Abstract
Aggressive natural killer cell leukemia (ANKL) is a systemic NK-cell neoplasm, almost always associated with EBV. Rare cases of EBV-negative ANKL have been described and some reports suggested more indolent behavior. We report the clinicopathologic, immunophenotypic and molecular characteristics of seven EBV-negative ANKL. All patients were adults, with median age of 63 years (range 22–83) and a M:F ratio of 2.5:1. Five patients were White, one Black and one Asian. All patients presented acutely, with fever (6/7), cytopenias (6/7) and splenomegaly (4/7). Four patients had lymphadenopathy, 4 had extranodal disease. Bone marrow involvement was present in 5, with hemophagocytosis in 3. Peripheral blood was involved in 4 with the neoplastic cells containing prominent azurophilic granules. By immunohistochemistry and/or flow cytometry, the tumor cells lacked surface CD3, and were positive for CD56 (7/7), CD2 (5/5), CD8 (3/7), CD30 (4/5) and Granzyme B (6/6). They were negative for CD4, CD5, Beta F1, TCRγ, LMP1 and EBER. PCR for TCRG clonality was polyclonal. Mutational analysis revealed missense mutations in the STAT3 gene in both cases studied. Median survival was 8 weeks from the onset of disease. One patient received allogeneic BMT and is alive with no disease (follow-up 15 months). EBV-negative ANKL, exists, but is rare. It tends to occur in older patients and is indistinguishable clinically and pathologically from EBV-positive ANKL, with a similar fulminant clinical course. The high prevalence of Asian patients seen with EBV-positive disease seems less evident with EBV-negative cases.
Keywords: aggressive NK leukemia/lymphoma, Epstein Barr virus, JAK/STAT pathway, epidemiology, NK- cells
Introduction
Aggressive NK leukemia (ANKL) is a rare, systemic neoplastic proliferation of natural killer (NK) cells, described commonly in young adults of Asian ethnicity 1–3. Patients present acutely with fever, cytopenias, liver failure, coagulopathy and fulminant clinical course with < 2 months survival 4–6. Although a highly complex karyotype, with unbalanced chromosomal abnormalities has been reported, no specific recurrent alterations have been identified 4, 7, 8.
Epstein Barr virus (EBV) infection has been linked to ANKL’s pathobiology and considered responsible for the aggressive clinical features. However, occasional reports of EBV-negative ANKL have been described 1, 5, 6, 9–11 and a slightly better outcome observed was attributed to an EBV-negative status6, 11. Nevertheless, the limited number of the cases reported hampers definitive conclusions. We identified 7 cases of EBV-negative ANKL for which we reviewed the clinico-pathological and immunophenotypic features. We also studied two cases with available DNA for recurrent mutations seen in T-cell and NK-cell neoplasms. Our results provide further insights into the pathogenesis of these aggressive NK-cell neoplasms, and their relationship to EBV-positive counterparts.
Material and Methods
The pathology data base of the Laboratory of Pathology, National Cancer Institute, was searched for NK/T cell lymphomas/leukemias reported to be EBV negative. Six such cases were identified in the Hematopathology consultation archives from 2000 to date. An additional case was contributed by one of the coauthors (CATC)). The cases were reviewed by three of the authors (ESJ, KG, AN) and a consensus in diagnosis was reached. The clinicopathological, immunophenotypic (including flow-cytometry) and molecular data were analyzed. This study was approved by the Institutional Review Board of the National Cancer Institute.
Immunohistochemistry and in situ hybridization studies
Immunohistochemistry (IHC) and in situ hybridization (ISH) studies were performed on available formalin-fixed paraffin-embedded tissue (FFPE) using the following antibodies: CD2, CD3, CD4, CD5, CD8, CD30, CD56, granzyme-B, βF1, TCRγ and LMP1. The clones, with dilution and source, are listed in Table 1. All cases were tested for Epstein-Barr Virus (EBV) - encoded RNA (EBER) by ISH. EBER1 DNP probe supplied by Ventana on an automated stainer (Ventana-Benchmark XT, Tucson, AZ) was used. ISH iView blue plus system with alkaline phosphatase and nitroblue tetrozolium and 5-bromo-4-chloro-3-indolyl phosphate substrate, with Fast Red as contrast was applied for visualization. To assess the appropriate staining, a positive control was run with the cases.
