins alone showed a 2-fold down-regulation of Oct4 gene expression and cells treated with DHA as well as MIF and/or neurotrophins showed a four-fold Oct4 down-regulation when compared with the undifferentiated conditions (Fig. 3a).
Table 1.
Primer Pairs for Assessing General and Inner Ear Specific Neuronal Differentiation
| Undifferentiated Cell marker | OCT4 | ||
|---|---|---|---|
| Early stage neuronal markers | Neurogenin1(Ngn1) | GAGCCGGCTGACAATACAAT | AAAGTACCCTCCAGTCCAG |
| NeuroD | AGGCTCCAGGGTTATGAGAT | TGTTCCTCGTCCTGAGAACT | |
| Neurofilament | GGAATTCGCCAGTTTCCTGA | GCAAGGTTCTCCCATGAACA | |
| Mature neuronal markers | Neurotrophicc tyrosine kinase receptor type 2 (Trkb) | ACCATAAACCACGGCATGAG | CCGTGAAGCAGAGTTCAACA |
| Neurotrophicc tyrosine kinase receptor type 3 (Trkc) | AACCATCACGAGAAAGCCTG | ACCATCTCCACCTCTGCTTA | |
| Peripherin Prph | TCGACAGCTGAAGGAAGAGA | AGAGGCAAAGGAATGAACCG | |
| Auditory neuronal markers | Gamma-aminobutyric acid A receptor (GABA-AR) | TCATCCTCAACGCCATGAAC | TCACCTCTCTGCTGTCTTGA |
| Vesicular glutamate transporter 1 (VgluT1) | CCAAGCTCATGAACCCTGTT | ACCTTGCTGATCTCAAAGCC | |
| Voltage gated sodium ion channel (SP19 orNAV1.1) | CCATGATGGTGGAAACGGAT | AGATGAGCTTGAGCACACAC |
Figure 3.
RTqPCR quantification of gene marker expression in the differentiated neuron-like cells (a). OCT4 expression in undifferentiated mES cells and neuron-like cells differentiated from mES cells. D3: Undifferentiated condition, statoacoustic ganglion: (SAG) spiral ganglion neuron culture (control). (b). Expression of proneural marker genes in neuron-like cells cells. Ngn1, NeuroD and Nfil expression is much higher in neuronally differentiated cells, but not detected in undifferentiated condition (D3ES). (c). Expression of mature neuronal marker genes: TrkB, TrkC, Prph in the neuron-like cells are upregulated in all the neuronal differentiated conditions and expression levels are much higher under conditions with DHA addition. F12: Neuronal basal media, C: CNTF differentiation condition, N: NGF differentiation condition, M: MIF differentiation condition, D: DHA differentiation condition, CD: CNTF+DHA differentiation condition, ND: NGF+DHA differentiation condition, MD: MIF+DHA differentiation condition. (d). Expression of auditory neuronal markers, GabaAR, VgluT1 and SP19 are highly upregulated in the neuronal differentiation conditions compared to undifferentiated conditions and F12 (basal) medium. VgluT1 expression is much higher in the MIF+DHA condition than all the other differentiation conditions, which was to be expected, given that MIF is the inner ear’s first differentiation “neurotrophin” (Holmes et al., 2011; Bank et al. 2012; Shen et al., 2012).
In general, neurotrophins (Flores-Otero et al. 2007; Bank et al., 2012) are thought to provide molecular signals that mediate the survival of neurons. In mice, ganglion neuron precursors and developing cochlear neurons express the proneural gene Ngn-1. NeuroD and neurofilament (Nfil) are upregulated early in inner ear development (Flores-Otero et al. 2007; Nayagam et al., 2011; Yang et al., 2011; Shibata et al., 2011: Reyes et al., 2008). In spiral ganglion neurons, three different forms of Nfil are expressed sequentially (Nayagam et al., 2011;Yang et al., 2011). In these studies, we found Ngn-1 expression was upregulated under all neuronal differentiation conditions when compared with cells grown in F12 basal media (undifferentiated conditions) (Fig. 3b). Expression of NeuroD was upregulated in cells treated with MIF alone, with CNTF+DHA, NGF+DHA and MIF+DHA (Fig. 3b). A rise in NeuroD expression levels suggests that MIF alone without DHA could support the differentiation and survival of stem cell-derived-neurons. CNTF, MIF, CNTF+DHA, NGF+DHA, MIF+DHA significantly increased NeuroD expression when compared with mES cells grown in the F12 basal medium.
Neurofilament (Nfil) expression was upregulated significantly under all the differentiation conditions compared with F12 basal medium. Nfil expression was upregulated in cells treated with CNTF+DHA, NGF+DHA and MIF+DHA when compared with conditions without DHA (Fig. 3b). Proneural gene marker upregulation in cells treated with MIF alone and MIF+DHA suggests that the cells’ expression profile is consistent with what can be expected of expression in statoacoustic ganglion neurons (the spiral ganglion neuronal precursors) (Fig. 3b).
A number of transcription factors (GATA3, Brn3a, Ngn1, NeuroD, islet1) as well as receptors for neurotrophins (TrkB and TrkC) have been defined as mature markers for differentiating auditory and vestibular neurons (Nayagam et al., 2011; Shibata et al., 2011; Yang et al., 2011). Peripherin is also expressed concomitantly with axonal growth during development in the inner ear, and its synthesis appears necessary for axonal regeneration in the adult (Nayagam et al., 2011; Shibata et al., 2011; Yang et al., 2011). TrkB, TrkC and Prphn were significantly upregulated under conditions of DHA supplementation of either of the two neurotrophins or MIF (Nayagam et al., 2011; Shibata et al., 2011; Yang et al., 2011). Upregulation of these “mature” neuronal markers and some selected auditory system neuronal markers are seen in Figs. 3c and 3d.
Differentiated neuron-like cells were stained for VgluT1 (a marker for spiral ganglion neurons of the inner ear), Neurofilament 200 (a neuronal marker) and myelin basic protein (MBP), which labels mature, neuron-interactive myelinating Schwann cells (Roth et al., 2007, 2008) (Fig. 1, Fig. 3). Differentiation conditions under which cells were treated with NGF, CNTF or MIF, either of the neurotrophins+DHA, or MIF+DHA exhibited more extensive neurite outgrowth than was seen in the F12 Basal medium. Differentiation conditions with neurotrophins or with MIF supplemented with DHA have extensive fasciculated bundles of neurites, which were positive for the glutamate transporter, VgluT1 and neurofilament (Nfil200kDa). By contrast, few neurites stained for both VgluT1 and Nfil200 under conditions in which CNTF, NGF, MIF or DHA alone were used as the inducing agents. All the differentiated neuron-like cells in the mid region of the device showed healthy neurite outgrowth over the lawn of Schwann cell-like cells as did cells treated with CNTF+DHA, NGF+DHA and MIF+DHA (see Fig. 2 for total neurite outgrowth measurements).
A substantial increase in glutamatergic properties (e.g. increased VgluT staining of neurites), robust neurite outgrowth with multiple branches as well as evidence of myelination in the device were all found in MIF-induced neuron-like cells. Although these properties do not exclusively define spiral ganglion neurons, development of these properties indicates that the cells were developing at least some of the properties of spiral ganglion neurons. Such criteria would be critical if the “neuronal” population were to prove adequate substitutes for lost or diseased spiral ganglion neurons in vivo.
Evidence of Early Steps in Myelination is seen in the Microfluidic Devices in CNTF and MIF-derived “neurons”
If NGF was used as the inducing agent, little evidence of myelination was seen during the time course of these experiments (up to 30 days), even in the presence of DHA (NGF+DHA: Fig 4 or the presence of Schwann cell-like cells. However, neuron-like cells induced by CNTF showed evidence of myelination with and without DHA in the presence of Schwann cell-like cells. When neuron-like cells were induced with the neurotrophic cytokine, MIF, the presence of DHA greatly enhanced evidence of myelination in the presence of the Schwann cell-like cells (Fig. 4).
The process of myelination is extremely difficult to follow at the cellular level in its entirely in (approximately) real time in vivo, without sacrificing large numbers of animals. Even in vitro, using primary cell cultures of neurons and Schwann cells, the process is difficult to observe (Callizot et al, 2011). However, in the microfluidic device, we have created a microenvironment in which the cells can be differentiated concomitantly into the two lineages. We can now begin to study the interaction between the two cell types by time lapse video and by stage-specific immuno-labelling (Roth et al., 2007) as they mature and mutually influence the other population’s development, as happens in vivo (Salzer, 2012, 2015; Glenn and Talbot, 2013; Kidd et al., 2013). Schwann cell myelin production requires the influence of neurons capable of carrying on the complex sequence of required cell-cell interactions and molecular cross talk (Bodhireddy et al., 1994; Roth et al., 2007; Salzer, 2012, 2015). We had previously found evidence of early steps in myelination in interactions between primary inner ear statoacoustic ganglion neurons and a “Schwann cell” lawn produced by inducing the Schwann cell phenotype in embryonic stem cells (Roth et al., 2008).
Importantly, the slow flow feature of our device, which closely approximates interstitial flow with respect to diffusion strength, is physiological for neuronal cells. Neurons usually develop in and remain separated from high flow rate environments (blood flow or even lymphatic flow in the body), which at least for the CNS, where oligodendrocytes and not Schwann cells myelinate neurons, is maintained by the blood-brain barrier. It is important to note that even in such flow-free environments, neurons still require a replenished nutrient supply and waste clearing by minimal convective flow, which is also achieved in our slow flow device. Stability of the flow in the channel is guaranteed since the operation of the flow is based simply on osmosis, a very stable phenomenon. The overall pattern of the two differentiated cell types in the device suggests that the neuron-like cells grown with the Schwann cell-like cells exhibited directional outgrowth towards the Schwann cell-like target cells (e.g. Fig. 1a Row 1 right). This observation recapitulates our earlier finding that neurites from primary ganglionic explants similarly ramify over such a stem cell-derived Schwann cell lawn (Roth et al., 2007). There is ample evidence of the early stages of myelination (Fig. 4) as these differentiating “neurons” and “Schwann cells” interact.
Properties of neuron-like cells differentiated by MIF or MIF+DHA could lend themselves to replacement strategies for inner ear neurons
We used whole-cell patch clamp electrophysiological recordings to determine whether the neuron-like cells derived from mES cells induced with MIF had excitable properties that showed voltage gated inward and outward currents. This would be the minimum requirement for action potentials and therefore render these cells capable of encoding frequency information from a cochlear implant electrode and conveying it to the central nervous system (CNS). After 3 weeks, an excitable population of neuron-like cells was identified in the 80% of cells that had taken on a neuronal morphology. The population with measurable excitable properties was approximately 25% of the cells with a neuronal morphology. By contrast, if the mESC were exposed to the F12 basal medium without any differentiation factors, no such properties could be documented.
To explore the underlying cause of these electrophysiological changes, we investigated the voltage-dependent properties of Na+ and K+ currents using whole-cell voltage-clamp electrophysiology. We detected voltage dependent inward and outward current with long active response times in the MIF/DHA exposed “neurons” (Fig. 5c) whereas the cells in F12 media alone (basal medium) showed no neuronal morphology and no active responses or any inward or outward currents. Electrophysiological recordings were also made in the NGF+DHA condition. However, no inward or outward currents were found. We also labelled the MIF and/or DHA-induced neuron-like cells for ion channel protein expression with antibodies for sodium ion channel SP19 and potassium ion channel Kv3.1 on the same cell preparation (double labeling). SP19 was distributed all over the somas and along the neurites, as well intra-cellularly (potentially nuclear staining); Kv3.1 was also expressed in the cell body and along the processes, although the staining was less well defined (data not shown). This finding does, however, agree with our RTqPCR data (see Table 1).
