Abstract
IgA nephropathy (IgAN) is the leading cause of primary glomerulonephritis in the world. The disease is characterized by the presence of immune complexes in the circulation and in mesangial deposits with ensuing glomerular injury. Although in humans there are two IgA subclasses, only IgA1 molecules are involved. The exclusivity of participation of IgA1 in IgAN prompted extensive structural and immunological studies of the unique hinge region (HR) of IgA1, which is absent in otherwise highly homologous IgA2. HR of IgA1 with altered O-glycans serves as an antigen recognized by autoantibodies specific for aberrant HR glycans leading to the generation of nephritogenic immune complexes. However, there are several unresolved questions concerning the phylogenetic origin of human IgA1 HR, the structural basis of its antigenicity, the origin of antibodies specific for HR with altered glycan moieties, the regulatory defects in IgA1 glycosylation pathways, and the potential approaches applicable to the disease-specific interventions in the formation of nephritogenic immune complexes. This review focuses on the gaps in our knowledge of molecular and cellular events that are involved in the immunopathogenesis of IgAN.
Keywords: IgA nephropathy, IgA subclasses, autoimmunity, animal models of IgA nephropathy, IgA hinge region, IgA glycans, IgA glycosylation
1. Introduction
Since the time of its discovery in 1968 [1], IgA nephropathy (IgAN) has been considered a chronic renal disease driven by mesangial deposition of immune complexes (IC). Up to 50% of patients with IgAN progress to end-stage renal disease, as there is no disease-specific treatment [2]. IgAN has a distinct world-wide geographic distribution, with the highest incidence in East Asia and the lowest incidence in central Africa. This differential geographic distribution can be at least partially explained by the variable number of genetic risk alleles of IgAN-associated loci and the local species abundance of parasites and bacteria [3–5]. It has been accepted that the pathogenesis of IgAN is driven by IgA-containing IC that form in the circulation and some of them deposit in the kidney to incite glomerular injury [6]. IgA in the pathogenic IC is of the IgA1 subclass and enriched for glycoforms deficient in galactose (Gal) at some hinge-region (HR) O-glycans (Gd-IgA1) [7–9]. Gd-IgA1 is bound in these complexes by autoantibodies recognizing glycans in the HR of Gd-IgA1 [9, 10]. Although there is limited information available concerning the characteristics of the IC present in the circulation and in mesangial deposits, as well as the association with clinical progression of disease, further studies of the origin of Gd-IgA1 and characteristics of the autoantibodies are needed to elucidate some of the basic mechanisms of this disease [2, 11, 12]. In this brief review, we attempt to highlight some of the unresolved issues and challenges to foster further progress in the clarification of molecular and cellular mechanisms involved in the disease pathogenesis. Inevitably, this is an incomplete and partially biased review of results and their interpretations, reflecting the many unanswered and challenging questions in the field.
2. Why IgA1?
Although immunoglobulins (Igs) structurally analogous to human IgA appear in the circulation of many species, IgA is the major Ig isotype produced in humans in quantities that by far exceed the combined production of Igs of all other isotypes [13]. Extensive comparative studies of Ig isotypes from various vertebrate species revealed several structural features intrinsic to IgA molecules, including the ability to produce IgA in monomeric (m) as well as in polymeric (p) forms, to bind covalently to the J chain through the penultimate Cys residue of the extended constant (C) regions of heavy (H) chains, to display unique glycan structures, and to participate in a selective, receptor-mediated transepithelial transport of J chain-containing pIgA into external secretions [13].
In humans and hominoid primates, there are two IgA subclasses, IgA1 and IgA2, which display a very high degree of structural homology in the C regions of α1 and α2 chains with the exception of a unique HR between Cα1 and Cα2 domains [14]. The molecular shape and dimensions of IgA1 and IgA2 are almost identical, except for the greater flexibility and length of the Fab arms of IgA1 due to the presence of extended HR when compared to IgA2 [13]. The amino-acid sequence homology of Cα1, Cα2 and Cα3 heavy chain domains of IgA1 and IgA2 is 90%, 93% and 98%, respectively [13]. In other words, the primary structures of the entire human α1 and α2 C regions differ only in 14 of 340 amino-acid residues, with the exception of HR.
Human Igs of the IgG, IgA and IgD isotypes contain HRs of variable length (from 12 to 64 amino-acid residues) and composition. There are some notable differences as well as similarities in HRs of IgA1, IgA2 and IgD. The most striking difference in the HR of α1 and α2 H chains is in the insertion of 13 amino-acid residues in HR of α1 H chains. A detailed comparison of human, chimpanzee, and gorilla IgA HRs with IgA HRs of 10 other species (including 13 subclasses of lagomorphs’ IgA) clearly demonstrates the structural uniqueness of human and hominoid primate IgA1 [13–15]. Due to the duplicated octapeptide with Pro, Ser, and Thr residues and the Ser/Thr-attached glycans, the HR of IgA1 is reminiscent of that of mucin glycopeptides with O-glycans. However, the origin of the gene segment encoding the HR of IgA1 remains unknown. Despite the structural similarities to mucin, it is unlikely that the two structures have a common ancestor [16]. The HR of IgA1 is susceptible to the cleavage by a large family of bacterial enzymes, IgA-specific proteases whose only known substrate in nature is the HR [16]. These enzymes constitute a functionally identical but enzymatically diverse group of proteases [16]. IgA1 HR-specific proteases have been identified in several human respiratory-tract mucosal pathogens and are thought to contribute to immune-evasion from human IgA1. Elongated IgA1 HR provides extended flexibility to both IgA1 Fab arms allowing, theoretically, interaction of IgA1 with a large range of spatially diverse epitopes. IgA1-specific proteases may thus represent an adaptation of several bacteria species to avoid the protective role of IgA1. Unique and sequentially specific cleavage of IgA1 HR by IgA1-specific protease has been employed for studies of IgA1 HR glycosylation and the Gd-IgA1 interaction with specific autoantibodies [9, 17, 18]. Furthermore, the ability of an IgA1 protease to cleave IgA1 into Fab and Fc regions and thus disrupt the formed IC has been considered in the treatment of IgAN (see below).