Table 1.
Antibodies used in the immunophenotypic analysis
| Antigen | Clone | Dilution | Source |
|---|---|---|---|
| CD2 | AB75 | 1:160 | Novocastra |
| CD3 | Polyclonal | 1:100 | Dako |
| CD4 | 1F6 | 1:40 | Novocastra |
| CD5 | 4C7 | 1:100 | Novocastra |
| CD8 | C8/144B | 1:50 | Dako |
| CD30 | 1G12 | 1:50 | Novocastra |
| CD56 | 1B6 | 1:50 | Novocastra |
| βF1 | 8A3 | 1:20 | Endogen |
| TCRγ | γ3.20 | 1:100 | Thermo Scientific |
| Granzyme-B | GrB-7+D170 | 1:100 | Monosan |
| LMP1 | CS1-4 | 1:400 | Dako |
Molecular studies
For T-cell receptor gamma (TRG) rearrangement, DNA was extracted from whole tissue FFPE tissue sections and amplified by PCR, as published previously 12. A single multiplexed PCR was done with primers directed against all known Vg family members, and the Jg1/2, JP1/2 and JP joining segments. The product was analyzed by capillary electrophoresis on an ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA).
Mutational analysis
Two cases with available DNA were analyzed for somatic mutations using a targeted next generation sequencing (NGS) strategy. The mutation panel included regions of 38 genes previously reported to be mutated in T-cell lymphomas, as well as regions of genes involved in T-cell signaling focused on the JAK/STAT signaling pathway. The amplicon libraries were generated with two custom primer pools (total 227 amplicons) and were sequenced on an Ion Torrent Personal Genome Machine (PGM) (Life Technologies). The NGS methods and the list of the genes analyzed were recently published 12
Results
Clinical features
The demographic, clinical and outcome data are detailed in Table 2. All patients were adults, with median age of 63 years (range 22–83) and a M:F ratio of 2.5:1. Five patients were White, one Black and one Asian. No patients were identified as being of Hispanic or Native American heritage. All patients presented acutely, with less than one-month duration of symptoms. One patient (case 2) had a 9-year history of lymphoplasmacytic lymphoma/Waldenstrom’s macroglobulinema and was diagnosed with ANKL just after completion of the 6th cycle of Rituxan, Fludarabine and Cytoxan for recurrent disease. Another patient (case 6) with hepatitis C, chronic kidney disease and intravenous drug abuse had a one-year history of lower extremity ulcers, which were thought clinically to be a manifestation of cryoglobulinemia and vasculitis.
Table 2.
Demographic features, presentation, management and outcome ANKL, EBV-
| Case No. | Age/ Sex | Ethnicity | Clinical presentation | Fever | Night sweats | Splenomegaly | Lymphadenopathy | Cytopenias | Therapy | Outcome | Duration disease/ follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22/F | White | Acute onset, skin rash, edema, ascites, hepatomegaly, jaundice, abnormal hepatic & kidney function, coagulopathy, high LDH | − | + | + | abdomen, chest | thrombocytopenia | CHOP, EPOCH, Campath + Cytosine arabinoside | DoD | 8 wks |
| 2 | 73/M | White | Fever and weakness, focal liver abnormalities | + | − | − | − | pancytopenia | N/A | DoD | 7 wks |
| 3 | 52/M | Asian | 3–4 wk history of abdominal distension, chills, weakness, ascites, pleural effusion, hypoxemia, abnormal liver function | + | + | + | mediastinum | − | 1xCHOP | DoD | 8 wks |
| 4 | 83/F | White | 4 week history of fever, episodic confusion | + | − | − | − | pancytopenia | 1xCHOP | DoD | 8 wks |