Coating of SC cells on Cochlear implants using hydrogel layering
We were sufficiently encouraged by the preliminary analyses of MIF-neuron-like cells that showed these cells had features that could potentially serve in the place of spiral ganglion neurons, to proceed to “coating” CIs with MIF-producing Schwann cell-like cells. We then followed the interaction of the MIF-induced neuron-like cells with these “coated” implants in vitro. Earlier work by Shepherd’s group (Pettingill et al., 2007) had demonstrated that CIs could be coated with neurotrophin-expressing Schwann cells that would attract SGN.
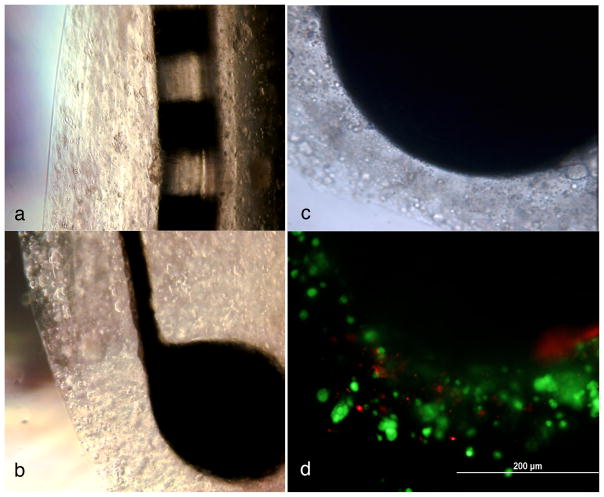
Two different types of platinum iridium electrodes (kindly provided by Dr. Bryan Pfingst, Dept. Otolaryngology Head and Neck Surgery, University of Michigan) were “coated” with Schwann cell-like cells or undifferentiated mESC using 1% sodium alginate hydrogel as a scaffold; both types of coated CIs were grown in culture for two weeks (Fig. 6). The cultures were supplemented with fresh media every week. Control electrodes were either uncoated, coated in hydrogel layers without cell inclusions or coated with undifferentiated mESC in the hydrogel layers. Fig. 6a is a photograph of a cochlear implant of the type used in human patients, coated with Schwann cell-like cells in the hydrogel layers (5 layers are readily discerned in this photograph). Figs. 6b through 6d are photographs of the type of cochlear implant electrode used in animal studies. All are photographs of the same electrode. Five layers of hydrogel containing Schwann cell-like cells are seen in Fig. 5b. Fig. 5c, is a close up phase contrast photograph of the electrode ball head. The encapsulated cells were stained for live and dead cells with ethidium bromide and calcein (LIVE/DEAD® Cell Viability Assays, Invitrogen, CA). Large numbers of healthy cells (green) and very few dead cells (orange) were found in the hydrogel layers coated with the Schwann cell-like cells (Fig. 6c) or undifferentiated ES cells (control; not shown) at 6 weeks in culture.
Figure 6.
Two types of cochlear implants coated with hydrogels with encapsulated Schwann cell-like cells: a. Platinum iridium eight array electrode of the type used in human patients. b. Platinum iridium electrode with a ball at the end of the type used in animal studies The differentiated Schwann cell-like cells were grown in layers on the electrodes in sodium alginate hydrogels for 2 weeks; Healthy Schwann cell-like cells are visible in 5–6 layers on both cochlear implants (a) and (b). (c) Higher magnification phase contrast imge of the hydrogel layers on a ball implant. (d) Fluorescence micrograph of the same cochlear impant pictured in (c). Live/Dead staining was used to distinguish living from dead cells. Ethidium homodimer was used to stain dead cells- (orange) and calcein AM was used to stain the live cells (green). The implant was coated with hydrogel-encapsulated Schwann cells, which were maintained for 2 weeks in the encapsulated form and then checked for viability. The scale bar in (d), the fluorescence image, applies to both (c) and (d).
Extension of primary mouse statoacoustic ganglion or spiral ganglion neurites towards the Schwann cell-like cell-coated cochlear implant but not a bare cochlear implant was seen by time lapse digital microscopy
Cochlear implant electrodes of the ball type seen in Fig. 5b–d were then co-cultured with a) intact primary embryonic mouse statoacoustic ganglia or intact post-natal spiral ganglia or b) neurons dissociated from these ganglia to determine whether the neurites would demonstrate directional outgrowth towards the cell coated cochlear implants. We monitored the entire interaction by time-lapse digital videography on a specially designed microscope with a gas-supplied (5% CO2) tissue culture incubator on the stage over 5–14 days. Neurite migration and directional outgrowth trajectories were also photographed every 24 hrs after addition of the cochlear implants to cultures of the primary ganglia.
We first tested whether primary statoacoustic ganglion neurons exhibited directed neurite outgrowth toward cochlear implants coated with hydrogels that contained Schwann cell-like cells. A cocultured SAG explant and the Schwann cell coated cochlear implant or a SAG explant and a bare implant were placed 1 cm apart in the tissue culture dish and a video camera was programmed to capture images at different locations over the dish at 20 min intervals over 5–14 days. We then analyzed the images to observe any evidence of neurite extension or directional outgrowth from the ganglion towards the implant. No directed outgrowth was seen towards bare cochlear implants under any conditions. By contrast, after 2 days, 87%+ 4% of the processes took the direction towards the Schwann cell-like cell-coated cochlear implant, even if during the first 2 days some outgrowth was random and non-directional. We also observed that the explant itself in some cases split in half and some of the neurons themselves (with and without underlying glial cells) migrated in the cochlear implant’s direction.
The long-term co-culture (2 wks) of Schwann cell-like cell-coated cochlear implant and statoacoustic ganglia showed directional outgrowth of neurites and extensive contact of neuronal processes on the Schwann cell-like cell-coated cochlear implant from both statoacoustic ganglion neurons explants and dissociated cells. No neurite outgrowth whatever was seen towards “bare” cochlear implants, those coated with hydrogel but not cells or implants coated with hydrogels containing undifferentiated mESC. Although some processes were seen emerging from the ganglia under the three “control” conditions (bare implant, hydrogel but not cell coated implant or undifferentiated mESC in the hydrogels), the neurite processes were short, emerged randomly from all sides of the explants and showed no directionality or appreciable extension even after 2 weeks.
In marked contrast, from day 3 onwards, neuronal processes emerging from the statoacoustic ganglia were observed to turn in the direction of the Schwann cell-like cell-coated cochlear implants and to migrate towards them. The emerging neurites and some migrating cell bodies could be positively stained for neurofilament marker protein throughout the experiment.
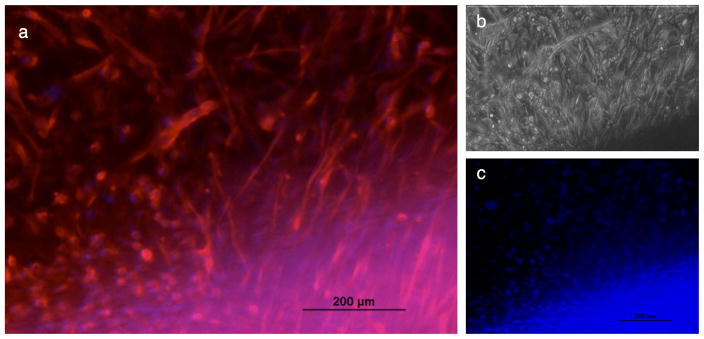
By two weeks, the statoacoustic ganglion neurites had reached the cochlear implant (Fig. 7) and had penetrated the hydrogel layers containing Schwann cell-like cells in the hydrogel layers. Fig. 7 a and b are fluorescence micrograph (Fig. 7a) and phase contrast micrograph (Fig. 7b) of the same part of the coated electrode surface. In Fig. 7a Neurofilament 150kDa (Nfil) (red) was used to label the neuronal processes and a few cell bodies. In this double-labelled photograph, the blue staining (DAPI) is associated with the Schwann cell-like cell nuclei. These are more clearly visible in Fig. 7c, where the DAPI stained nuclei are heavily concentrated close to the electrode head and seen to belong to the Schwann cell like cells (compare Fig. 7c with 7a). A few neuronal cell bodies that stained with DAPI are seen in the area above the concentrated Schwann cell layers (Fig. 7c).
Figure 7.
Neurites of embryonic mouse (Eday 12.5) statoacoustic ganglion interact with Schwann cell-like cells in the hydrogel layers on a ball type cochlear implant at 2 weeks in culture. At the beginning of the experiment the statoacoustic ganglion was placed 1 cm away from the Schwann cell-like coated cochlear implant on a porcine gel coated tissue culture dish. Interactions with the cell coats on the impants were followed by time lapse digital cinematography over the entire 2 week period. The processes and some neuronal cell bodies reached the cochlear implant after 4–5 days. These photographs were taken at 13 days. (a) Neurites (and some cell bodies) were labelled by immunocytochemistry for Neurofilament 150kDa (Nfil) protein marker and a secondary antibody (AlexaFlor 580 (red) labels the extensive processes that had reached the cochlear implant. The blue crescent of labelling at the bottom represents the DAPI labelled Schwann cell-like cell nuclei. (b) the phase contrast micrograph view of these processes/cells from the statoacoustic ganglion and the implant (same field as (a). (c) DAPI-stained nuclei of the Schwann cell-like cells are seen in blue within the hydrogel “coats” in this micrograph of the “blue” channel. No directional statoacoustic ganglion neurite outgrowth was seen when the statoacoustic ganglion explants were co-cultured with a bare cochlear implant or with a cochlear implant coated with hydrogel layers containing undifferentiated stem cells.
Directional outgrowth of neurites from SGN was seen towards a variety of targets
Spiral ganglia isolated from the 6-day old postnatal mouse cochlea were also cocultured with bare cochlear implants, or with hydrogel coated cochlear implants containing Schwann cell-like cells or undifferentiated mESC. We used the same culturing techniques, cinematographic and photographic techniques to observe neurite extension and document any directional outgrowth both under the light microscope and by time-lapse live cell imaging. At the experiments end, the cultures were stained for neurofilament and DAPI. Again, we found that directional neurite outgrowth was only seen towards the Schwann cell-like cell coated-cochlear implants. Initially, as with the primary statoacoustic ganglia, at least some outgrowth of neurites was observed 360° around the cluster of spiral ganglion neuronal cell bodies. After the 3rd day of co-culture, the majority (91+6 %) of the neurites showed directional outgrowth towards the Schwann cell-like cell-coated cochlear implant and processes reached the implant by 8–9 days in culture. Neurite outgrowth from spiral ganglion neurons in the presence of a bare cochlear implant or one coated with undifferentiated mES cells showed little extension and no directional outgrowth at all even after 2 weeks in co-culture (data not shown).
These migration and directional outgrowth studies demonstrate that a Schwann cell-like cell-encapsulated cochlear implant could potentially deliver directional molecular cues to attract neurites of an endogenous adult spiral ganglion. Importantly, we had previously demonstrated that receptors for MIF remain on spiral ganglion neurons into adulthood (Bank et al., 2012).
Neuron- like mESC-derived cells’ directional outgrowth towards targets
The MIF-induced/DHA enhanced neuron-like cells were assessed after a week of co-culture for directional migration and directional outgrowth towards: a) Organ of Corti explants derived from 6 day old postnatal mouse cochleae (wild type or derived from a MIF knock out mouse) as described in our previous studies (Bank et al., 2012); b) A bare cochlear implant; c) A cochlear implant coated with Schwann cell-like cells; d) A cochlear implant coated with undifferentiated mouse embryonic stem cells (mESC); e) A hydrogel coated cochlear implant without cells. The neuron-like cells exhibited few emerging processes and no directional neurite outgrowth toward the bare cochlear implant, the hydrogel-coated (no encapsulated cells) cochlear implant or the mESC-coated cochlear implant. In contrast neuron-like cells co-cultured with the Schwann cell-like cell-cochlear implant showed long processes, rapid migration and directional outgrowth towards the cochlear implant (71+11% of the processes were oriented towards the cochlear implant). However, these processes became so fasciculated that measurements of total “oriented” processes became difficult over time.