3. What is the origin and molecular form of nephritogenic Gd-IgA1?
It has been shown that Gd-IgA1 is produced by some IgA1-producing cells due to altered expression and activity of key glycosyltransferases [19]. However, a highly challenging question, potentially related to future therapeutic approaches, is related to the tissue origin of Gd-IgA1-producing cells. Several indirect pieces of evidence indicate that cells producing Gd-IgA1 may originate from mucosal tissues. First, Gd-IgA1 in the glomerular deposits of IgAN patients is polymeric, which is a form typical for mucosal IgA [13]. Second, macroscopic hematuria episodes typical for IgAN are often associated with upper-respiratory tract and/or gastrointestinal tract infections [2, 11, 20], indicating that mucosal inflammatory conditions associated with local cytokine stimulation (as discussed below) could induce in predisposed individuals enhanced production of Gd-IgA1 [21, 22]. The explanation of how pGd-IgA1 produced in mucosal tissue could reach the systemic compartment could be based on observation that a proportion of pIgA1 produced by lamina propria plasma cells may be directed to systemic compartment instead of being targeted to secretions through the interaction with receptor for polymeric immunoglobulins (pIgR) on the epithelial cells [23]. Mutation in the pIgR gene was identified in a population of Japanese IgAN patients and was proposed as the potential contributor to IgAN [24]. Another explanation for the presence of pIgA in plasma is consistent with alterations in the homing of IgA1-producing cells migrating from the inductive to the effector sites through systemic circulation [25]. Nevertheless, pIgA- and J chain-producing cells have been also detected in the bone marrow from IgAN patients [26–30]. Although these studies suggested the close association between increased serum IgA levels and numbers of IgA plasma cells in the bone marrow of IgAN patients, such correlation was not confirmed for the IgA1 subclass [27]. Another report indicated that number of IgA plasma cells was elevated in the bone marrow of IgAN patients, but these cells did not produce pIgA1 [30]. Thus, a clear evidence for the bone-marrow origin of pGd-IgA1-producing plasma cells is missing.
In the circulation of IgAN patients, Gd-IgA1 is bound by unique Gd-IgA1-specific autoantibodies that can result in the formation of pathogenic IC [9, 10]. The IC activate mesangial cells in vitro, inducing cellular proliferation and overproduction of extracellular matrix components and cytokines/chemokines (for review see [31]). To activate mesangial-cell proliferation, Gd-IgA1 must be present in IC; uncomplexed Gd-IgA1 does not stimulate proliferation of mesangial cells (for reviews see [11, 32]. Moreover, it was observed that the levels of IgA-containing IC correlated with disease activity [33] and the circulatory levels of the components of these complexes, Gd-IgA1 and anti-Gd-IgA1 autoantibodies, predict disease progression and, in IgAN patients with transplanted kidney, the disease recurrence [34–36].
3.1. What are the epitopes for Gd-IgA1-specific autoantibodies?
Autologous antibodies to IgA have been detected not only in sera of patients with IgA deficiency but also in apparently healthy individuals [37–43]. Detailed studies of the specificity and isotype association revealed that such antibodies are mainly of the IgG isotype but are also present, albeit at lower levels, in the IgM and even IgA isotypes [8]. Furthermore, these antibodies were found at elevated levels in IgAN patients [9, 10, 43]. With respect to their specificity, the antibodies react with native molecules of IgA1 myeloma proteins, polyclonal secretory IgA from human milk, as well as Fab but not Fc fragments obtained by cleavage of IgA1 myeloma protein with IgA-specific protease from Haemophilus influenzae [9]. After this treatment with H. influenzae protease, most of HR remained associated with the Fab [9]; because the enzymatic removal of HR O-glycans reduced autoantibody binding, we concluded that anti-IgA1 antibodies required O-glycans of HR [9]. Furthermore, the removal of sialic acid (SA) and Gal significantly enhanced the antibody binding [10]. This observation indicated that N-acetylglucosamine (GalNAc) represented the dominant epitope [9]. However, the location and context of the involved GalNAc residue(s) have not been conclusively established. Based on the earlier experiments [43] and the progress in the analysis of IgA1 clustered O-glycans by high-resolution mass spectrometry (for review see [44]), GalNAc residues attached to Thr 228 and/or Ser 230 are the likely candidates [9, 45, 46]. Although the enzymatic removal of SA and Gal enhances the reactivity with antibodies, further studies are necessary to determine the precise identity of the epitope. Notably, Gd-IgA1 bound in IC by corresponding antibody reacts with lectins specific for GalNAc, thus indicating that not all GalNAc residues are covered by Gd-IgA1-specific antibodies.
3.2. What is the origin of anti-Gd-IgA1 antibodies?
The origin of anti-GalNAc antibodies has not been conclusively established. It is not clear whether Gd-IgA1 is the antigen solely responsible for inducing such antibodies, because anti-Gd-IgA1 antibodies are found also in sera of healthy individuals. Moreover, sera of several animal species (pigs, rabbits, cows, camels, llamas, donkeys, goats, sheep, mice, and rats) have anti-Gd-IgA1 antibodies [47]. By analogy with other glycan-specific antibodies, such as isohemagglutinins specific for terminal Gal or GalNAc of blood group substances, it appears that antibodies binding to GalNAc-containing glycoconjugates may be induced by cross-reacting antigens of microbial origin [48]. In the case of IgAN, surface glycoproteins and glycoconjugates with terminal GalNAc from Epstein-Barr virus, respiratory syncytial virus, herpes simplex virus, and streptococci [47, 49–53] may be responsible for their initial induction of Gd-IgA1-specific autoantibodies [9]. The cross-reacting antigens of Gram-negative bacteria are also involved in the induction of IgM-associated antibodies against Tn antigen (likely consisting of three vicinary GalNAc residues) on O-glycans expressed on surfaces of erythrocytes, platelets, monocytes, lymphocytes, and polymorphonuclear leukocytes of patients with Tn syndrome [54], a disease analogous to IgAN [55]. Some enterobacteria express highly immunogenic T antigen (Gal-GalNAc) and Tn antigen (GalNAc), and the corresponding antibodies are elicited by normal microbiota, as confirmed in germ-free animals [48]. Thus, anti-Tn antibodies could be elicited in human infants and adults after feeding or inhalation of live or killed Escherichia coli (O86) [48].