| 5 | 64/M | White | Acute onset, nausea, vomiting, diarrhea | + | + | + | diffuse | pancytopenia | 6xSMILE, allogeneic MUD BMT | Alive, no disease | 15 months (8 months after transplant) |
| 6 | 63/M | Black | Acute onset, chest pain, skin plaques/nodules, edema, sinus changes, pericardium, pancreas, gastric wall. 1 yr history of lower extremity ulcers, Hep C, IVDA, CKD. | + | + | − | diffuse | anemia | Palliative Care | DoD | 8 wks |
| 7 | 60/M | White | Acute onset, skin rash/patches/plaques/nodules, edema, ascites, fever, chills, night sweats, weakness, weight loss, hepatomegaly, high LDH, abnormal kidney function | + | + | + | − | pancytopenia | 2xHyper- CVAD, Bortezomib, intrathecal CHT | DoD | 7 mo |
Abbreviations: LPL - lymphoplasmacytic lymphoma, IVDA - intravenous drug abuse, Hep - hepatitis C, CKD – chronic kidney disease
CHT, chemotherapy, CHOP - cyclophosphamide, adriamycin, vincristine, prednisone
CVAD - cyclophosphamide, vincristine, doxorubicin, dexamethasone
EPOCH - etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin
SMILE - dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide
UD BMT - matched unrelated donor bone marrow transplant
DoD - died of disease; wks - weeks; yrs -years, M - male; F - female
Six patients presented with fever, 5 with night sweats, 4 with lower extremity edema and ascites. Three patients had skin lesions manifesting as rash (case 1) or patches, ulcerated plaques and nodules (cases 6, 7). One patient had disseminated intravascular coagulation (case 1). Clinical examination and CT/PET scan showed splenomegaly in 4 and lymphadenopathy in 4 patients. One patient had extensive omental disease (case 3) and one showed foci of increased metabolic activity in the sinuses, pericardium, pancreas and gastric wall (case 6). Four patients had abnormal liver function tests and/or hepatomegaly. Except one, all showed various degree of cytopenias; none showed leukocytosis.
Three patients received CHOP and one a CHOP-like regimen as initial therapy. In addition, case 1 received EPOCH, Campath and high dose cytosine arabinoside and case 7 received Bortezomib, with no evident response. Five patients died within 2 months and one in 7 months from the onset of symptoms (median survival 8 weeks). One patient (case 5) who received 6 cycles of SMILE13 and a matched unrelated donor bone marrow transplant is alive with no disease (follow-up 15 months).
Pathologic findings
A summary of the morphological, immunohistochemical and molecular findings is shown in Table 3 and representative images are displayed in Figure 1. Bone marrow biopsies were available for evaluation in five cases; cellularity ranged from hypercellular (cases 1, 4) to hypocellular (case 2). Core biopsies showed an atypical lymphocytic infiltrate with an interstitial pattern, which varied in extent from subtle (cases 2, 7) to extensive (cases 1, 4). In addition, loose lympho-histiocytic aggregates (cases 2, 3) and a rich granulomatous background (case 4) were seen. Normal hematopoiesis was overall reduced with progressive maturation present. Hemophagocytic activity was noted in three cases and most prominent in case 2.
Table 3.
Morphology, Immunophenotype, EBER-ISH and T-cell clonality by PCR in ANKL, EBV-
| Case No. | Biopsy site (s) | Tumor cell morphology | HPS | CD2 | CD3 | CD4 | CD8 | CD5 | CD7 | CD56 | CD30 | βF1 | TCRγ | GZB | LMP1 | EBER | PCR TRG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bone marrow | monotonous, medium size | − | na | + c | na | + | − | na | + | na | − | − | + | − | − | No amp |