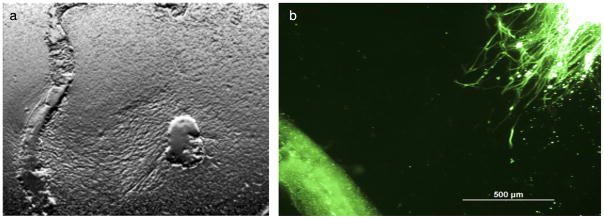
MIF-induced neuron-like cells co-cultured with the 6-day old postnatal wild type mouse Organ of Corti explants showed clusters of neurites with growth cones and multiple branches growing towards the wild type Organ of Corti (Fig. 8b). Compare this outgrowth with the robust neurite outgrowth emerging from a wild type primary spiral ganglion neurons towards the wild-type Organ of Corti explant seen in Fig. 8a. Little outgrowth, random emergence of processes and no directional outgrowth of neurites was seen if the Organ of Corti explant came from a MIF knock out mouse. These data recapitulate our finding with primary neurons and Organ of Corti explants. Neither statoacoustic ganglion neurites nor spiral ganglion neurites emerged towards a MIF knock out Organ of Corti explant (Bank et al., 2012). These studies indicate that the MIF/DHA-induced neuron-like cells derived after 3 weeks exposure to the neurotrophic cytokine MIF and DHA can migrate towards appropriate targets in vitro. Functionality is being tested electrophysiologically. However, directional outgrowth and electrophysiological function remain to be thoroughly tested when such electrodes are implanted in vivo in animal models.
Figure 8.
Interaction of primary spiral ganglion neurites (mouse post natal day 3 (a) and processes from MIF-induced neuron-like cell neurites (b) with wild type Organ of Corti explants in culture. (a) Polarized image of a primary mouse spiral ganglion (right) extending extensive neurites directionally toward a wild type Organ of Corti explant (at left). No directional outgrwoth was seen towards a MIF knock out mouse Organ of Corti (not shown). This was also demonstrated in separate experiments detailed in Bank et al., 2012, (Fig. 6 in that paper). (b) MIF-induced neuron-like cells stained with acetylated tubulin (green) cultured with a wild type Organ of Corti explant for a week showed fasciculated neurites (approaching form the right) growing towards the wild-type Organ of Corti (at lower left) No such outgrowth was seen towards OC explants from MIF-KO mice (as was also demonstrated for primary spiral ganglion neuronal processes in Bank et al., 2012 Fig. 6).
DISCUSSION
Concomitant differentiation of neuron-like cells and Schwann cell-like cells in a microfluidic device
Two novel stem cell-based therapeutic strategies for producing cell populations that could be used to alleviate sensorineural hearing loss have been assessed in these studies. We have differentiated a common population of mouse embryonic stem cells (mESC) (Doetschman et al., 1985; Roth et al., 2007, 2008, Bank et al., 2012) into neuron-like and Schwann cell-like cells simultaneously in a slow flow microfluidic device.
These studies were based on earlier work in which we used conventional tissue culture for such differentiation studies. Our lab had produced the first embryonic stem cell based model of both the myelinating and non-myelinating Schwann cell in 2007 (Roth et al., 2007, 2008). We also induced mouse embryonic stem cells to take on neuronal phenotypes with different characteristics depending on which neurotrophin was used as the inducing agent (NGF, CNTF: Roth et al., 2007, 2008; MIF: Bank et al., 2012). In these studies, we also used Neuregulin (as in Roth et al., 2007, 2008) to induce the mESC to become Schwann cell-like on the “Schwann cell” side of the microfluidic device.
Although both NGF and CNTF served as the “control” neuron-inducing agents in the microfluidic studies, we used MIF as the inducing neurotrophic cytokine in most of the studies detailed here both in the microfluidic model and in additional tissue culture based experiments. This was because we had previously demonstrated that MIF is the first neurotrophin expressed in the developing inner ear (Holmes et al, 2011; Shen et al., 2012; Bank et al., 2012), that it is responsible for the earliest steps in both neurogenesis and directional neurite outgrowth and that it is a neuronal survival factor (Bank et al., 2012). We had also previously demonstrated that inner ear spiral ganglion neurons retain the appropriate receptors for MIF into adulthood (Bank et al., 2012). The neuron-like cells induced by MIF in either conventional tissue culture dishes (Bank et al. 2012) or, as we show here in microfluidic devices, can bear significant resemblance to inner ear spiral ganglion neurons (Bank et al., 2012). The use of DHA enhances the maturation of such “neurons” in conventional tissue culture and in the microfluidic devices.
In the microfluidic devices, concomitant neuronal cell-type specific differentiation was seen and documented in morphological, molecular and electrophysiological assays in the devices themselves. These devices spare both cells and reagents and are labour-saving because the reservoirs supply a continuously delivered source of medium and factors (see the cartoons in Figs. 1c, d). Neuron-like cells extended processes over the Schwann cell lawns (Fig. 1a) and interactions between the ‘neurons’ and ‘Schwann cells’ resulted in the early steps of myelination (Fig. 4). We had previously demonstrated such directional outgrowth and early steps in myelination in conventional tissue culture experiments (Roth et al., 2008). The proximity of the two differentiating cell populations in the device is as critical for development of mature properties in both populations as it is in vivo. Without intimate contact with neurons, Schwann cells do not develop myelin components; nor, of course do they myelinate any cells in their environment except neurons. The role of MIF in myelination is under study in a number of systems. Disrupting pathways known to be downstream of MIF, such as Jab1, are known to cause dysmyenination (Porrello et al., 2011). Such pathways can be easily studied in these microfluidic devices and in model organisms such as the zebrafish (Weber et al., in preparation) as in more comprehensive studies of the events in myelination (Han et al., 2013; Glenn and Talbot, 2013).
The extremely slow flow maintained in the microfluidic device over 3 weeks allows for the accumulation of autocrine and paracrine factors that would ordinarily be washed through the device if the flow rate were accelerated (Park et al., 2009). This slow flow also allows for interactions between the two differentiating cell populations that emerge from the common population of ES cells—neuron-like and Schwann cell like cells. The two cell populations differentiate “side by side” and interactions between them can be documented.
We addressed the role of MIF in the observed directional outgrowth of neuron-like cells to the “Schwann cell” side of the device by inhibiting MIF’s down stream effects in one of two ways: use of the biochemical MIF inhibitor 4-iPP as we had done previously (Shen et al., 2012) or by using a function-blocking antibody to MIF, again as we had done in previous studies (Bank et al., 2012). We found that blocking MIF by either of these means on the Schwann cell side of the device once the embryonic stem cells had differentiated into Schwann cell-like cells, we could significantly reduce directional neurite outgrowth over the Schwann cell lawn, no matter which neurotrophin was used to induce the neuron-like phenotype (Fig. 2). However, the greatest effect was seen if MIF was the inducing agent or if MIF were enhanced with DHA. Note that blocking MIF did not totally eliminate directed neurite outgrowth over the Schwann cell lawn. This is because Schwann cells are known to produce another neurotrophic cytokine, monocyte chemoattractant protein 1 (MCP1) which also is produced by sensory hair cells of the inner ear (Bank et al., 2012) and which we have shown to affect both directional neurite outgrowth from inner ear neurons and their survival (Bianchi et al., 2005; Bank et al., 2012). The effect of blocking MCP1 was not tested in these experiments.
Meeting a long-term objective: Producing a stem-cell-derived neuronal population that phenotypically has some resemblance to that of the inner ear’s spiral ganglion neuron
One objective of these studies is to produce a stem cell-derived population of neuron-like cells that could potentially replace lost or damaged spiral ganglion neurons in human patients, potentially using a patient’s own induced pluripotent stem cells (Oshima et al., 2010) as a source of such neurons that could alleviate sensorineural hearing loss.
Researchers have previously attempted to develop glutamatergic neurons from ES cells by transient expression of neurogenin 1 and supplementation with BDNF and GDNF. These neurons were found to have some characteristics of spiral ganglion neurons, including electrophysiological functionality (Reyes et al., 2008). The ‘neurons’ were also implanted in an animal model (Reyes et al., 2008), although no functional improvement of a malfunctioning auditory system was demonstrated.
More promising later studies from the Rivolta group demonstrated that some significant improvement in hearing function could be achieved in a deafened gerbil model (Chen, Jongkamonwiwat et al., 2012) when human embryonic inner ear stem cells were implanted into the deafened animals. However, the derivation of neurons from human inner ear stem cells required a complex multi-step many week in vitro protocol for neuronal differentiation before implanting the cells. By contrast to these complex differentiation protocols, the ES to neuron conversion described here and in our previous studies requires only a single step with recombinant MIF (Bank et al. 2012). It is worth emphasizing that MIF is the inner ear’s earliest expressed neurotrophin and that this neurotrophic cytokine controls gangliogenesis in the vertebrate inner ear (Shen et al., 2012; Bank et al., 2012). Its use in eliciting a neuronal population is preferable to the use of other nerve growth factors (NGF, CNTF) or cytokines, largely because we have also demonstrated that ADULT SGN retain receptors for MIF (Bank et al., 2012).
Therapeutic Potential: A neuronal replacement strategy
Neuron-like cells derived from MIF and DHA-induced ES cells could potentially be used in the future to replace lost or damaged sensory neurons of the spiral ganglion in adults. However, if this paradigm is to be successful, several important hurdles will have to be met, some of which would be incurred in optimizing the neuronal cell implant procedure itself and some of which involve meeting critical criteria for monitoring functional connections of the “neuronal” population to the Organ of Corti if the implant were to be successful.
First, the cells must have cellular, molecular and electrophysiological properties sufficiently similar to the cells they replace to be functionally “competent” in situ. Second any implanted substitute “neuronal” population would have to remain viable and capable of extending “neurites” in vivo, towards a source of molecular cues that could provide directional signals and sustain their survival.
MIF is exactly such a neuronal directional outgrowth and survival factor (Holmes et al., 2011; Shen et al., 2012; Bank et al., 2012). MIF is produced in the native Organ of Corti by the supporting cells that underlie each sensory hair cell and by the Schwann cells of the inner ear (Bank et al., 2012). The neurite outgrowth documented in these microfluidic studies demonstrates that the neuron-like cells also respond to chemoattractant-producing (MIF-producing) Schwann cells in the microfluidic devices. Either a function-blocking antibody to MIF (Bank et al., 2012) or the biochemical MIF inhibitor, 4-iodo-6-phenylpyrimidine (4-IPP, Shen et al., 2012) can prevent directed outgrowth of these neuron-like cells towards the Schwann cell target cells, demonstrating that MIF production by the Schwann cell like cells is a key feature of the neuron-like cells’ directed outgrowth. A MIF-producing Schwann cell like cell-coated cochlear implant could also provide such cues and is discussed later in this section.
The number of surviving “neuron-like” cells has to be sufficient to provide functionally viable “neurons” over however many days are required for their neuronal processes to reach the target tissue (surviving hair cells in the Organ of Corti presumably via the MIF-producing supporting cells that cup each sensory hair cell) or the cochlear implant.
If the target tissue is the native Organ of Corti, the implant site would have to be optimized and two additional critical criteria would have to be met: the implanted cells cannot form a tumor or dedifferentiate and proliferate, even if the cell bolus that eventually forms is benign.
