Abstract
Relatively little is known about serotonergic involvement in pair-bonding despite its putative role in regulating social behavior. Here we sought to determine if pharmacological elevation of serotonin 1A (5-HT1A) receptor activity would lead to changes in social behavior in pair-bonded male titi monkeys (Callicebus cupreus). Adult males in established heterosexual pairs were injected daily with the selective 5-HT1A agonist 8-OH-DPAT or saline for 15 days using a within-subjects design. Social behavior with the female pair-mate was quantified, and plasma concentrations of oxytocin, vasopressin, and cortisol were measured. When treated with saline, subjects showed reduced plasma oxytocin concentrations, while 8-OH-DPAT treatment buffered this decrease. Treatment with 8-OH-DPAT also led to decreased plasma cortisol 15 minutes post-injection and decreased social behavior directed toward the pair-mate including approaching, initiating contact, lipsmacking, and grooming. The reduction in affiliative behavior seen with increased activity at 5-HT1A receptors indicates a substantial role of serotonin activity in the expression of social behavior. In addition, results indicate that the effects of 5-HT1A agonism on social behavior in adulthood differ between rodents and primates.
Keywords: Pair-bonding, oxytocin, vasopressin, cortisol, monogamy, 8-OH-DPAT, social behavior, primate
Introduction
Interpersonal relationships are greatly important for human health and wellbeing, and yet we know relatively little about the neurobiology that underlies such relationships. Social bonds play a significant role in human mental health; in adulthood these most commonly take the form of a close romantic relationship. Such relationships are one form of an attachment similar to that seen in filial or parental relationships (Hazan and Shaver, 1987). This “pair-bond”, the specific and enduring attachment relationship between an adult male and female, is found in many socially monogamous species. Animals which possess the capacity to form pair-bonds thus hold great utility for studying the underlying processes which are involved in pair-bonding as a means to understand these processes in humans.
The pair-bond has been extensively studied in rodents, particularly in the monogamous prairie vole (Aragona and Wang, 2004; Carter, 1998; Insel et al., 1998; Young et al., 2011; Young et al., 2001). This work indicates critical roles of multiple neurochemical systems in the regulation of behaviors associated with pair-bonding including oxytocin (Bales et al., 2007b; Keebaugh et al., 2015; Ross et al., 2009; Williams et al., 1994), vasopressin (Lim et al., 2004; Lim and Young, 2004; Pitkow et al., 2001), dopamine (Aragona and Wang, 2009; Hostetler et al., 2011; Resendez et al., 2016), and opioid systems (Burkett et al., 2011; Resendez et al., 2013; Resendez et al., 2016; Resendez et al., 2012). In contrast, no work to our knowledge has examined serotonergic involvement in pair-bonding in the prairie vole, and little work has examined serotonergic influences on social behavior more generally in this species. Of the few studies which have examined social behavior, two indicate that developmental interaction of oxytocin and serotonin underlie the neural substrates of social behavior (Eaton et al., 2012; Martin et al., 2012).
Serotonin plays important roles in a multitude of processes arising from the central nervous system including aggression, anxiety, mood, cognition, stress responses, and sexual behavior (Olivier, 2015), and is intimately involved in shaping social responses (Kiser et al., 2012). This variety of functions of serotonin is rooted in its widespread distribution throughout the brain and a diversity of receptor subtypes. There are 7 classes of serotonin receptors, 5-HT1-5-HT7, and all are g-protein coupled receptors except for 5-HT3. 5-HT1A is the primary inhibitory receptor in the serotonin system and exists both as a presynaptic autoreceptor in the raphe nuclei and as a postsynaptic heteroreceptor in higher brain areas. As a somatodendritic autoreceptor in the raphe nuclei, 5-HT1A receptors operate as part of a negative feedback loop which functions to inhibit further serotonin signaling. This makes the 5-HT1A receptor a powerful mediator of serotonergic activity throughout the brain, and has been theorized to underlie developmental changes to sociality in autism spectrum disorder (Khatri et al., 2014; Whitaker-Azmitia, 2005). However, the majority of studies in rodent species have indicated 5-HT1A agonism as having anxiolytic (Cheeta et al., 2001; Feighner and Boyer, 1989) and prosocial (Bell and Hobson, 1994; Olivier et al., 1989; Picazo et al., 1995; Thompson et al., 2007) effects during adulthood which are linked to acute oxytocin release (Jorgensen et al., 2003; Osei-Owusu et al., 2005; Uvnas-Moberg et al., 1996). It remains to be determined how serotonergic manipulations affect social behavior in a pair-bonding rodent.