| 2 | Bone marrow, Liver, PB | monotonous, medium size, azurophilic granules | + | na | + c | − | − | − | na | + | + | na | − | + | − | − | PC |
| 3 | Bone marrow, Omentum, PB | monotonous, small- medium size, azurophilic granules | + | + | + c | − | + | na | + | + | na | na | na | na | na | − | na |
| 4 | Bone marrow | pleomorphic, range in cell size | − | + | − | − | + f,w | − | − | + | − | na | − | + | na | − | na |
| 5 | LN axilla, PB | monotonous, medium size, azurophilic granules | + | + | + c | − | − | − | − | + f | + | − | − | + | − | − | PC |
| 6 | Skin, PB | monotonous, medium size, azurophilic granules | − | + | +c | − | − | − | − | + | +w | − | − | + | − | − | PC |
| 7 | Skin, Liver, Bone marrow, PB | monotonous, medium size, azurophilic granules | − | + | −s | − | − | − | − | + | +f | − | − | + | na | − | PC |
EBER - Epstein–Barr virus-encoded small RNAs; ISH - in situ hybridization
BM - Bone marrow; PB - peripheral blood; LN - lymph node; HPS - hemophagocytic syndrome; Ly -lymphocytes
GZB - granzyme B; PCR - polymerase chain reaction; TRG - T-cell receptor γ chain gene
No amp - no amplification; PC - polyclonal
na - not available; c - cytoplasmic; f - focal; w - weak; s-surface
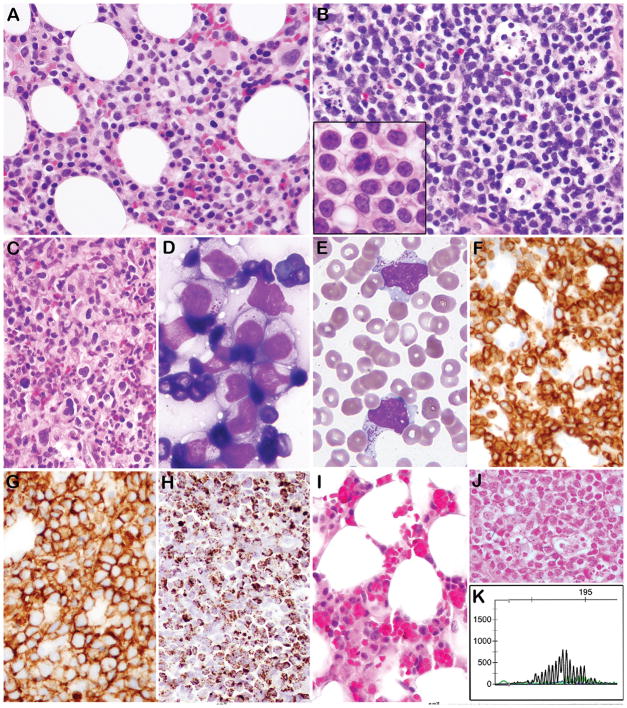
Figure 1. Morphological, immunophenotypic, and genotypic features of EBV negative ANKL.
A. Bone marrow biopsy contains atypical interstitial infiltrate composed of medium sized lymphocytes with fine chromatin and abundant pale cytoplasm (Case 3). B. A lymph node is diffusely infiltrated by atypical lymphocytes with abundant apoptotic debris in tingible body macrophages. The tumor cells on touch prep were medium in size with round/irregular nuclei, pale cytoplasm and easily identifiable mitoses (B, inset) (Case 5). C. The bone marrow from Case 4 contains more pleomorphic lymphocytes ranging in cell size, with reactive histiocytes in the background. D. A touch prep from an omental mass showed cytologically atypical lymphocytes with pale cytoplasm containing prominent azurophilic granules (Case 3). E. Circulating cells with prominent azurophilic granules are present (Case 5). By Immunohistochemistry the cells express cytoplasmic CD3 (F), CD56 (G), and Granzyme B (H). I. Bone marrow (Case 2) shows reduced hematopoiesis and numerous histiocytes with hemophagocytosis. J. All cases were negative for EBER by ISH (Case 3) K. PCR for TCRG showed a polyclonal rearrangement pattern (Case 5).
The liver biopsy in case 2 showed atypical portal lympho-histiocytic aggregates with scattered abnormal lymphocytes in the parenchyma and multiple foci of confluent hepatocellular necrosis without a zonal distribution pattern. By contrast the liver involvement in case 7 was predominantly intrasinusoidal. A lymph node biopsy (case 5) showed diffuse effacement by tumor cells, which also percolated into surrounding adipose tissue. A prominent starry sky pattern with tingible body macrophages was seen. Skin biopsies (cases 6, 7) showed a patchy, atypical dermal lymphoid infiltrate with a perivascular and interstitial pattern of infiltration. Apoptotic bodies were present, but the lesions lacked overt necrosis. The omental biopsy (case 3) demonstrated a panniculitis-like lymphoid infiltrate, with presence of rimming by the atypical lymphocytes.