In the future, in order to prevent tumor formation in the inner ear, once the spiral ganglion neuronal processes have contacted the cochlear implant, a cell suicide cassette could be triggered to eliminate the Schwann cell-like cells (e.g. Li, Zhang et al., 2012), the cell debris of which would presumably be removed by macrophages. Slow release gels (e.g. Inaoka et al., 2009) that release recombinant MIF could also be used to coat the cochlear implants, but even with slow release, the supply of MIF in these gels would eventually be exhausted. It is not known how long it would be necessary to supply MIF to ensure that a maximum number of spiral ganglion neuronal processes reach the implant and form functional contacts. Using a stem cell-based source of MIF could provide the cytokine for a much longer time. The possibility of infection must also be minimized.
How far have we come towards realizing these criteria? MIF and DHA are capable of inducing some properties in the mESC-derived neurons that are similar to those of spiral ganglion neurons under the conditions of the experiment. These include: 1) VgluT1 (a marker for spiral ganglion neurons of the inner ear) transporter expression and protein, which was documented in MIF and DHA induced neuron-like cells.
However, the transporter is also expressed to the same extent in nerve growth factor- (NGF-) or ciliary neurotrophic factor- (CNTF-) induced mESC-derived neurons, each of which could also provide a viable source of ‘neurons’. 2) Electrophysiological excitability and the possibility of action potentials: Ion channel expression was assessed by RTqPCR and at the protein level by immunocytochemistry. The neuron-like cells’ physiological properties were evaluated by whole cell patch clamping. We were encouraged by the preliminary electrophysiological experiments showing that MIF+DHA could induce a neuronal population with promising electrophysiological properties. No inward or outward currents were found when electrophysiological recordings were made from the neuron-like cells induced by either NGF or NGF+DHA.
The cells also expressed ion channels (sodium and potassium channels) that are expected for spiral ganglion neurons (Greenwood et al., 2007; Xie et al., 2007), but sodium channel protein expression was better defined in the immunohistochemistry labeling experiments than potassium ion channel expression on the neurites.
These experiments demonstrate that these neuron-like cells express some functionally mature properties in common with spiral ganglion neurons under in vitro conditions. We have not yet documented action potentials under any of these conditions; such recordings will be attempted in the future.
Functionally mature neuron-like mESC-derived cells were experimentally tested for directional neurite outgrowth when cocultured with a cochlear implant or primary explanted Organ of Corti targets under in vitro conditions. They exhibited directional outgrowth towards both the Schwann cell-like coated cochlear implant and wild type but not the MIF knock out mouse Organ of Corti explants in culture. Therefore, neuron-like cells derived from mESC using MIF and DHA could potentially be implanted as a stem cell-based therapy in patients with low spiral ganglion counts who are not presently candidates for cochlear implant therapy.
Potential of ESC-derived Schwann cell-like cells for alleviating sensorineural hearing loss
The second embryonic stem cell based therapeutic approach described in these studies is the production of mESC-derived Schwann cell-like cells. These cells are potentially capable of continuous production of molecular cues, including MIF and MCP1, that could improve the number of connections between any remaining spiral ganglion neurons and a Schwann cell-like cell-coated cochlear implant.
In these studies, Schwann cell-like cell-coated cochlear implants in vitro were studied for their ability to “attract” neurites from both primary embryonic statoacoustic ganglion and the mature spiral ganglion. Eventually, such a strategy might be employed to enhance the functionality of a cochlear implant. If successful, this could lead to improved hearing or better speech perception in patients experiencing hearing loss.
Schwann cells are known to be important for peripheral nerve regeneration (Bhatheja and Field, 2006; Gambarotta et al., 2013). These properties depend on their state of differentiation (e.g. quiescent, proliferating or mature Schwann cells) (Baehr and Bunge, 1989; Kidd et al, 2013; Salzar, 2012, 2015; Grigoryan and Birchmeier, 2015).
In earlier studies by other investigators, the effects of factors secreted by Schwann cells were examined on a quasi-neuronal model composed of PC12 cells, which are, in some investigators’ estimations a model for neuronal cells, which were tested for cell survival and “neurite” outgrowth (Bampton and Taylor, 2005). In these experiments, Schwann cell-conditioned medium was tested on “neurite” outgrowth from PC12 cells against a range of isolated factors known to be secreted by Schwann cells. This conditioned medium showed clear neuritogenic effects and suggested that Schwann cells are likely candidates for promoting neuronal regeneration (Bampton and Taylor, 2005; see also Wissel et al., 2008).
Other groups have reported enhanced survival of spiral ganglion neurons in animal models of hearing loss by implanting Schwann cells, which were molecularly engineered to provide sustained delivery of neurotrophins such as BDNF (Bunge, 1993; Girard et al., 2005; Golden et al., 2007; Pettingill et al., 2008). Such an engineered population of Schwann cells was used to coat a cochlear implant, with some success in attracting spiral ganglion neurons to the implant (Pettingill et al., 2008).
However, the group attributed the attraction of the spiral ganglion neurons to the Schwann cells on the implant to the engineered neurotrophin expression and release by the Schwann cells. While this could indeed be the reason, it is also possible that since all Schwann cell express MIF, MIF was either the main source of or contributed to the attraction.
If this cell coated cochlear implant strategy were to be successful, initially after cochlear implant was introduced, electrical impedance should be higher due to the presence of the hydrogel encapsulated cells, but the biocompatible and biodegradable hydrogel might release the cells in the perilymph or area surrounding the cochlear implant, which could potentially provide sustained neurotrophin release, which would also serve to protect the spiral ganglion neurons. Once the hydrogel has completely degraded after releasing all the cells, the cochlear implant’s own electrical stimulation would presumably function normally, but this has not been examined either in vitro or in a living animal.
Combining stem cell based strategies
Various combinations of these approaches could be used. Neuron-like (especially potentially spiral ganglion-like) embryonic stem cell-derived cells could be implanted into an inner ear in which hair cell degeneration has begun but not yet progressed to complete loss. Potential stem cell sources could include a human patient’s own induced pluripotent stem cells (iPSC) (Oshima et al., 2010). Alternatively, stem cell-derived Schwann cell-like cells that are capable of sustained delivery of critical supporting factors like MIF could be used to coat a cochlear implant. Recall that all Schwann cells, including these engineered Schwann cell-like cells produce MIF (Huang et al., 2002, Bank et al., 2012). The research studies outlined here are limited to in vitro conditions; in the future this approach can be applied in animal models in in vivo studies.
Conclusions
These studies provide first steps towards producing MIF-induced stem cell derived neurons that could resemble spiral ganglion neurons sufficiently to replace lost or damaged spiral ganglion neurons. Furthermore, Neuregulin-induced Schwann cell-like cells that produce MIF could be used to “coat” a cochlear implant that provides a source of MIF to which both immature and mature auditory system neurons respond. We had earlier demonstrated that both neuronal subtypes retain MIF receptors (Bank et al., 2012). Thus a common population of “stem cells”, which in this report are mouse embryonic stem cells, but which in the future could be host derived iPS cells, can be induced to form two populations of cells that could provide viable approaches for auditory system repair.
EXPERIMENTAL PROCEDURES
Culturing mES cells
Cell lines: The cell line used for these studies was the D3 mES cell line (ATCC-CRL-1934; Doetschman et al., 1985). 10,000 mESC D3 cells were plated onto 0.1% porcine gel-coated (Sigma) 48 well Primaria® plates (Falcon) and cells were differentiated over a three-week period, using our previously-reported methods (Roth et al., 2007; 2008). Undifferentiated mES cells (controls) were fed basal medium daily, while cells in neuronal differentiation media and Schwann cell differentiation medium were fed every third day.
Media Preparation: Proliferating/undifferentiated (ES medium): 81% DMEM (Invitrogen, Carlsbad, CA, USA) without phenol red, but including 1% L-Glut, 1% Pen/Strep, 1% nonessential amino acids (Invitrogen), 15% FBS (Atlanta Biological, Lawrenceville, GA, USA), 1% sodium pyruvate (2% stock, Sigma Aldrich, St. Louis, MO, USA), 7 μL/L mercaptoethanol (Sigma Aldrich), 1,000 U/mL ESGRO (Chemicon, Temecula, CA, USA).
Differentiation of mES cell into Schwann cell-like cells in vitro
Our previously published methods were used to produce Schwann cell differentiation (Roth et al., 2007; 2008). The medium consisted of 500 mL alpha modified MEM (GIBCO 41061–029), 6% Day 11 chick embryo extract (prepared in house), 10% Heat Inactivated FBS, 58 μL Gentamycin (Invitrogen), 10 μL Neuregulin1 (NRG1) (R&D Systems).
Differentiation of mES cell into various neuron-like cell types in vitro
Neuronal differentiation: 95% F-12 (Invitrogen), 1% Pen/Strep (Invitrogen), 1% N2 (Invitrogen), 2% B27 (Invitrogen), 2% sodium pyruvate (2% stock), 0.5 μg/L bFGF (R&D Systems, Minneapolis, MN, USA), 1 μg/L IL-7, 5 μg/L IGF-1 (Sigma), or for specific neuronal cell types: 1 μg/L ciliary neurotrophic factor (CNTF; R&D Systems) for motor neuron-like neurons (Roth et al., 2007); 1 μg/L nerve growth factor (NGF; Millipore, Billerica MA, CA) (sensory neuron-like cells; Roth et al., 2007); macrophage migration inhibitory factor (MIF) 1 μg/mL (R&D Systems), + 5 μM docosahexaenoic acid (DHA; Nu-check Prep Inc., Elysian, MN, USA). DHA supplemented media included: CNTF and 5 μM DHA, NGF and 5 μM DHA, MIF and 5 μM DHA (Bank et al., 2012). To block MIF release from Schwann cell-like cells, either the biochemical MIF inhibitor, 4-iodo-6-phenylpyrimidine (4-IPP; Specs, Delft, Netherlands) at 0.1μM (Shen et al., 2012) or the MIF function-blocking monoclonal antibody under our previously described conditions (Bank et al., 2012) were added to the Schwann cell reservoir. These MIF blocking strategies were used ONLY on the Schwann cell side of the device once the cells had differentiated.
Assessing directed differentiation of D3 cells into neuron-like cells
After three weeks of growth, cells were labeled for expression of neurofilament marker (NF150) (neuronal differentiation marker). Antibodies (Chemicon, MA) were diluted in 5% normal goat serum (Chemicon) in 0.1% tween20/phosphate buffered saline (PBS) (Sigma). Cells were fixed in 4% paraformaldehyde (Sigma), and treated with primary antibody NF150 (R&D systems) at 1:500 dilution followed by secondary antibody Alexa Fluor 590 at 1:200 dilution (InVitrogen, CA) and DAPI (1:1000 dilution) stain for the cell nucleus. Photomicrographs were taken with a light microscope or using appropriate filters on a Leica inverted fluorescence microscope. We repeated each set of experimental conditions at least three times in separate experiments. The differentiated cells were labeled by our previously reported ICC techniques (Roth et al., 2007) for ion channel protein expression with antibodies (Abcam, CA) to sodium channel (SP19) and potassium channel (Kv3.1).
Isolation and culture of primary mouse Statoacoustic Ganglia Neuron (statoacoustic ganglion)
Statoacoustic ganglia from Balb/C mouse embryos (E15.5–17.5) were isolated from the 2 ears as previously described (Germiller et al., 2004; Bank et al., 2012) and rinsed in PBS (Bianchi and Raz, 2004; Bank et al. 2012). The whole ganglia were then plated on 0.1% porcine gel-treated Primaria@ plates or 0.1% porcine gel coated glass cover slips (Roth et al., 2008) with the addition of 0.5ml F12 neuronal basal media (InVitrogen) and incubated overnight for attachment at 37°C in a cell culture incubator with 5% CO2. Under some conditions, the statoacoustic ganglia were dissociated and plated as individual neurons as previously described (Bianchi et al., 2005; Bank et al., 2012).