4. What potential approaches are explored for the disease-specific treatment of IgAN?
Currently, there is no disease-specific therapy available that can selectively prevent formation or enhance removal of nephritogenic IC [2]. Several novel approaches have been considered and designed to interfere with the formation of nephritogenic IC based on immunological and biochemical strategies (for review see [6]).
4.1. Inhibition of Gd-IgA1 production
The elimination of cells producing Gd-IgA1 would be one of desirable goals that would prevent formation of nephritogenic Gd-IgA1-containing IC. However, the tissue origin and phenotype of such cells has not been determined. We do not know whether cells that produce Gd-IgA1 also express the same glycoform of IgA1 on their surfaces; furthermore IgA1 on the B cells surface is likely to be covered by anti-Gd-IgA1 antibodies and, thus, not necessarily accessible for differential recognition. There is a possibility that cells, ultimately producing Gd-IgA1 after their differentiation, do not express IgA on their surfaces and proceed in their differentiation from sIgM+ cells directly to the IgA-secreting cells [56]. Moreover, we do not know how such Gd-IgA1-secreting cells may be affected by human microbiome and how are such interactions genetically regulated [57].
Alternatively, it would be desirable to “correct” the glycosylation defect in IgA1-producing cells. Several glycosyltransferases were identified to be involved in O-glycosylation of IgA1 HR and at least three of them are abnormally expressed in Gd-IgA1-producing cells, including core 1 β1,3-galactosyltransferase (C1GalT1), α-N-acetylgalactosaminide α-2,6-sialyltransferase 2 (ST6GalNAc-II), and an O-glycosylation-initiating enzyme (N-acetylgalactosaminyltransferase 14; GalNAcT14) (Fig. 1) [19, 58–61]. Recently, it was shown that the pro-inflammatory cytokine IL-6 and to a lesser extent IL-4, could enhance production of Gd-IgA1 in cells from IgAN patients by affecting the expression level and activity of C1GalT1 and ST6GalNAc-II [22]; it is possible that these enzymes can be therapeutically targeted by agents blocking IL-6 signaling, particularly mediated through Jak-STAT pathway [62].
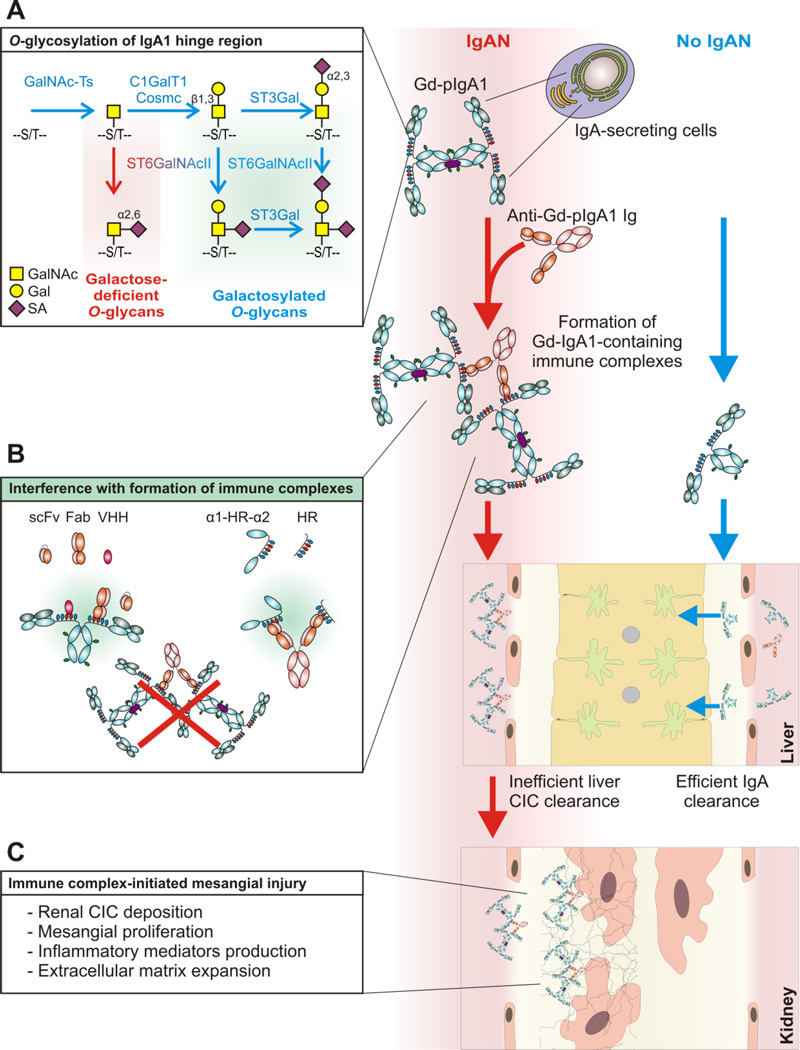
Fig. 1. Proposed pathogenesis of IgA nephropathy and potential therapeutic approaches.