Cytologically, in 6 cases the tumor cells were monotonous, medium-sized with a moderate amount of pale cytoplasm, round or irregular nuclei, finely clumped chromatin and small nucleoli. Case 4 showed atypical cells ranging in cell size with some large pleomorphic forms, dense chromatin and moderate to abundant pale cytoplasm. The atypical cells in this case were admixed with histiocytes. Peripheral blood smears, available for five patients, showed atypical lymphoid cells with irregular nuclei and basophilic cytoplasm with prominent azurophilic granules. The same cytological features were also observed in the bone morrow aspirates (cases 1, 2, 3, 4, 7) and touch preps obtained from the omental mass (case 3).
Immunophenotype
The immunohistochemical stains and the flow cytometry studies (available in 6/7 cases) demonstrated an absence of surface CD3 (Figure 2). The cells expressed CD56 (7/7), CD2 (5/5), CD8 (3/7), Granzyme B (6/7) and CD30 (4/5). They were negative for CD4, CD5, Beta F1, TCRγ and LMP1. Two cases analyzed showed absence of CD57 by flow cytometry. All cases were negative for EBER by ISH.
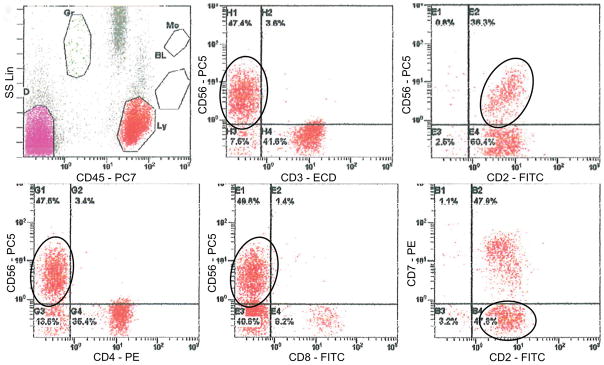
Figure 2. Flow cytometric analysis of EBV negative ANKL.
The atypical cells are negative for surface CD3, positive for CD56 and CD2, and negative for CD4, CD8, CD7, and CD5 (not shown) (Case 5).
T-cell Receptor gene rearrangement and mutational analysis
Polyclonal TCRG rearrangement was present in all four cases analyzed with adequate DNA. One case showed no amplification products.
Two cases (5 and 6) were analyzed with a custom NGS mutation panel. Case 5 was found to have STAT3 (c.1919A>T; p.Tyr640Phe, allele frequency (AF) 20.3%) and PTPN2 (c.671G>T, p.Gly224Val, AF 24.2%) mutations. Case 6 showed a missense mutation in STAT3 (c.1981G>T, p.Asp661Tyr, AF 41.9%), but differed from that seen in Case 5.
Discussion
ANKL is an extremely rare entity with less than 200 cases published to date 2, 4, 5. It has a strong association with EBV infection, which is considered part of disease definition. Like other EBV-positive T-cell and NK-cell neoplasms, it has a higher incidence in Asia, and is also encountered among indigenous populations in the Americas. Since its description by Fernandez et al. in 1986 14, sporadic EBV negative ANKL cases have been reported . EBV-negativity has been speculated to correlate with a less aggressive clinical outcome15,11. However, the rarity of the reported cases precludes firm conclusions. In the current study we describe the clinicopathological features, clinical management and outcome in seven ANKL EBV negative cases and compare the findings with those reported for ANKL EBV-positive.
ANKL EBV-negative is clinically comparable to ANKL EBV-positive with a similar clinical presentation and course. However, patients with EBV-negative disease tend to be older (median age 63 years) compared to patients with EBV-positive ANKL (median age approximately 36–38 years) 2, 4, 16. The older age at onset might correlate with other epidemiological features. Interestingly, five of our patients were White (not of Hispanic heritage), providing further epidemiological contrast with ANKL EBV-positive. Similar to EBV-positive ANKL, our patients presented acutely with fever, splenomegaly, and cytopenias 2, 4, 5. Besides, bone marrow and peripheral blood involvement, lymphadenopathy was a common feature observed in 57% of cases, similar to what has been reported for EBV-positive disease 1, 2, 4. Historically, the terminology of “leukemia/lymphoma” has been used for this disease,17 reflecting its systemic nature and extensive multi-organ involvement8, 17 .