Electrophysiological recordings of mESC-derived neuron-like cells; patch clamp whole cell recording
Whole-cell current- and voltage-clamp recordings were performed on cells selected for a bipolar neuron-like morphology with long processes. These recordings were made at room temperature using an Axopatch 200B amplifier with a Digidata 1200 or 1322A digitizer (Molecular Devices, Sunnyvale, CA). Data were acquired using pCLAMP 9 software (Molecular Devices). Glass electrodes were pulled from borosilicate capillaries (World Precision Instruments, Sarasota, FL) to achieve an electrical resistance of 3–6 M. Electrodes were filled with an internal solution containing (in mM): 112 KCl, 2 MgCl2, 0.1 CaCl2, 11 EGTA, 10 HEPES, 5 Na2+ ATP, and buffered to pH 7.2 with KOH. The external solution contained (in mM): 137 NaCl, 5 KCl, 1.7 CaCl2, 1 MgCl2, 10 HEPES, 16 glucose and buffered to pH 7.2 with NaOH.
Isolation of Organ of Corti and explant culture
A modification of the method of Parker et al., (2010) was used to isolate mouse OC as previously described (Bank et al., 2012). Day 6 neonatal Balb/C mice were first decapitated and the outer skin was removed using a scalpel blade. The skull and the brain were bisected to expose the temporal bone and the tissue and the bones surrounding the temporal bone were removed. The temporal bone was rinsed in sterile PBS and transferred to a new plastic 50 mm dish and the cochlea was removed under a surgical microscope. The bony labyrinth was carefully separated and the apex of the organ of Corti was exposed to visualize the basal cochlea after the removal of the labyrinth. Using fine forceps the base was held and the OC carefully removed and unwound. The isolated OC explants were positioned on 1% porcine gel coated Primaria (Falcon) plates with 0.5mL F12 basal media and incubated at 5%CO2 in a tissue culture incubator overnight.
Isolation of primary Spiral Ganglia and Spiral Ganglion Neurons
Once the OC explant was isolated, the modiolus of the cochlea was cut in half and the tissue was minced using a scalpel blade. SGN tissue explants were placed on 0.1% porcine gel coated Primaria tissue culture plates with 0.5mL F12 media and incubated overnight in order to promote attachment as previously described (Bank et al., 2012).
Simultaneous differentiation of neuron-like cells and Schwann cell-like cells in a microfluidic device
Device Preparation and Maintenance: The microfluidic device itself and all tubes, pipette tips (used as reservoirs) and equipment were sterilized by the same autoclaving and UV sterilizing procedures used in previous studies (Park et al., 2009). PDMS microfluidic chambers were autoclaved and subsequently were oxygen-plasma treated, so that the channel was hydrophilic. Assembly and operation of the microfluidic system was done in a tissue culture hood under positive pressure to prevent possible contamination. To prevent air bubble formation initially, a PBS solution-filled 1000 μL pipette-tip was inserted into the outlet port, and by the force of gravity; PBS was allowed to flow slowly into the main channel without producing any air bubbles in the channel. Air bubble formation at all stages of loading, cell seeding and growth was carefully examined microscopically. None of the many devices used in these experiments developed air bubbles/locks either initially or during the course of the experiment or the ensuing analysis.
The microfluidic devices were designed according to previous specifications (Fig. 1) (Park et al., 2009). Microfluidic chambers and tubing were made hydrophilic and sterilized by oxygen plasma and UV treatment, respectively. Devices were pre-filled with PBS by inserting a 1000 μL pipette tip into the outlet and allowing PBS to fill the chamber by gravity-driven flow. Air bubbles were avoided by maintaining liquid (PBS, culture medium) on top of the device, thereby preventing bubble formation due to evaporation or due to connection of pipette tips. Devices were coated with 0.1% porcine gel for 2 h under UV light before cell seeding. mESchwann cellells were trypsinized and seeded into devices at a density of 10,000 cells/mL and allowed to adhere overnight at 37°C and 5% CO2. When the mESC were fully attached, an osmotic pump was connected to tubing at the outlet channel and 1 mL pipette tip reservoirs containing either neuronal differentiation or Schwann cell differentiation media were inserted into the appropriate inlets (Park et al., 2009). Devices, including osmotic pumps and inlet and PEG reservoirs, were incubated at 37°C and 5% CO2 over three weeks with the PEG and inlet reservoirs being replaced once every 7 days.
Assessment of cell type differentiation and myelination in a microfluidic device
At various time points during the 3-week period, cells were fixed with 4% PFA and labeled with either (a) antibody to neurofilament heavy chain protein (200kDa), a general marker for neurons, (b) VgluT1, a putative marker for mature “auditory system-like” neurons, and (c) myelin basic protein, a marker for Schwann cell myelination (Bodhireddy et al., 1994; Roth et al., 2007, 2008; Salzer, 2012, 2015). Fluorescent images were taken of 5 sections of the microfluidic device to observe the overall pattern of neurite outgrowth from the neuron-like cells derived from the mESC and neurite extension over the mESC-derived Schwann cell-like cell lawns in the other side of the device as well as any evidence of myelination.
COMSOL simulation
A COMSOL simulation was performed to correlate simulated concentration profiles of soluble factors to the observed growth patterns in the device. Microfluidic device geometry was imported into COMSOL Multiphysics 4.2 (COMSOL, Inc.). Fluid density and viscosity were set based on previous measurements and laminar flow average velocities were set at the two inlets (1.4 μm/s) and outlet (2.8 μm/s) based on volumetric flow measurements as described in “Medium viscosity measurement” (Supplemental Materials). To ensure a fully developed profile, entrance and exit lengths were set to 1000 times greater than 0.06ReD, where Re and D are Reynolds number of flow and tubing diameter, respectively. A laminar flow solver was used to generate flow profiles, which, due to the extremely slow flow allowed diffusive mixing of growth factors and cytokines at the interface of the two separate laminar streams. Concentration profiles of relevant soluble factors were analyzed by the dilute species transport model. Diffusion coefficients were selected to match previous results for molecules with similar molecular weights: 1.5×10−10, 4.25×10−10, and 1.27×10−10 m2/s for MIF (Durand et al., 2009), DHA (Culbertson et al., 2002) and NGF( Lawrence et al., 2009) (or CNTF, since molecular weights are similar), respectively. A quad mesh system with minimum element size of 3.1×10−7 and maximum element size of 0.154 mm was generated to fully capture flow and concentration profiles within the channel for steady laminar flow.
Neurite measurement
Either the MetaMorph© “Neurite Outgrowth Measurement” program or NIH ImageJ software (downloaded from the internet: https://imagej.nih.gov/ij/) was used to measure total neurite outgrowth and length of “neurites” under a variety of conditions (Figs. 1 and 2). Briefly, thresholds were adjusted so that intensity levels for fluorescent detection were 50% above the background grayscale (MetaMorph® instruction manual (Universal imaging corporation, documented: T20029). Settings were then optimized for cell body size before calculating the above parameters.
Even though neurites extended only micrometers in the microfluidic device, photographs were taken at magnification of 20× in the Leica inverted microscope. Using MetaMorph® or ImageJ software we were able to analyze the photomicrographs at the maximum pixel size, representing the neurite measurement in microns in length. The mean outgrowth could not be normalized to cell numbers because neurite outgrowth extends from clusters of the mES cells that are individually indistinguishable.
Statistical analysis
For quantification of the MetaMorph and ImageJ data, all the experiments were run at the minimum in triplicate (e.g. n=8 for all conditions analyzed in Fig. 2). Differences between treatment groups were analyzed using Mann-Whitney U tests performed in Matlab. Single tailed tests were performed using the ranksum function and significance levels were computed exactly using the ‘method’ ‘exact’ option.
Coating a cochlear implant with hydrogel-encapsulated Schwann cell-Like cells
The Schwann cell-like cells were differentiated from mESC following our previously published procedures (Roth et al., 2007) and trypsinized using 0.5% trypsin EDTA. The cells were counted and diluted to 10,000 cells/mL and centrifuged at 1000RPM for 5 min. The cell pellet was resuspended in 1 mL of Schwann cell differentiation media and 1 mL 2% Sodium alginate (hydrogel) tissue culture grade (Pronovo SLG-100, Novomatrix) to bring the hydrogel concentration to 1%. To optimize cell growth in alginate gels we had first tested different concentrations of sodium alginate from 0.5 – 2.0%, which was used for encapsulating the SCs at 10,000 cells/mL. We also tested gel polymerization achieved with different concentrations of calcium chloride (50mM – 300mM). Optimized conditions for “Schwann cell” viability and gel firmness were determined to be 1% sodium alginate and 200mM calcium chloride for gel polymerization.
The cells were thoroughly mixed with alginate and placed in an ice bath. The cochlear implant was coated with hydrogel in order to support Schwann cell growth by a modification of the method of Winter et al. (2007) and each implant was monitored daily under a light microscope to determine if Schwann cell growth was limited to the vicinity of the electrode. The electrode was initially dipped in an ice-cold 200mM CaCl2 solution, followed by dipping in the hydrogel – cell mixture solution. This step was repeated to cover hydrogel-cell mixture on the cochlear implant up to 2–3mms up to 5–6 layers of coating. Then the whole hydrogel – cell and cochlear implant setup was placed in an ice-cold 200mM CaCl2 solution for 10 min. Once polymerization was complete, the electrode was placed on a tissue culture plate (35mm Falcon) and the encapsulated SC allowed to grow for two days with Schwann cell medium supplementation (Roth et al., 2007). After we observed that the Schwann cell encapsulation was successful, the Schwann cell population was tested for survivability. The encapsulated cells were checked for viability using the Live/Dead stain (Invitrogen, CA) following the manufacturer’s protocol. The cells were stained for Ethidium homodimer (orange indicates dead cells) and Calcein (green for the live cells). The Schwann cell coated cochlear implant was then used for co culture studies with primary statoacoustic ganglion or spiral ganglion neurons or with mESC-derived “neurons” induced by the various inducing agents/cytokine.
Cocultures of neuron-like cells- with OC explants, with bare cochlear implant or “Schwann cell”-coated cochlear implants
Neuron-like cells were differentiated from mES cells with MIF on porcine-gel-coated glass coverslips for 2–3 weeks; mouse Organ of Corti explants (prepared as described) were placed in the same 35mm Falcon Primaria TC plate at a distance of 1 cm to study directional neurite outgrowth towards the target (OC). The cells were cultured and fed with F12 basal media for 5 days and neurite outgrowth was observed both by time-lapse digital micrography and, at end stage, by ICC staining for TuJ1 for neuron-specific class III beta-tubulin. Interactions between the neuron-like cells and the “Schwann cell”-coated cochlear implant were monitored in “real time” by placing the mESC-derived Schwann cell-like cell coated cochlear implant or bare cochlear implant with the differentiated neuron-like cells for 5 days. Directional outgrowth was observed and photomicrographs were taken in time-lapse mode at various locations in the culture dish every 20 minutes. ICC at “end stage” was performed by fixing the preparation in 4%PFA followed by neurofilament marker as primary mouse antibody followed by Tuj1 primary antibody followed by Alexa Fluor 488-conjugated anti mouse secondary antibody (Invitrogen, CA). Photomicrographs were taken using a Leica inverted fluorescence microscope.
Coculture of MIF-induced neuron-like cells or spiral ganglia with bare cochlear implant, (ES)-cochlear implant and (Schwann cell-coated) cochlear implant
Spiral ganglion (mouse P3) explants were cultured alone for 2 days to promote strong attachment to the 0.1% porcine gel-coated plate and then a bare cochlear implant or the “Schwann cell”-encapsulated cochlear implant was placed 1 cm away from the SG explant. Neurite outgrowth and neurite contact with (Schwann cell) cochlear implant or bare cochlear implant were monitored for 5 days and photomicrographs were taken at 24 hr intervals using a light microscope. Schwann cell (cochlear implant) and spiral ganglion neuron interactions and neurite outgrowth was also monitored in live time lapse imaging for 2–4 days post positioning of the ganglion explant using a live cell imaging station -Delta vision microscope in the Microscopy and Image Analysis Laboratory of the Cell and Developmental Biology Department. The camera was assigned (by computer) to visit different locations repeatedly around the plate. The camera was programmed to revolve around the explant and capture time-lapse images at 20 min intervals. The actual setup of the experiment is shown in Fig. 6.