A hypothesis on the multi-hit process of the pathogenesis of IgAN explains autoimmune features of the disease, wherein Gd-IgA1 is recognized by anti-Gd-IgA1 autoantibodies, resulting in the formation of immune complexes, some of which deposit in the kidney and incite glomerular injury [6]. Mucosal infection-associated inflammatory mediators may in genetically predisposed subjects activate plasma-cell precursors, possibly originating from mucosal tissues, to produce pGd-IgA1 at higher proportions in IgAN patients (red arrows) relative to healthy controls (blue arrows).
Gal deficiency is associated with aberrant O-glycosylation of IgA1 HR (A)O-glycosylation is initiated by a family of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases (GalNAc-Ts) which catalyze the transfer of GalNAc from UDP-GalNAc to the hydroxyl group on Ser or Thr residues (S/T). GalNAc-T2 was the identified as the key enzyme initiating glycosylation of IgA1 [75]. GalNAc-T14 is another candidate GalNAc-T involved in IgA1 HR O-glycosylation [12, 76]. Attachment of GalNAc is followed by addition of Gal from UDP-Gal catalyzed by C1GalT1. Synthesis of the stable enzyme C1GalT1 depends on Core 1 β1,3-galactosyltransferase-specific chaperone (Cosmc). The Core 1 disaccharide can be decorated with sialic acid residue(s) transferred from CMP-neuraminic acid (CMP-NeuAc) by sialyltransferases to either Gal or GalNAc or both. The reactions are catalyzed by a Galβ1,3GalNAc α2,3-sialyltransferase (ST3Gal) and a α2,6-sialyltransferase (ST6GalNAc), respectively. In IgA1-producing cells, sialylation of GalNAc is catalyzed exclusively by ST6GalNAc II [19, 58]. Patients with IgAN have elevated serum levels of aberrantly glycosylated IgA1, with some of their O-glycans Gal-deficient and, thus, composed of GalNAc or sialylated GalNAc [19, 58]. This aberrancy is associated with decreased activity of C1GalT1 and increased activity of ST6GalNAc II, in the IgA1-producing cells from the patients with IgAN [19]. Sialylated GalNAc (α2,6) represents a terminal step of the O-glycosylation pathway, as it presents a block for further O-glycan processing [22, 77].
pGd-IgA1 in the circulation is recognized by autoantibodies specific for Gd-IgA1 HR glycans or glycopeptides, leading to the formation of nephritogenic circulating immune complexes (CIC). pGd-IgA1-containing CIC are not effectively eliminated by liver clearance (hepatocytes) because of its large size precluding entry into the space of Disse and the reduced binding to liver receptor for IgA, the asialoglycoprotein receptor (ASGPR), due to occupation of Gd-IgA1 O-glycans by the autoantibody. In contrast, normal circulatory IgA1, mostly monomeric, is effectively eliminated by liver hepatocytes after binding to ASGPR (blue arrows).
Therapeutic outlook for interference with immune-complex formation (B). Formation of CIC in IgAN could be prevented by monovalent analogs of Gd-IgA1-specific autoantibodies (scFv, Fab, VHH) or by analogs of Gd-IgA1 hinge region (α1-HR-α2, HR). scFv, Fab, VHH would block the epitopes on Gd-IgA1, whereas α1-HR-α2 or HR must be prepared as GalNAc-osylated polypeptides to neutralize Gd-IgA1-specific autoantibodies.
Nephritogenic CIC are inefficiently eliminated by liver clearance and ultimately may deposit in the mesangium after passing through fenestrae in glomerular capillary endothelial cells. CIC bind to mesangial cells, involving transferrin receptor (CD71), and activate them. This process may be enhanced by alternative complement pathway activation. Subsequently, mesangial cells proliferate and produce inflammatory mediators and extracellular matrix proteins, thus inciting glomerular injury (C)
4.2. Interference with generation of nephritogenic IC
Inhibition of the formation of pathogenic Gd-IgA1-containing ICs is another attractive possibility for disease-specific therapy (for reviews see [6, 11]. The current view of the properties of IC suggests that they consist of two dimeric molecules of Gd-IgA1 bound by one or two molecules of IgG autoantibody [12]. Circulating ICs (CICs) with the molecular mass of ∼800–900 kDa display nephritogenic characteristics (activate cellular proliferation of mesangial cells), whereas the smaller CICs tend to be less active (for review see [12, 32, 44]. There are two potential approaches to prevent the formation of such complexes (Fig. 1). To interfere with the cross-linking of Gd-IgA1 by IgG autoantibodies, it is possible to use, for example, IgA1 HR-derived E. coli-expressed recombinant or synthetic peptides with GalNAc attached enzymatically [6, 46]. Such an approach does not require detailed information about particular epitope recognized by patient’s Gd-IgA1-specific autoantibodies. However, several questions arise, namely concerning stability and potential immunogenicity of such glycoconjugates.
In the second approach we proposed [6, 11, 31] to generate monovalent fragments of antibodies specific for HR GalNAc and, thus, prevent the formation of pathogenic ICs. This goal can be achieved by generation of single chain Fv fragments (variable regions of H and light chains linked by a peptide chain) of antibodies specific for Gd-IgA1. Even more attractive is the possibility to generate such reagents by a novel technology based on the unexpected uniqueness of some camelid antibodies, which comprise two covalently linked H chains, and the antigen-binding site consists only of the variable-regions’ domains without light chain participation [63, 64]. Through the use of molecular genetic manipulations, monoclonal, highly specific monovalent antibodies containing only the variable region of H chain (designated VHH or “nanobodies”) can be generated [11, 65]. Both approaches are currently pursued in our laboratories.
4.3. Inhibition of IC-induced signaling in mesangial cells
As detailed before, we proposed a multi-hit hypothesis for pathogenesis of IgAN [6]. Specifically, Gd-IgA1 produced in elevated amounts in patients with IgAN (Hit 1) is recognized in the circulation by unique autoantibodies (Hit 2), resulting in the formation of pathogenic IC (Hit 3), some of which ultimately deposit in the glomerular mesangium, activate mesangial cells and induce renal injury (Hit 4) (for review see [32]). Gd-IgA1-containing IC activates cultured human mesangial cells, as evidenced by phosphorylation of multiple proteins [66–68]. Consequently, strategies using inhibitors of specific protein kinases can be a useful strategy for patients at risk of progressive renal disease [32].