We observed hepatomegaly and/or abnormal liver function tests in 57% of patients. It has been shown that besides tumor cell involvement, malignant NK cells constitutively express FASL, which triggers apoptosis of hepatocytes or other cell types that express FAS 18. Indeed, in case 2, we observed multifocal areas of necrosis and a high rate of apoptosis of individual hepatocytes. Furthermore, similar to EBV-positive cases, there is risk for involvement of the central nervous system; one of our patients presented with episodic confusion. This feature can be attributed to CD56/Neuronal Cell Adhesion Molecule (NCAM) expression on the tumor cells, which leads to cell-adhesion and recognition of other cell types expressing NCAM, such as brain, nerve and muscle 2, 19.
ANKL EBV negative is a rapidly progressive disease with high mortality within weeks of diagnosis. Five of our patients died within 8 weeks from the onset of symptoms; the only long term survivor received an allogeneic bone marrow transplant. A similarly aggressive outcome was reported by Suzuki et al. in two other patients with EBV-negative ANKL 5. However, a few case reports encompassing four patients described longer survival with a range from 11 months to 4 years9, 10, 15. It has been speculated that lack of EBV and its upregulation of the multidrug resistance genes 6 might account for an initial therapeutic response and the slightly better survival observed15. The favorable outcome of our singular patient who underwent allogeneic bone marrow transplant points to a possible role of this management to control the disease. Newer regimens containing asparaginase have also proved effective in other EBV-positive NK-cell neoplasms, and may be suitable to explore13.
No morphological or immunophenotypic features reliably discriminate between EBV positive and negative ANKL. Five of our cases showed blast-like morphology with monotonous, medium sized lymphocytes with fine chromatin and coarse azurophilic granules; only two cases contained pleomorphic cells, with hyperchromatic nuclei. A similar cytological spectrum has been described in EBV-positive ANKL 4. Additionally, the observed immunophenotype with expression of CD2, CD56 and lack of surface CD3 and CD7 is similar to that of EBV-positive ANKL8 . Clinical features aid in the differential diagnosis with extranodal NK/T-cell lymphoma, nasal type, which presents with more localized extranodal disease, lacking the systemic dissemination of both EBV-positive and negative ANKL. The presence of large geographic areas of necrosis is a helpful pathological feature, not seen in ANKL 20.
Prompt and accurate diagnosis of EBV-negative ANKL is required. The diagnosis is often delayed due to the absence of EBV, a feature leading to all of the cases being submitted for consultation. Based on the acute clinical course, we excluded the possibility of chronic lymphoproliferative disorder of NK-cells (CLPD-NK), an indolent process immunophenotypically similar to EBV-negative ANKL. More challenging is the distinction of EBV-negative ANKL from hepatosplenic T-cell lymphoma (HSTL) due to significant clinical, morphological and immunophenotypic overlap, as seen for case 7, initially suspected as HSTL in the liver biopsy. Lack of surface CD3 by flow cytometry is useful in assigning a NK lineage; however, this feature, although rare, has been described in HSTL at relapse as well as in a de novo case reported recently by Kapur et al. 4 Abnormalities of chromosome 7 7 and trisomy 8 4 , typical of HSTL, have been described in exceptional cases of EBV-positive ANKL, but no case with both abnormalities have been reported. In case 7 multiple features favor EBV-negative ANKL over HSTL, including systemic disease with lymphadenopathy, skin lesions, interstitial bone marrow involvement, and lack of clonal TCR gene rearrangement.
Little is known about the molecular pathogenesis of either EBV-positive or EBV-negative ANKL. A recent report from Gao et al. found evidence of STAT5B mutations in 1/5 cases studied of EBV-positive ANKL, but all five cases were negative for mutations in STAT38. We were able to identify STAT3 mutations by targeting sequencing in both EBV-negative cases analyzed, although different hotspots were seen [Y640F (case 5) and D661V (case 6)]. The same type of STAT3 mutation has been identified in T-cell and NK-cell large granular lymphocyte (LGL) leukemia 21, 22, where it has been shown to increase the transcriptional activity of STAT3 with upregulation of its downstream targets 23. These observations suggest that EBV-negative ANKL might arise by transformation from NK-cell LGL, a phenomenon that has been reported rarely 24 . However, none of our patients had clinical evidence of prior low-grade disease. Cytological atypia, seen in all of our cases of EBV-negative ANKL, is one key to this critical differential diagnosis. In addition, 3 cases showed evidence of hemophagocytic activity, which is a common feature of both EBV-positive and EBV-negative ANKL, but is not seen in NK-cell LGL20, 25.