RTqPCR Assessment of putative SGN marker expression
The expression of a variety of SGN-”specific” markers was performed by RTqPCR using our previously published methods (Germiller et al., 2004; Bank et al., 2012 and modification of methods in Chen et al., 2011), using the primer pairs specified in those publications.
Total RNA was extracted from: a) whole statoacoustic ganglion; b) whole spiral ganglia; c) mESC-derived neuronal cultures (+ DHA as described above) at roughly 3 day intervals over a 30 day period; or d) from co-cultures of mESC-derived “neurons” grown on mESC-derived SC lawns (as in Roth et al., 2008). Total RNA was extracted using an RNeasy mini kit (Qiagen), reverse-transcribed with Superscript II RNase H-minus reverse transcriptase (Invitrogen), and amplified using SYBR Green- based PCR (Applied Biosystems). Controls included DNase I treatment during RNA isolation) and reverse transcriptase-minus and non-template controls during PCR). The sequences of the PCR products was confirmed in the University of Michigan’s DNA Sequencing Core.
Bullet Points.
We have used a simple “ultra-slow flow” microfluidic device to differentiate a single population of mouse embryonic stem cells into neuron-like cells and Schwann cell-like cells, using one of three different neuron-inducing agents and Neuregulin, a Schwann cell inducing agent.
The agents used to induce the neuronal phenotype were nerve growth factor (NGF), ciliary neurotrophic factor (CNTF) or macrophage migration inhibitory factor (MIF), the neurotrophic cytokine that is critical for inner ear development in vertebrates. Use of different agents resulted in differences in neuronal properties and influenced the observed early steps in myelination.
We tested the influence of MIF on directional neurite outgrowth from neuron-like cells to the Schwann cell-like cells by inhibiting MIF biochemically or by blocking its effects with a function-blocking monoclonal antibody. Statistically significant reductions in directed neurite outgrowth were observed under all conditions, but especially if MIF was used as the neural inducing agent.
We then “coated” cochlear implants with alginate hydrogel layers containing the MIF-producing Schwann cell-like cells. Primary mouse statoacoustic ganglion neurons, spiral ganglion neurons and MIF-induced neuron-like cells exhibited directed outgrowth in vitro to two types of cochlear implants: implants meant for human implantation and implants used in animal (e.g. gerbil) studies. No directed outgrowth from any of the “neuronal” subtypes was seen toward a “bare” or hydrogel coated cochlear implant or implants coated with undifferentiated embryonic stem cells.
Macrophage migration inhibitory factor (MIF)-induced neuron-like-cells exhibited directed neurite outgrowth to a wild type mouse Organ of Corti explant in culture but not towards a MIF-knock out organ of Corti explant.
Acknowledgments
Grant Sponsors: NIH (NIH/NIDCD 2RO1DC04184-04, 3R01DC004184-08W12 and R01 DC006436-04A2 (KFB)), NSF (IOS 0930096 (KFB)); Deafness Research Foundation (DRF) (KFB); NIHT32 DE007057 (TEAM) (PR); NIH 5T32EB005582-05 (Microfluidics in Biotechnology) (PR), NSF DGE 0718128 (JBW, ID: 2010101926).
This work was supported by grants from the NIH (NIH/NIDCD 2RO1DC04184-04, 3R01DC004184-08W12 and R01 DC006436-04A2 (KFB)), NSF (IOS 0930096 (KFB)); Deafness Research Foundation (DRF) (KFB); NIHT32 DE007057 (TEAM) (PR); NIH 5T32EB005582-05 (Microfluidics in Biotechnology) (PR), NSF DGE 0718128 (JBW, ID: 2010101926). The authors would like to thank Dr. Robin Davis, Department of Neuroscience, Rutgers University for extensive discussions and advice on the design of primer pairs for identification of SGN-specific markers, as well as providing primer pairs for some of the ion channels analyzed in her studies; Dr. Steven H. Green of the University of Iowa for extensive discussions on the development of Spiral Ganglion neurons and their stage-specific characteristics; Dr. Ethan M. Jewett, UC Berkeley Departments of Statistics and Computer Science for the statistical study design and computations, for re-design of some of the figures and for editing the manuscript; members of the Barald and Takayama labs for comments on the manuscript and suggestions for additional experimental approaches using microfluidics.
References
- Altschuler RA, O’Shea KS, Miller JM. Stem cell transplantation for auditory nerve replacement. Hearing research. 2008;242:110–116. doi: 10.1016/j.heares.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Annals of the New York Academy of Sciences. 1999;884:305–311. doi: 10.1111/j.1749-6632.1999.tb08650.x. [DOI] [PubMed] [Google Scholar]
- Baehr M, Bunge RP. Functional status influences the ability of Schwann cells to support adult rat retinal ganglion cell survival and axonal regrowth. Exp Neurol. 1989;106:27–40. doi: 10.1016/0014-4886(89)90141-6. [DOI] [PubMed] [Google Scholar]
- Bampton ET, Taylor JS. Effects of Schwann cell secreted factors on PC12 cell neuritogenesis and survival. Journal of neurobiology. 2005;63:29–48. doi: 10.1002/neu.20119. [DOI] [PubMed] [Google Scholar]
- Bank LM, Bianchi LM, Ebisu F, Lerman-Sinkoff D, Smiley EC, Shen Y, Ramamurthy P, Thompson DL, Roth TM, Beck CR, Flynn MJ, Teller RS, Feng L, Llewellyn GN, Holmes BB, Sharples C, Coutinho-Budd J, Linn SA, Chervenak AP, Dolan DF, Benson J, Kanicki A, Martin C, Altschuler R, Jewett EM, Koch A, Germiller JA, Barald KF. The cytokine Macrophage Migration Inhibitory Factor (MIF) acts as a neurotrophin for neurons in the developing mammalian and avian inner ears. Development. 2012;139(24):4666–4674. doi: 10.1242/dev.066647. (Cited in Faculty of 1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131(17):4119–30. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Current opinion in neurobiology. 2003;13:119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Daruwalla Z, Roth TM, Attia NP, Lukacs NW, Richards AL, White IO, Allen SJ, Barald KF. Immortalized mouse inner ear cell lines demonstrate a role for chemokines in promoting the growth of developing statoacoustic ganglion neurons. JARO. 2005;6(4):355–67. doi: 10.1007/s10162-005-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. The international journal of biochemistry & cell biology. 2006;38:1995–1999. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Raz Y. Methods for providing therapeutic agents to treat damaged spiral ganglion neurons. Curr Drug Targets CNS Neurol Disord. 2004;3:195–199. doi: 10.2174/1568007043337454. [DOI] [PubMed] [Google Scholar]
- Bodhireddy SR, Lyman WD, Rashbaum WK, Weidenheim KM. Immunohistochemical detection of myelin basic protein is a sensitive marker of myelination in second trimester human fetal spinal cord. Journal of neuropathology and experimental neurology. 1994;53:144–9. doi: 10.1097/00005072-199403000-00005. [DOI] [PubMed] [Google Scholar]
- Bodmer D. Protection, regeneration and replacement of hair cells in the cochlea: implications for the future treatment of sensorineural hearing loss. Swiss Med Wkly. 2008;138:708–712. doi: 10.4414/smw.2008.12260. [DOI] [PubMed] [Google Scholar]
- Boomkamp SD, Riehle MO, Wood J, Olson MF, Barnett SC. The development of a rat in vitro model of spinal cord injury demonstrating the additive effects of Rho and ROCK inhibitors on neurite outgrowth and myelination. Glia. 2012;60:441–56. doi: 10.1002/glia.22278. [DOI] [PubMed] [Google Scholar]
- Breuskin I, Bodson M, Thelen N, Thiry M, Nguyen L, Belachew S, Lefebvre PP, Malgrange B. Strategies to regenerate hair cells: identification of progenitors and critical genes. Hear Res. 2008;236:1–10. doi: 10.1016/j.heares.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bunge R, Moya F, Bunge M. Observations on the role of Schwann cell secretion in Schwann cell--axon interactions. Adv Biochem Psychopharmacol. 1981;28:229–242. [PubMed] [Google Scholar]
- Bunge RP. Schwann cells in central regeneration. Ann N Y Acad Schwann celli. 1991;633:229–233. doi: 10.1111/j.1749-6632.1991.tb15614.x. [DOI] [PubMed] [Google Scholar]
- Bunge RP. Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Curr Opin Neurobiol. 1993;3:805–809. doi: 10.1016/0959-4388(93)90157-t. [DOI] [PubMed] [Google Scholar]
- Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242:S19–21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- Burns JC, Corwin JT. A historical to present-day account of efforts to answer the question: “what puts the brakes on mammalian hair cell regeneration?”. Hear Res. 2013;297:52–67. doi: 10.1016/j.heares.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callizot N, Combes M, Steinschneider R, Poindron P. A new long term in vitro model of myelination. Experimental cell research. 2011;317:2374–83. doi: 10.1016/j.yexcr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. Journal of neurochemistry. 2009;111:510–21. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490(7419):278–82. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Xue HZ, Hsu YL, Liu Q, Patel S, Davis RL. Complex distribution patterns of voltage-gated calcium channel α-subunits in the spiral ganglion. Hearing Research. 2011;278(1–2):52–68. doi: 10.1016/j.heares.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Davis RL. Voltage-gated and two-pore-domain potassium channels in murine spiral ganglion neurons. Hearing Research. 2006;222(1–2):89–99. doi: 10.1016/j.heares.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Chen WC, Xue HZ, Hsu YL, Liu Q, Patel S, Davis RL. Subunits of voltage-gated calcium channels in murine spiral ganglion cells. Acta oto-laryngologica. 2007;127(1):8–12. doi: 10.1080/00016480600627927. [DOI] [PubMed] [Google Scholar]
- Coleman B, Fallon JB, Pettingill LN, de Silva MG, Shepherd RK. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp Cell Res. 2007;313:232–243. doi: 10.1016/j.yexcr.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J Neurobiol. 2006;66:1489–1500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson CT, Jacobson SC, Ramsey JM. Diffusion coefficient measurements in microfluidic devices. Talanta. 2002;56:365–73. doi: 10.1016/s0039-9140(01)00602-6. [DOI] [PubMed] [Google Scholar]
- Davis RL, Liu Q. Complex primary afferents: What the distribution of electrophysiologically-relevant phenotypes within the spiral ganglion tells us about peripheral neural coding. Hear Res. 2011;276:34–43. doi: 10.1016/j.heares.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe MM, Feijoo Redondo AF, Garcia-Sancho J, Schimmang T, Alonso MB. Cell- and gene-therapy approaches to inner ear repair. Histology & Histopathology. 2011;26(7):923–40. doi: 10.14670/HH-26.923. [DOI] [PubMed] [Google Scholar]
- Defourny J, Lallemend F, Malgrange B. Structure and development of cochlear afferent innervation in mammals. American Journal of Physiology - Cell Physiology. 2011;301(4):C750–61. doi: 10.1152/ajpcell.00516.2010. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Emb Exp Morph. 1985;87:27–45. [PubMed] [Google Scholar]
- Duran Alonso MB, Feijoo-Redondo A, Conde de Felipe M, Carnicero E, Garcia AS, Garcia-Sancho J, Rivolta MN, Giraldez F, Schimmang T. Generation of inner ear sensory cells from bone marrow-derived human mesenchymal stem cells. Regenerative Medicine. 2012;7(6):769–83. doi: 10.2217/rme.12.65. [DOI] [PubMed] [Google Scholar]