4.4. Proteolytic digestion of IgA1 in the glomerular deposits
The HR of human IgA1 is susceptible to the digestion by several enzymes from many strains and species of pathogenic or commensal bacteria resulting in the generation of Fab and Fc fragments [16]. It appears that not only the free IgA1 but also IgA1 in IC is susceptible to the proteolytic cleavage, resulting in their solubilization and at least partial elimination from mesangial of mice injected with human IC containing IgA1 [69]. Although attractive, this approach has little chance of success in the treatment of IgAN for several reasons. If injected in humans, IgA1 proteases would first encounter an enormous excess of its substrate, IgA1 in the circulation, before reaching the mesangium. This approach is applicable only to experimental animals whose endogenous IgA is not susceptible to the cleavage by IgA1 proteases and may therefore exhibit their activity in murine mesangium with human IgA1-containing complexes [70]. The inevitable generation of large quantities of Fab and Fc fragments in the circulation of IgA1-protease-treated patients is likely to have unacceptable consequences [71]. Furthermore, the majority of bacterial IgA1 proteases induce, as microbial antigens, potent humoral responses manifested by the presence of neutralizing antibodies [16]. IgA1 proteases produced by selected oral bacteria are the only exception, probably due to the fact that such bacteria colonize oral cavities of children shortly after the birth and may thus elicit low immune responses due to the induction of mucosal tolerance [16, 72, 73]. Therefore, the anticipated frequently repeated use of IgA1 proteases required for solubilization of continuously generated complexes is not likely to be successful.
5. How useful are animal models of IgAN?
Although several small-animal models of IgAN have been described, their shortcomings limit their utility and interpretation relevant to human disease [74]. Most importantly, IgA present in sera and secretions of experimental animals - such as mice, rats, and ferrets - resemble human IgA2 and do not contain HR with O-glycans [11]. Therefore, the putative antigen, Gal-deficient HR glycan essential for the formation of IC is absent. Moreover, the IgA systems in humans and experimental animals display marked differences in the dominant molecular forms, biosynthesis, catabolism and transport [15]. In contrast to humans, almost all IgA in the murine circulation is present in the polymeric form and is effectively removed either in the free form or as IC from the circulation by the pIgR expressed on hepatocytes, which thus participate in the hepatobiliary transport [15]. However, murine models may be useful in assessing some aspects of IgAN, including the testing of various inhibitors of immune-complex formation or activity (for review, see [12]).
6. Conclusions
The available data concerning the marked structural and immunological differences between human IgA1 and IgA2 and the critical role of HR of IgA1 with its aberrant glycosylation provide a plausible explanation for the exclusive association of the IgA1 with the formation of nephritogenic IC in patients with IgAN. Additional studies are required to determine the roles of specific glycosyltransferases and the defects in the regulation of HR glycosylation to provide the physiological basis for the disease-specific interventions and rational therapy.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health DK078244, DK082753, DK099228, DK105124, and GM098539 and by the grant of Ministry of Health, Czech Republic NV 15-33686A and Ministry of School and Education of the Czech Republic, Grant number LO1304.
Abbreviations
- ASGPR
Asialoglycoprotein receptor
- CIC
Circulating immune complexes
- Gd-IgA1
Galactose-deficient IgA1
- GalNAc
N-acetylgalactosamine
- HR
Hinge region
- IC
Immune complexes
- IgAN
IgA nephropathy
- mIgA
Monomeric IgA
- pIgA
Polymeric IgA
- pIgR
Polymeric immunoglobulin receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J. Urol. Nephrol. (Paris) 1968;74:694–695. [PubMed] [Google Scholar]
- 2.Wyatt RJ, Julian BA. IgA nephropathy. N. Engl. J. Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 3.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, Snyder HJ, Choi M, Hou P, Scolari F, Izzi C, Gigante M, Gesualdo L, Savoldi S, Amoroso A, Cusi D, Zamboli P, Julian BA, Novak J, Wyatt RJ, Mucha K, Perola M, Kristiansson K, Viktorin A, Magnusson PK, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Boland A, Metzger M, Thibaudin L, Wanner C, Jager KJ, Goto S, Maixnerova D, Karnib HH, Nagy J, Panzer U, Xie J, Chen N, Tesar V, Narita I, Berthoux F, Floege J, Stengel B, Zhang H, Lifton RP, Gharavi AG. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8:e1002765. doi: 10.1371/journal.pgen.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiryluk K, Novak J, Gharavi AG. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu. Rev. Med. 2013;64:339–356. doi: 10.1146/annurev-med-041811-142014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerova D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Paczek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat. Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin. Exp. Immunol. 1995;100:470–474. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 9.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J. Clin. Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, Novak L, Matousovic K, Novak J. IgA nephropathy: molecular mechanisms of the disease. Annu. Rev. Pathol. Mech. Dis. 2013;8:217–240. doi: 10.1146/annurev-pathol-011110-130216. [DOI] [PubMed] [Google Scholar]