Notably, activating mutations in STAT3 also have been recently identified in 26.5% of extranodal NK/T-cell lymphoma nasal type 26 and regarded as an intrinsic mechanism of STAT3 activation in addition to an extrinsic one elicited by the EBV proteins 26. Besides a STAT3 missense mutation, case 5 also harbored a mutation in the PTPN2. PTPN2 (protein tyrosine phosphatase non-receptor type 2) is a tumor suppressor gene and a negative regulator of JAK/STAT pathway 27. Biallelic inactivation of PTPN2 by deletion of the entire gene locus or by mutation has been identified with low frequency in T-cell acute lymphoblastic leukemia and peripheral T cell lymphoma not otherwise specified 27, 28. Although no additional functional studies are available, it is likely that a combination of PTPN2 inactivation and STAT3 mutation would result in uncontrolled activation of the JAK/STAT pathway. Our data provide further evidence for deregulation of the JAK/STAT pathway in NK derived malignancies, independent of EBV status.
In conclusion, we provide further evidence for the existence of EBV-negative AKNL, which shares most clinical and pathological features with EBV-positive ANKL, including an aggressive clinical course. The high incidence of non-Asian patients in this Western series suggests that EBV-negative ANKL may not share the racial predilections of the EBV-positive cases. Deregulation of the JAK/STAT pathway appears to play an important role, as it does in other cytotoxic T-cell and NK-cell malignancies. Future studies to compare EBV-positive and EBV-negative ANKL would be of interest in order to better assess their relationship and common pathobiology.
Acknowledgments
This study was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
The authors would like to thank the following physicians who contributed clinical information or case materials utilized in this report: Dr. Damian McManus, Belfast, Northern Ireland; Dr. Michael Glowalla, Kaiser Permanente, Los Angeles, CA; Dr. Dervila O’Reilly Jonas, Inova Loudon Hospital, VA, Dr. Richard G. Emanuelson, Moses Taylor Hospital, Scranton, PA; Dr. Aaron P. Rapaport, University of Maryland, St, Joseph Medical Center, MD.
Footnotes
Disclosures: The author(s) have no conflicts of interest or other funding to disclose
References
- 1.Zhang Q, Jing W, Ouyang J, et al. Six cases of aggressive natural killer-cell leukemia in a Chinese population. Int J Clin Exp Pathol. 2014;7:3423–3431. [PMC free article] [PubMed] [Google Scholar]
- 2.Ruskova A, Thula R, Chan G. Aggressive Natural Killer-Cell Leukemia: report of five cases and review of the literature. Leuk Lymphoma. 2004;45:2427–2438. doi: 10.1080/10428190400004513. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Tian Y, Wang J, et al. Abnormal immunophenotype provides a key diagnostic marker: a report of 29 cases of de novo aggressive natural killer cell leukemia. Transl Res. 2014;163:565–577. doi: 10.1016/j.trsl.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Kapur LH, Khaled Y, Solh M, et al. De novo CD3 negative hepatosplenic T-cell lymphoma: diagnostic challenges and pitfalls. Arch Pathol Lab Med. 2014;138:969–973. doi: 10.5858/arpa.2013-0074-CR. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki R, Suzumiya J, Nakamura S, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. 2004;18:763–770. doi: 10.1038/sj.leu.2403262. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Kim WS, Park C. SNARK, a novel downstream molecule of EBV latent membrane protein 1, is associated with resistance to cancer cell death. Leuk Lymphoma. 2008;49:1392–1398. doi: 10.1080/10428190802087454. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima Y, Tagawa H, Suzuki R, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosomes Cancer. 2005;44:247–255. doi: 10.1002/gcc.20245. [DOI] [PubMed] [Google Scholar]