- Durand NF, Saveriades E, Renaud P. Detecting proteins complex formation using steady-state diffusion in a nanochannel. Analytical and bioanalytical chemistry. 2009;394:421–5. doi: 10.1007/s00216-008-2550-6. [DOI] [PubMed] [Google Scholar]
- Evans AJ, Thompson BC, Wallace GG, Millard R, O’Leary SJ, Clark GM, Shepherd RK, Richardson RT. Promoting neurite outgrowth from spiral ganglion neuron explants using polypyrrole/BDNF-coated electrodes. Journal of biomedical materials research Part A. 2009;91:241–250. doi: 10.1002/jbm.a.32228. [DOI] [PubMed] [Google Scholar]
- Flores-Otero J, Xue HZ, Davis RL. Reciprocal regulation of presynaptic and postsynaptic proteins in bipolar spiral ganglion neurons by neurotrophins. J Neurosci. 2007;27:14023–14034. doi: 10.1523/JNEUROSCI.3219-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarotta G, Fregnan F, Gnavi S, Perroteau I. Neuregulin 1 role in Schwann cell regulation and potential applications to promote peripheral nerve regeneration. International Review of Neurobiology. 2013;108:223–56. doi: 10.1016/B978-0-12-410499-0.00009-5. [DOI] [PubMed] [Google Scholar]
- Géléoc GS, Holt JR. Sound strategies for hearing restoration. Science. 2014;344(6184):1241062. doi: 10.1126/science.1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germiller JA, Smiley EC, Ellis AD, Hoff JS, Deshmukh I, Allen SJ, Barald KF. Molecular characterization of conditionally immortalized cell lines derived from mouse early embryonic inner ear. Dev Dyn. 2004;231(4):815–27. doi: 10.1002/dvdy.20186. [DOI] [PubMed] [Google Scholar]
- Gingras M, Beaulieu MM, Gagnon V, Durham HD, Berthod F. In vitro study of axonal migration and myelination of motor neurons in a three-dimensional tissue-engineered model. Glia. 2008;56:354–64. doi: 10.1002/glia.20617. [DOI] [PubMed] [Google Scholar]
- Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait-Oumesmar B, Baron-Van Evercooren A, Lachapelle F. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25:7924–7933. doi: 10.1523/JNEUROSCI.4890-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TD, Talbot WS. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Current Opinion in Neurobiology. 2013;23(6):1041–8. doi: 10.1016/j.conb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden KL, Pearse DD, Blits B, Garg MS, Oudega M, Wood PM, Bunge MB. Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp Neurol. 2007;207:203–217. doi: 10.1016/j.expneurol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman I, Golden G, Flitman S, Xie K, McConville M, Levy S, Zimmerman E, Lebedeva Z, Richter R, Minagar A, Averback P. A multi-center blinded prospective study of urine neural thread protein measurements in patients with suspected Alzheimer’s disease. Journal of the American Medical Directors Association. 2007;8:21–30. doi: 10.1016/j.jamda.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Greenwood D, Jagger DJ, Huang LC, Hoya N, Thorne PR, Wildman SS, King BF, Pak K, Ryan AF, Housley GD. P2X receptor signaling inhibits BDNF-mediated spiral ganglion neuron development in the neonatal rat cochlea. Development. 2007;134:1407–1417. doi: 10.1242/dev.002279. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Birchmeier W. Molecular signaling mechanisms of axon-glia communication in the peripheral nervous system. Bioessays. 2015;37(5):502–13. doi: 10.1002/bies.201400172. [DOI] [PubMed] [Google Scholar]
- Han H, Myllykoski M, Ruskamo S, Wang C, Kursula P. Myelin-specific proteins: A structurally diverse group of membrane-interacting molecules. Biofactors. 2013;39(3):233–241. doi: 10.1002/biof.1076. [DOI] [PubMed] [Google Scholar]
- Heinen A, Kremer D, Hartung HP, Kury P. p57 kip2’s role beyond Schwann cell cycle control. Cell Cycle. 2008;7:2781–6. doi: 10.4161/cc.7.18.6629. [DOI] [PubMed] [Google Scholar]
- Holmes KE, Wyatt MJ, Shen YC, Thompson DA, Barald KF. Direct delivery of MIF morpholinos into the zebrafish otocyst by injection and electroporation affects inner ear development. J Vis Exp. 2011 Jan 7;(47) doi: 10.3791/2466. pii: 2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SH, Ni HC. Fabrication of the microgrooved/microporous polylactide substrates as peripheral nerve conduits and in vivo evaluation. Tissue engineering Part A. 2009;15:1381–90. doi: 10.1089/ten.tea.2008.0175. [DOI] [PubMed] [Google Scholar]
- Houle JD, Ye JH. Survival of chronically-injured neurons can be prolonged by treatment with neurotrophic factors. Neuroscience. 1999;94:929–936. doi: 10.1016/s0306-4522(99)00359-0. [DOI] [PubMed] [Google Scholar]
- Hu Z, Ulfendahl M, Olivius NP. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain research. 2004a;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Hu Z, Ulfendahl M, Olivius NP. Survival of neuronal tissue following xenograft implantation into the adult rat inner ear. Experimental neurology. 2004b;185:7–14. doi: 10.1016/j.expneurol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hu Z, Ulfendahl M, Olivius NP. NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiology of disease. 2005;18:184–192. doi: 10.1016/j.nbd.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Huang T, Qin J, Xiong S, Yu L, Huo X, Liao H, Li J, Liu D. Expression of macrophage migration inhibitory factor mRNA in Schwann cells. Zhonghua wai ke za zhi [Chinese journal of surgery] 2002;40:699–701. [PubMed] [Google Scholar]
- Huang T, Qin JQ, Huo XK, Yu L, Xiong SH, Liu DY, Zhong SZ. Changes in content of macrophage migration inhibitory factor secreted by Schwann cells after peripheral nerve injury. Zhonghua wai ke za zhi [Chinese journal of surgery] 2002;40:702–707. [PubMed] [Google Scholar]
- Hurley PA, Crook JM, Shepherd RK. Schwann cells revert to non-myelinating phenotypes in the deafened rat cochlea. European J Neuroscience. 2007;26(7):1813–1821. doi: 10.1111/j.1460-9568.2007.05811.x. [DOI] [PubMed] [Google Scholar]
- Inaoka T, Nakagawa T, Kikkawa YS, Tabata Y, Ono K, Yoshida M, Tsubouchi H, Ido A, Ito J. Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in guinea pigs. Acta oto-laryngologica. 2009;129:453–457. doi: 10.1080/00016480902725197. [DOI] [PubMed] [Google Scholar]
- Ito J, Endo T, Nakagawa T, Kita T, Kim TS, Iguchi F. A new method for drug application to the inner ear. ORL; journal for oto-rhino-laryngology and its related specialties. 2005;67:272–275. doi: 10.1159/000089407. [DOI] [PubMed] [Google Scholar]
- Ito K, Yoshiura Y, Ototake M, Nakanishi T. Macrophage migration inhibitory factor (MIF) is essential for development of zebrafish, Danio rerio. Dev Comp Immunol. 2008;32:664–672. doi: 10.1016/j.dci.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Jarjour AA, Zhang H, Bauer N, Ffrench-Constant C, Williams A. In vitro modeling of central nervous system myelination and remyelination. Glia. 2012;60:1–12. doi: 10.1002/glia.21231. [DOI] [PubMed] [Google Scholar]
- Jhaveri SJ, Hynd MR, Dowell-Mesfin N, Turner JN, Shain W, Ober CK. Release of nerve growth factor from HEMA hydrogel-coated substrates and its effect on the differentiation of neural cells. Biomacromolecules. 2009;10:174–183. doi: 10.1021/bm801101e. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun SB, Hynd MR, Dowell-Mesfin NM, Al-Kofahi Y, Roysam B, Shain W, Kim SJ. Modulation of cultured neural networks using neurotrophin release from hydrogel-coated microelectrode arrays. J Neural Eng. 2008;5:203–213. doi: 10.1088/1741-2560/5/2/011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Farghaly WM, Amry Sel D, Osman AA. Neural maturation of breastfed and formula-fed infants. Acta paediatrica. 2004;93:734–738. doi: 10.1111/j.1651-2227.2004.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Kidd GJ, Ohno N, Trapp BD. Biology of Schwann cells. Handbook of Clinical Neurology. 2013;115:55–79. doi: 10.1016/B978-0-444-52902-2.00005-9. [DOI] [PubMed] [Google Scholar]
- Kondo T, Johnson SA, Yoder MC, Romand R, Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc Natl Acad Schwann celli USA. 2005;102:4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;38(6112):1353–6. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- Landry TG, Wise AK, Fallon JB, Shepherd RK. Spiral ganglion neuron survival and function in the deafened cochlea following chronic neurotrophic treatment. Hearing Research. 2011;282(1):303–313. doi: 10.1016/j.heares.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BJ, Devarapalli M, Madihally SV. Flow dynamics in bioreactors containing tissue engineering scaffolds. Biotechnology and bioengineering. 2009;102:935–47. doi: 10.1002/bit.22106. [DOI] [PubMed] [Google Scholar]
- Liazoghli D, Roth AD, Thostrup P, Colman DR. Substrate Micropatterning as a New in Vitro Cell Culture System to Study Myelination. ACS chemical neuroscience. 2012;3:90–95. doi: 10.1021/cn2000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nature Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Schwann celli USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang G, Liu T, Gu H, Yan L, Chen B. Construction of a novel vector expressing the fusion suicide gene yCDglyTK and hTERT-shRNA and its antitumor effects. Exp Ther Med. 2012;4(3):442–448. doi: 10.3892/etm.2012.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen P, Wang J. Molecular mechanisms and potentials for differentiating inner ear stem cells into sensory hair cells. Dev Biol. 2014;390(2):93–101. doi: 10.1016/j.ydbio.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Mellado Lagarde MM, Wan G, Zhang L, Gigliello AR, McInnis JJ, Zhang Y, Bergles D, Zuo J, Corfas G. Spontaneous regeneration of cochlear supporting cells after neonatal ablation ensures hearing in the adult mouse. Proc Natl Acad Schwann celli. 2014;111(47):16919–24. doi: 10.1073/pnas.1408064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena MA, Garcia de Yebenes J. Glial cells as players in parkinsonism: the “good,” the “bad,” and the “mysterious” glia. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2008;14:544–60. doi: 10.1177/1073858408322839. [DOI] [PubMed] [Google Scholar]
- Merkus P, Di Lella F, Di Trapani G, Pasanisi E, Beltrame MA, Zanetti D, Negri M, Sanna M. Indications and contraindications of auditory brainstem implants: systematic review and illustrative cases. [Review] European Archives of Oto-Rhino-Laryngology. 2014;271(1):3–13. doi: 10.1007/s00405-013-2378-3. [DOI] [PubMed] [Google Scholar]
- Miller JM, Miller AL, Yamagata T, Bredberg G, Altschuler RA. Protection and regrowth of the auditory nerve after deafness: neurotrophins, antioxidants and depolarization are effective in vivo. Audiology & neuro-otology. 2002;7:175–179. doi: 10.1159/000058306. [DOI] [PubMed] [Google Scholar]
- Monzack EL, Cunningham LL. Lead roles for supporting actors: critical functions of inner ear supporting cells. Hear Res. 2013;303:20–9. doi: 10.1016/j.heares.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namgung U. The Role of Schwann Cell-Axon Interaction in Peripheral Nerve Regeneration. Cells Tissues Organs. 2015;200(1):6–12. doi: 10.1159/000370324. [DOI] [PubMed] [Google Scholar]