- 12.Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, Raska M, Renfrow MB, Julian BA, Novak J. The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front. Immunol. 2016;7:117. doi: 10.3389/fimmu.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woof JM, Mestecky J. Mucosal Immunoglobulins. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroute H, Lambrecht BN, editors. Mucosal Immunology. 4th. Amsterdam: Elsevier/Academic Press; 2015. pp. 287–324. [Google Scholar]
- 14.Kawamura S, Omoto K, Ueda S. Evolutionary hypervariability in the hinge region of the immunoglobulin alpha gene. J. Mol. Biol. 1990;215:201–206. doi: 10.1016/S0022-2836(05)80336-5. [DOI] [PubMed] [Google Scholar]
- 15.Kaetzel CS, Russell MW. Phylogeny and comparative physiology of mucosal immunoglobulins. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroute H, Lambrecht BN, editors. Mucosal Immunology. 4th. Amsterdam: Elsevier/Academic Press; 2015. pp. 325–348. [Google Scholar]
- 16.Kilian M, Russell MW. Microbial evasion of IgA functions. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroute H, Lambrecht BN, editors. Mucosal Immunology. 4th. Amsterdam: Elsevier/Academic Press; 2015. pp. 455–470. [Google Scholar]
- 17.Novak J, Tomana M, Kilian M, Coward L, Kulhavy R, Barnes S, Mestecky J. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol. Immunol. 2000;37:1047–1056. doi: 10.1016/s0161-5890(01)00019-0. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Wall SB, Suzuki H, Smith AD, Hall S, Poulsen K, Kilian M, Mobley JA, Julian BA, Mestecky J, Novak J, Renfrow MB. Clustered O-glycans of IgA1: Defining macro- and micro-heterogeneity by use of electron capture/transfer dissociation. Mol. Cell. Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcon GS, Kimberly RP, Tomino Y, Mestecky J, Novak J. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J. Clin. Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Ozono Y, Harada T, Hara K. Changes in circulating immune complex and charge distribution with upper respiratory tract inflammation in IgA nephropathy. Nephron. 1995;69:384–390. doi: 10.1159/000188507. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Raska M, Moldoveanu Z, Julian BA, Wyatt RJ, Lifton RP, Tomino Y, Mestecky J, Gharavi AG, Novak J. Mechanisms of aberrant glycosylation of IgA1 in patients with IgA nephropathy (IgAN) J. Am. Soc. Nephrol. 2009;20:301A. [Google Scholar]
- 22.Suzuki H, Raska M, Yamada K, Moldoveanu Z, Julian BA, Wyatt RJ, Tomino Y, Gharavi AG, Novak J. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J. Biol. Chem. 2014;289:5330–5339. doi: 10.1074/jbc.M113.512277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker K, Blumberg RS, Kaetzel CS. Immunoglobulin transport and immunoglobulin receptors. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroute H, Lambrecht BN, editors. Mucosal Immunology. 4th. Amsterdam: Elsevier/Academic Press; 2015. pp. 349–407. [Google Scholar]
- 24.Obara W, Iida A, Suzuki Y, Tanaka T, Akiyama F, Maeda S, Ohnishi Y, Yamada R, Tsunoda T, Takei T, Ito K, Honda K, Uchida K, Tsuchiya K, Yumura W, Ujiie T, Nagane Y, Nitta K, Miyano S, Narita I, Gejyo F, Nihei H, Fujioka T, Nakamura Y. Association of single-nucleotide polymorphisms in the polymeric immunoglobulin receptor gene with immunoglobulin A nephropathy (IgAN) in Japanese patients. J. Hum. Genet. 2003;48:293–299. doi: 10.1007/s10038-003-0027-1. [DOI] [PubMed] [Google Scholar]
- 25.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 26.Allen A, Harper S, Feehally J. Origin and structure of pathogenic IgA in IgA nephropathy. Biochem. Soc. Trans. 1997;25:486–490. doi: 10.1042/bst0250486. [DOI] [PubMed] [Google Scholar]
- 27.Harper SJ, Allen AC, Layward L, Hattersley J, Veitch PS, Feehally J. Increased immunoglobulin A and immunoglobulin A1 cells in bone marrow trephine biopsy specimens in immunoglobulin A nephropathy. Am. J. Kidney Dis. 1994;24:888–892. doi: 10.1016/s0272-6386(12)81056-0. [DOI] [PubMed] [Google Scholar]
- 28.Harper SJ, Allen AC, Pringle JH, Feehally J. Increased dimeric IgA producing B cells in the bone marrow in IgA nephropathy determined by in situ hybridisation for J chain mRNA. J. Clin. Pathol. 1996;49:38–42. doi: 10.1136/jcp.49.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper SJ, Feehally J. The pathogenic role of immunoglobulin A polymers in immunoglobulin A nephropathy. Nephron. 1993;65:337–345. doi: 10.1159/000187509. [DOI] [PubMed] [Google Scholar]
- 30.van den Wall Bake AW, Daha MR, Radl J, Haaijman JJ, Van der Ark A, Valentijn RM, Van Es LA. The bone marrow as production site of the IgA deposited in the kidneys of patients with IgA nephropathy. Clin. Exp. Immunol. 1988;72:321–325. [PMC free article] [PubMed] [Google Scholar]
- 31.Novak J, Julian BA, Tomana M, Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin. Nephrol. 2008;28:78–87. doi: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, Glassock RJ. IgA nephropathy. Nat. Rev. Dis. Primers. 2016;2 doi: 10.1038/nrdp.2016.1. article number 16001. [DOI] [PubMed] [Google Scholar]