- 8.Gao LM, Zhao S, Liu WP, et al. Clinicopathologic Characterization of Aggressive Natural Killer Cell Leukemia Involving Different Tissue Sites. Am J Surg Pathol. 2016;40:836–846. doi: 10.1097/PAS.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 9.Matano S, Nakamura S, Annen Y, et al. Monomorphic agranular natural killer cell lymphoma/leukemia with no Epstein-Barr virus association. Acta Haematol. 1999;101:206–208. doi: 10.1159/000040955. [DOI] [PubMed] [Google Scholar]
- 10.Osuji N, Matutes E, Morilla A, et al. Prolonged treatment response in aggressive natural killer cell leukemia. Leuk Lymphoma. 2005;46:757–763. doi: 10.1080/10428190500032273. [DOI] [PubMed] [Google Scholar]
- 11.Park JA, Jun KR, Nam SH, et al. Favorable outcome in a child with EBV-negative aggressive NK cell leukemia. Int J Hematol. 2013;97:673–676. doi: 10.1007/s12185-013-1319-7. [DOI] [PubMed] [Google Scholar]
- 12.Menon MP, Nicolae A, Meeker H, et al. Primary CNS T-cell Lymphomas: A Clinical, Morphologic, Immunophenotypic, and Molecular Analysis. Am J Surg Pathol. 2015 doi: 10.1097/PAS.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410–4416. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez LA, Pope B, Lee C, et al. Aggressive natural killer cell leukemia in an adult with establishment of an NK cell line. Blood. 1986;67:925–930. [PubMed] [Google Scholar]
- 15.Ko YH, Park S, Kim K, et al. Aggressive natural killer cell leukemia: is Epstein-Barr virus negativity an indicator of a favorable prognosis? Acta Haematol. 2008;120:199–206. doi: 10.1159/000193225. [DOI] [PubMed] [Google Scholar]
- 16.Cheung MM, Chan JK, Wong KF. Natural killer cell neoplasms: a distinctive group of highly aggressive lymphomas/leukemias. Semin Hematol. 2003;40:221–232. doi: 10.1016/s0037-1963(03)00136-7. [DOI] [PubMed] [Google Scholar]
- 17.Imamura N, Kusunoki Y, Kawa-Ha K, et al. Aggressive natural killer cell leukaemia/lymphoma: report of four cases and review of the literature. Possible existence of a new clinical entity originating from the third lineage of lymphoid cells. Br J Haematol. 1990;75:49–59. doi: 10.1111/j.1365-2141.1990.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 19.Kern WF, Spier CM, Hanneman EH, et al. Neural cell adhesion molecule-positive peripheral T-cell lymphoma: a rare variant with a propensity for unusual sites of involvement. Blood. 1992;79:2432–2437. [PubMed] [Google Scholar]
- 20.Chan JK. Natural killer cell neoplasms. Anat Pathol. 1998;3:77–145. [PubMed] [Google Scholar]
- 21.Poullot E, Zambello R, Leblanc F, et al. Chronic natural killer lymphoproliferative disorders: characteristics of an international cohort of 70 patients. Ann Oncol. 2014;25:2030–2035. doi: 10.1093/annonc/mdu369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajala HLM, Eldfors S, Kuusanmäki H, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. 2013;121:4541–4550. doi: 10.1182/blood-2012-12-474577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120:3048–3057. doi: 10.1182/blood-2012-06-435297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Q, Chang KL, Gaal KK, et al. An aggressive extranodal NK-cell lymphoma arising from indolent NK-cell lymphoproliferative disorder. Am J Surg Pathol. 2005;29:1540–1543. doi: 10.1097/01.pas.0000168510.54867.9a. [DOI] [PubMed] [Google Scholar]
- 25.Quintanilla-Martinez L, Jaffe ES. Commentary: aggressive NK cell lymphomas: insights into the spectrum of NK cell derived malignancies. Histopathology. 2000;37:372–374. doi: 10.1046/j.1365-2559.2000.01029.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Park HY, Kang SY, et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget. 2015;6:17764–17776. doi: 10.18632/oncotarget.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleppe M, Tousseyn T, Geissinger E, et al. Mutation analysis of the tyrosine phosphatase PTPN2 in Hodgkin's lymphoma and T-cell non-Hodgkin's lymphoma. Haematologica. 2011;96:1723–1727. doi: 10.3324/haematol.2011.041921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleppe M, Lahortiga I, El Chaar T, et al. Deletion of the protein tyrosine phosphatase gene PTPN2 in T-cell acute lymphoblastic leukemia. Nat Genet. 2010;42:530–535. doi: 10.1038/ng.587. [DOI] [PMC free article] [PubMed] [Google Scholar]