- Nayagam BA, Muniak MA, Ryugo DK. The spiral ganglion: connecting the peripheral and central auditory systems. Hearing Research. 2011;278(1–2):2–20. doi: 10.1016/j.heares.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annual Review of Cell & Developmental Biology. 2014;30:503–33. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Nishihira J, Ishibashi T, Kato H, Minami A. Role of macrophage migration inhibitory factor (MIF) in peripheral nerve regeneration: anti-MIF antibody induces delay of nerve regeneration and the apoptosis of Schwann cells. Mol Med 2002. 2002 Sep;8(9):509–20. [PMC free article] [PubMed] [Google Scholar]
- O’Leary SJ, Richardson RR, McDermott HJ. Principles of design and biological approaches for improving the selectivity of cochlear implant electrodes. Journal of neural engineering. 2009a;6:055002. doi: 10.1088/1741-2560/6/5/055002. [DOI] [PubMed] [Google Scholar]
- O’Leary SJ, Richardson RR, McDermott HJ. Principles of design and biological approaches for improving the selectivity of cochlear implant electrodes. J Neural Eng. 2009b;6:055002. doi: 10.1088/1741-2560/6/5/055002. [DOI] [PubMed] [Google Scholar]
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paino CL, Fernandez-Valle C, Bates ML, Bunge MB. Regrowth of axons in lesioned adult rat spinal cord: promotion by implants of cultured Schwann cells. J Neurocytol. 1994;23:433–452. doi: 10.1007/BF01207115. [DOI] [PubMed] [Google Scholar]
- Paivalainen S, Nissinen M, Honkanen H, Lahti O, Kangas SM, Peltonen J, Peltonen S, Heape AM. Myelination in mouse dorsal root ganglion/Schwann cell cocultures. Molecular and cellular neurosciences. 2008;37:568–78. doi: 10.1016/j.mcn.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Pan GJ, Chang ZY, Schöler HR, Pei D. Stem cell pluripotency and transcription factor Oct4. Cell Res. 2002 Dec 12;2002(5–6):321–9. doi: 10.1038/sj.cr.7290134. [DOI] [PubMed] [Google Scholar]
- Park JY, Kim SK, Woo DH, Lee EJ, Kim JH, Lee SH. Differentiation of neural progenitor cells in a microfluidic chip-generated cytokine gradient. Stem Cells. 2009;27:2646–54. doi: 10.1002/stem.202. [DOI] [PubMed] [Google Scholar]
- Parker M, Brugeaud A, Edge AS. Primary culture and plasmid electroporation of the murine organ of Corti. Journal of visualized experiments: JoVE. 2010 doi: 10.3791/1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins CW. The bionic ear: principles and current status of cochlear prostheses. Neurosurgery. 1985;16:853–865. doi: 10.1227/00006123-198506000-00025. [DOI] [PubMed] [Google Scholar]
- Pettingill LN, Minter RL, Shepherd RK. Schwann cells genetically modified to express neurotrophins promote spiral ganglion neuron survival in vitro. Neuroscience. 2008;152:821–828. doi: 10.1016/j.neuroscience.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettingill LN, Richardson RT, Wise AK, O’Leary SJ, Shepherd RK. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng. 2007a;54:1138–1148. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettingill LN, Wise AK, Geaney MS, Shepherd RK. Enhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pig. PLoS One. 2011;6:e18733. doi: 10.1371/journal.pone.0018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Zhou N, Colesa DJ, Watts MM, Strahl SB, Garadat SN, Schvartz-Leyzac KC, Budenz CL, Raphael Y, Zwolan TA. Importance of cochlear health for implant function. Hearing Research. 2015;322:77–88. doi: 10.1016/j.heares.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan PA, Tadros SF, Kim Y, Birnbaumer L, Housley GD. Developmental regulation of TRPC3 ion channel expression in the mouse cochlea. Histochemistry and cell biology. 2010;133(4):437–48. doi: 10.1007/s00418-010-0686-x. [DOI] [PubMed] [Google Scholar]
- Porrello E, Dina G, Panattoni M, Triolo D, Cerri F, Del Carro U, Comi F, Wrabetz L, Feltri ML, Pardi R, Quattrini A, Previtali SC. CSN5/Jab1 inactivation in Schwann cells causes dysmyelinating peripheral neuropathy. J Peripheral Nervous System. 2011;16(Supplement 3):S111–S112. [Google Scholar]
- Ramekers D, Versnel H, Grolman W, Klis SF. Neurotrophins and their role in the cochlea. Hearing Research. 2012;288(1–2):19–33. doi: 10.1016/j.heares.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Reyes JH, O’Shea KS, Wys NL, Velkey JM, Prieskorn DM, Wesolowski K, Miller JM, Altschuler RA. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci. 2008;28:12622–12631. doi: 10.1523/JNEUROSCI.0563-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Current opinion in otolaryngology & head and neck surgery. 2005;13:294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Roehm PC, Xu N, Woodson EA, Green SH, Hansen MR. Membrane depolarization inhibits spiral ganglion neurite growth via activation of multiple types of voltage sensitive calcium channels and calpain. Mol Cell Neurosci. 2008;37(2):376–387. doi: 10.1016/j.mcn.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TM, Ramamurthy P, Muir D, Wallace MR, Zhu Y, Chang L, Barald KF. Influence of hormones and hormone metabolites on the growth of Schwann cells derived from embryonic stem cells and on tumor cell lines expressing variable levels of neurofibromin. Dev Dyn. 2008;237(2):513–24. doi: 10.1002/dvdy.21430. [DOI] [PubMed] [Google Scholar]
- Roth TM, Ramamurthy P, Ebisu F, Lisak RP, Bealmear BM, Barald KF. A mouse embryonic stem cell model of Schwann cell differentiation for studies of the role of neurofibromatosis type 1 in Schwann cell development and tumor formation. Glia. 2007;55(11):1123–33. doi: 10.1002/glia.20534. [DOI] [PubMed] [Google Scholar]
- Salzer JL. Axonal regulation of Schwann cell ensheathment and myelination. J Peripheral Nervous System. 2012;17(Suppl 3):14–9. doi: 10.1111/j.1529-8027.2012.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Schwann cell myelination. Cold Spring Harbor perspectives in biology. 2015;7(8):a020529. doi: 10.1101/cshperspect.a020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Doi K, Taniguchi M, Yamashita T, Kubo T, Tohyama M. Progressive hearing loss in mice carrying a mutation in the p75 gene. Brain Res. 2006;1091:224–234. doi: 10.1016/j.brainres.2005.12.104. [DOI] [PubMed] [Google Scholar]
- Shen Y, Thompson DL, Kuah MK, Wong KL, Wu KL, Linn SA, Jewett EM, Shu-Chien AC, Barald KF. The cytokine macrophage migration inhibitory factor (MIF) acts as a neurotrophin in the developing inner ear of the zebrafish, Danio rerio. Dev Biol. 2012;363(1):84–94. doi: 10.1016/j.ydbio.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Edge ASB. Prospects for replacement of auditory neurons by stem cells. Hearing Res. 2013;297:106–112. doi: 10.1016/j.heares.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Budenz CL, Bowling SA, Pfingst BE, Raphael Y. Nerve maintenance and regeneration in the damaged cochlea. Hearing Research. 2011;281(1–2):56–64. doi: 10.1016/j.heares.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. Neurogenic drugs and compounds to treat CNS diseases and disorders. Central nervous system agents in medicinal chemistry. 2011;11:35–37. doi: 10.2174/187152411794961103. [DOI] [PubMed] [Google Scholar]
- Torisawa YS, Chueh BH, Huh D, Ramamurthy P, Roth TM, Barald KF, Takayama S. Efficient formation of uniform-sized embryoid bodies using a compartmentalized microchannel device. Lab Chip. 2007;7(6):770–6. doi: 10.1039/b618439a. [DOI] [PubMed] [Google Scholar]
- Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids. 2001;36:885–895. doi: 10.1007/s11745-001-0798-1. [DOI] [PubMed] [Google Scholar]
- Wan G, Corfas G, Stone JS. Inner ear supporting cells: rethinking the silent majority. Semin Cell Dev Biol. 2013 May 24;2013(5):448–59. doi: 10.1016/j.semcdb.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Gong K, Zheng Z, Liu L, Wang A, Zhang L, Ao Q, Gong Y, Zhang X. Schwann -like cell differentiation of rat adipose-derived stem cells by indirect co-culture with Schwann cells in vitro. Cell proliferation. 2010;43:606–16. doi: 10.1111/j.1365-2184.2010.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JO, Cogan SF, Rizzo JF. Neurotrophin-eluting hydrogel coatings for neural stimulating electrodes. J Biomed Mater Res B Appl Biomater. 2007;81:551–563. doi: 10.1002/jbm.b.30696. [DOI] [PubMed] [Google Scholar]
- Winter JO, Gokhale M, Jensen RJ, Cogan SF, Rizzo JF. Tissue Engineering Applied to the Retinal Prosthesis: Neurotrophin-Eluting Polymeric Hydrogel Coatings. Mater Schwann celli Eng C Mater Biol Appl. 2008;28:448–453. doi: 10.1016/j.msec.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Fallon JB, Neil AJ, Pettingill LN, Geaney MS, Skinner SJ, Shepherd RK. Combining Cell-Based Therapies and Neural Prostheses to Promote Neural Survival. Neurotherapeutics. 2011a doi: 10.1007/s13311-011-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Fallon JB, Neil AJ, Pettingill LN, Geaney MS, Skinner SJ, Shepherd RK. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2011b;8:774–787. doi: 10.1007/s13311-011-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O’Leary SJ, Shepherd RK, Richardson RT. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–1122. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissel K, Stover T, Hofmann NS, Chernajovsky Y, Daly G, Sasse S, Warnecke A, Lenarz T, Gross G, Hoffmann A. Fibroblast-mediated delivery of GDNF induces neuronal-like outgrowth in PC12 cells. Otology & neurotology. 2008;29:475–481. doi: 10.1097/MAO.0b013e318164d110. [DOI] [PubMed] [Google Scholar]
- Xie D, Hu P, Xiao Z, Wu W, Chen Y, Xia K. Subunits of voltage-gated calcium channels in murine spiral ganglion cells. Acta Otolaryngol. 2007;127(1):8–12. doi: 10.1080/00016480600627927. [DOI] [PubMed] [Google Scholar]
- Yang IH, Gary D, Malone M, Dria S, Houdayer T, Belegu V, McDonald JW, Hakor N. Axon myelination and electrical stimulation in a microfluidic, compartmentalized cell culture platform. Neuromolecular medicine. 2012;14:112–8. doi: 10.1007/s12017-012-8170-5. [DOI] [PubMed] [Google Scholar]
- Yang L, Su J, Zhang X, Jiang C. Hypercapnia modulates synaptic interaction of cultured brainstem neurons. Respiratory physiology & neurobiology. 2008;160:147–59. doi: 10.1016/j.resp.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hearing Research. 2011;278(1–2):21–33. doi: 10.1016/j.heares.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin CS. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. The journal of sexual medicine. 2011;8:437–46. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zeng Y, Zhang W, Wang J, Wu J, Li J. Co-transplantation of neural stem cells and NT-3-overexpressing Schwann cells in transected spinal cord. Journal of Neurotrauma. 2007;24:1863–1877. doi: 10.1089/neu.2007.0334. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Zeng X, He LM, Ding Y, Li Y, Zeng YS. NT-3 gene modified Schwann cells promote TrkC gene modified mesenchymal stem cells to differentiate into neuron-like cells in vitro. Anat Schwann celli Int. 2010;85:61–67. doi: 10.1007/s12565-009-0056-8. [DOI] [PubMed] [Google Scholar]
- Zhong SX, Liu ZH. Immunohistochemical localization of the epithelial sodium channel in the rat inner ear. Hearing Research. 2004;193(1–2):1–8. doi: 10.1016/j.heares.2004.03.001. [DOI] [PubMed] [Google Scholar]