- 33.Coppo R, Basolo B, Martina G, Rollino C, De Marchi M, Giacchino F, Mazzucco G, Messina M, Piccoli G. Circulating immune complexes containing IgA, IgG and IgM in patients with primary IgA nephropathy and with Henoch-Schönlein nephritis. Correlation with clinical and histologic signs of activity. Clin. Nephrol. 1982;18:230–239. [PubMed] [Google Scholar]
- 34.Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82:790–796. doi: 10.1038/ki.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J. Am. Soc. Nephrol. 2012;23:1579–1587. doi: 10.1681/ASN.2012010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berthelot L, Robert T, Vuiblet V, Tabary T, Braconnier A, Drame M, Toupance O, Rieu P, Monteiro RC, Toure F. Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int. 2015;88:815–822. doi: 10.1038/ki.2015.158. [DOI] [PubMed] [Google Scholar]
- 37.Rivat L, Rivat C, Daveau M, Ropartz C. Comparative frequencies of anti-IgA antibodies among patients with anaphylactic transfusion reactions and among normal blood donors. Clin. Immunol. Immunopathol. 1977;7:340–348. doi: 10.1016/0090-1229(77)90068-x. [DOI] [PubMed] [Google Scholar]
- 38.Burks AW, Sampson HA, Buckley RH. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. Detection of IgE antibodies to IgA. N. Engl. J. Med. 1986;314:560–564. doi: 10.1056/NEJM198602273140907. [DOI] [PubMed] [Google Scholar]
- 39.Wilson ID, Soltis RD, Williams RC., Jr Naturally occurring human antibodies to pepsin-digested IgA. Blood. 1970;36:390–398. [PubMed] [Google Scholar]
- 40.Petty RE, Sherry DD, Johannson JM. IgG anti-IgA1 and anti-IgA2 antibodies: their measurement by an enzyme-linked immunosorbent assay and their relationship to disease. Int. Arch. Allergy. Appl. Immunol. 1986;80:337–341. doi: 10.1159/000234078. [DOI] [PubMed] [Google Scholar]
- 41.Jackson S, Montgomery RI, Julian BA, Galla JH, Czerkinsky C. Aberrant synthesis of antibodies directed at the Fab fragment of IgA in patients with IgA nephropathies. Clin. Immunol. Immunopathol. 1987;45:208–213. doi: 10.1016/0090-1229(87)90035-3. [DOI] [PubMed] [Google Scholar]
- 42.Jackson S, Montgomery RI, Mestecky J, Czerkinsky C. Normal human sera contain antibodies directed at Fab of IgA. J. Immunol. 1987;138:2244–2248. [PubMed] [Google Scholar]
- 43.Jackson S, Montgomery RI, Mestecky J, Julian BA, Galla JH, Czerkinsky C. Antibodies directed at Fab of IgA in the sera of normal individuals and IgA nephropathy patients. Adv. Exp. Med. Biol. 1987;216B:1537–1544. [PubMed] [Google Scholar]
- 44.Novak J, Julian BA, Mestecky J, Renfrow MB. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin. Immunopathol. 2012;34:365–382. doi: 10.1007/s00281-012-0306-z. [DOI] [PubMed] [Google Scholar]
- 45.Novak J, Moldoveanu Z, Renfrow MB, Yanagihara T, Suzuki H, Raska M, Hall S, Brown R, Huang WQ, Goepfert A, Kilian M, Poulsen K, Tomana M, Wyatt RJ, Julian BA, Mestecky J. IgA nephropathy and Henoch-Schoenlein purpura nephritis: aberrant glycosylation of IgA1, formation of IgA1-containing immune complexes, and activation of mesangial cells. Contrib. Nephrol. 2007;157:134–138. doi: 10.1159/000102455. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi K, Suzuki H, Yamada K, Hall S, Moldoveanu Z, Poulsen K, Mestecky J, Julian BA, Renfrow MB, Novak J. Molecular characterization of IgA1 secreted by IgA1-producing cell lines from patients with IgA nephropathy. J. Am. Soc. Nephrol. 2012;23:853A. [Google Scholar]
- 47.Mestecky J, Suzuki H, Yanagihara T, Moldoveanu Z, Tomana M, Matousovic K, Julian BA, Novak J. IgA nephropathy: current views of immune complex formation. Contrib. Nephrol. 2007;157:56–63. doi: 10.1159/000102305. [DOI] [PubMed] [Google Scholar]
- 48.Springer GF, Tegtmeyer H. Origin of anti-Thomsen-Friedenreich (T) and Tn agglutinins in man and in White Leghorn chicks. Br. J. Haematol. 1981;47:453–460. doi: 10.1111/j.1365-2141.1981.tb02813.x. [DOI] [PubMed] [Google Scholar]
- 49.Wertz GW, Krieger M, Ball LA. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J. Virol. 1989;63:4767–4776. doi: 10.1128/jvi.63.11.4767-4776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 51.Kief E. Epstein-Barr virus and its replication. 3rd. Philadelphia: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 52.Cisar JO, Sandberg AL, Reddy GP, Abeygunawardana C, Bush CA. Structural and antigenic types of cell wall polysaccharides from viridans group streptococci with receptors for oral actinomyces and streptococcal lectins. Infect. Immun. 1997;65:5035–5041. doi: 10.1128/iai.65.12.5035-5041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson DC, Spear PG. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983;32:987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berger EG. Tn-syndrome. Biochim. Biophys. Acta. 1999;1455:255–268. doi: 10.1016/s0925-4439(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 55.Mestecky J, Tomana M. Structural heterogeneity of glycans in immunoglobulin molecules: Implications in IgA nephropathy. Nephrology. 1997;3:S653–S657. [Google Scholar]
- 56.Conley ME, Bartelt MS. In vitro regulation of IgA subclass synthesis. II. The source of IgA2 plasma cells. J. Immunol. 1984;133:2312–2316. [PubMed] [Google Scholar]
- 57.Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. J. Clin. Invest. 2014;124:2325–2332. doi: 10.1172/JCI74475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raska M, Moldoveanu Z, Suzuki H, Brown R, Kulhavy R, Andrasi J, Hall S, Vu HL, Carlsson F, Lindahl G, Tomana M, Julian BA, Wyatt RJ, Mestecky J, Novak J. Identification and characterization of CMP-NeuAc:GalNAc-IgA1 α2,6-sialyltransferase in IgA1-producing cells. J. Mol. Biol. 2007;369:69–78. doi: 10.1016/j.jmb.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raska M, Yamada K, Horynova M, Takahashi K, Suzuki H, Moldoveanu Z, Novakova J, Kasperova A, Julian BA, Kiryluk K, Mestecky J, Renfrow MB, Gharavi AG, Novak J. Role of GalNAc-transferases in the synthesis of aberrant IgA1 O-glycans in IgA nephropathy. J. Am. Soc. Nephrol. 2011;22:625A. [Google Scholar]
- 60.Novakova J, Stewart T, Yamada K, Suzuki H, Moldoveanu Z, Julian BA, Novak J, Raska M. Overexpression of N-acetylgalactosaminyltransferase-14 contributes to galactose-deficient IgA1 production: relevance for IgA nephropathy. J. Am. Soc. Nephrol. 2013;24:492A. [Google Scholar]
- 61.Stuchlova Horynova M, Raska M, Clausen H, Novak J. Aberrant O-glycosylation and anti-glycan antibodies in an autoimmune disease IgA nephropathy and breast adenocarcinoma. Cell. Mol. Life Sci. 2013;70:829–839. doi: 10.1007/s00018-012-1082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada K, Reily C, Huang ZQ, Anderson JC, Raska M, Suzuki H, Julian BA, Willey CD, Novak J. Characterization of a signaling network that enhances production of galactose-deficient IgA1 in IgA1-secreting cells from patients with IgA Nephropathy. J. Am. Soc. Nephrol. 2015:591A. [Google Scholar]
- 63.Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, Cauerhff A, Danquah W, Rissiek B, Scheuplein F, Schwarz N, Adriouch S, Boyer O, Seman M, Licea A, Serreze DV, Goldbaum FA, Haag F, Koch-Nolte F. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 2009;198:157–174. doi: 10.1007/s00430-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vincke C, Muyldermans S. Introduction to heavy chain antibodies and derived Nanobodies. Methods. Mol. Biol. 2012;911:15–26. doi: 10.1007/978-1-61779-968-6_2. [DOI] [PubMed] [Google Scholar]
- 65.De Meyer T, Muyldermans S, Depicker A. Nanobody-based products as research and diagnostic tools. Trends. Biotechnol. 2014;32:263–270. doi: 10.1016/j.tibtech.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Novak J, Raskova Kafkova L, Suzuki H, Tomana M, Matousovic K, Brown R, Hall S, Sanders JT, Eison TM, Moldoveanu Z, Novak L, Novak Z, Mayne R, Julian BA, Mestecky J, Wyatt RJ. IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured human mesangial cells. Nephrol. Dial. Transplant. 2011;26:3451–3457. doi: 10.1093/ndt/gfr448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamouza H, Chemouny JM, Raskova Kafkova L, Berthelot L, Flamant M, Demion M, Mesnard L, Paubelle E, Walker F, Julian BA, Tissandie E, Tiwari MK, Camara NO, Vrtovsnik F, Benhamou M, Novak J, Monteiro RC, Moura IC. The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int. 2012;82:1284–1296. doi: 10.1038/ki.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim MJ, McDaid JP, McAdoo SP, Barratt J, Molyneux K, Masuda ES, Pusey CD, Tam FW. Spleen tyrosine kinase is important in the production of proinflammatory cytokines and cell proliferation in human mesangial cells following stimulation with IgA1 isolated from IgA nephropathy patients. J. Immunol. 2012;189:3751–3758. doi: 10.4049/jimmunol.1102603. [DOI] [PubMed] [Google Scholar]
- 69.Lechner SM, Abbad L, Boedec E, Papista C, Le Stang MB, Moal C, Maillard J, Jamin A, Bex-Coudrat J, Wang Y, Li A, Martini PG, Monteiro RC, Berthelot L. IgA1 protease treatment reverses mesangial deposits and hematuria in a model of IgA nephropathy. J. Am. Soc. Nephrol. 2016 doi: 10.1681/ASN.2015080856. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamm ME, Emancipator SN, Robinson JK, Yamashita M, Fujioka H, Qiu J, Plaut AG. Microbial IgA protease removes IgA immune complexes from mouse glomeruli in vivo: potential therapy for IgA nephropathy. Am. J. Pathol. 2008;172:31–36. doi: 10.2353/ajpath.2008.070131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eitner F, Floege J. Bacterial protease for the treatment of IgA nephropathy. Nephrol. Dial. Transplant. 2008;23:2173–2175. doi: 10.1093/ndt/gfn155. [DOI] [PubMed] [Google Scholar]
- 72.Reinholdt J, Kilian M. Titration of inhibiting antibodies to bacterial IgA1 proteases in human sera and secretions. Adv. Exp. Med. Biol. 1995;371A:605–608. doi: 10.1007/978-1-4615-1941-6_127. [DOI] [PubMed] [Google Scholar]
- 73.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen EV. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 74.Scivittaro V, Amore A, Emancipator SN. Animal models as a means to study IgA nephropathy. Contrib. Nephrol. 1993;104:65–78. doi: 10.1159/000422398. [DOI] [PubMed] [Google Scholar]
- 75.Iwasaki H, Zhang Y, Tachibana K, Gotoh M, Kikuchi N, Kwon YD, Togayachi A, Kudo T, Kubota T, Narimatsu H. Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-a-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2. J. Biol. Chem. 2003;278:5613–5621. doi: 10.1074/jbc.M211097200. [DOI] [PubMed] [Google Scholar]
- 76.Stuchlova Horynova M, Vrablikova A, Stewart TJ, Takahashi K, Czernekova L, Yamada K, Suzuki H, Julian BA, Renfrow MB, Novak J, Raska M. N-acetylgalactosaminide α2,6-sialyltransferase II is a candidate enzyme for sialylation of galactose-deficient IgA1,the key autoantigen in IgA nephropathy. Nephrol. Dial. Transplant. 2015;30:234–238. doi: 10.1093/ndt/gfu308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Novak J, Julian BA, Tomana M, Mestecky J. Progress in molecular and genetic studies of IgA nephropathy. J. Clin. Immunol. 2001;21:310–327. doi: 10.1023/a:1012284402054. [DOI] [PubMed] [Google Scholar]