Likewise, little is known about serotonergic involvement in pair-bonding in primates. Much of our understanding of pair-bonding in primates comes from the study of the socially monogamous titi monkey (Callicebus cupreus), which forms selective and enduring pair-bonds between a single adult male and female (Mason, 1966). This relationship, and attachment relationships more broadly, involve specific preference for and proximity maintenance to the attachment figure (Carp et al., 2015), physiological and affective response to involuntary separation from the attachment figure (Hennessy, 1997), and social buffering of stress responses in the presence of the attachment figure that does not occur in the presence of another individual (Mason and Mendoza, 1998).
The neurochemical systems involved in the pair-bond in titi monkeys indicate that pair-bonding in primates employs many of the same neural substrates as in pair-bonding in rodents (Bales et al., 2007a; Jarcho et al., 2011; Ragen et al., 2013). To our knowledge, no work has yet examined the involvement of serotonin in social behavior of a pair-bonding primate. This was addressed in the current study by treating adult male titi monkeys with the selective 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT) or saline vehicle in two experiments to examine its impact on behavior and peripheral hormone release.
Methods and Materials
Subjects
Subjects were nine adult male titi monkeys (Callicebus cupreus) housed at the California National Primate Research Center. Four males were used in experiment 1, and eight males were used in experiment 2. Three of the four males used in experiment 1 were also subjects in experiment 2.
Subjects had a mean age of 6.89 years (range = 2.9–14.4) at the start of participation in the experiment and had been housed with their female pair-mate for a mean (±SEM) of 23.07±6.28 months (range = 3.0–47.9). Female pair-mates were a mean age of 7.1 years (range = 2.0–14.0). Subjects’ mean (±SEM) weight at the start of the experiment was 1.28±0.04 kg. All males were housed continuously with their mates. Two males also had dependent offspring at the start of the study and during the course of experiment 2, two females gave birth causing their male pair-mates to be dropped from the study on the day of parturition. Table 1 provides information on group composition of all subjects.
Table 1.
Social composition and birth timing information.
| ♂ Subject | ♀ Pair-mate | Offspring sex | Offspring age (months) | Birth timing | |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| 34438 | 37623 | - | - | - | |
| 35915 | 37808 | - | - | - | |
| 36152 | 39529 | - | - | - | |
| 38640 | 39618 | - | - | - | |
| Experiment 2: | |||||
| 30410 | 36993 | - | - | - | |
| 34438 | 37623 | - | - | - | |
| 35383 | 30557 | - | - | - | |
| 36152 | 39529 | ♂ | 0* | 8-OH-DPAT day 2** | |
| 37728 | 31274 | ♂; ♂ | 8.1; 0* | Before start; Saline day 11** | |
| 38064 | 30496 | ♀ | 2.5 | Before start | |
| 38640 | 39618 | - | - | - | |
| 39552 | 40487 | - | - | - | |
infant birth, experiment in progress.
Testing ceased.
Animals were housed indoors identical to that reported in Mendoza (1999) in cages sized 1.2m x 1.2m x 3.1m on a 12:12 light: dark cycle with lights turned on at 0600 and off at 1800 (Valeggia et al., 1999). Subjects were fed a diet of new world monkey chow, apple, carrot, rice cereal, and banana at 0800 and 1300 daily. Water was available ad libitum. All procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee.
Experiment 1
The purpose of experiment 1 was to assess the behavioral and hormonal response to acute doses of 5-HT1A receptor agonist 8-OH-DPAT in male titi monkeys in order to determine an appropriate dose of 8-OH-DPAT to be used in experiment 2. Furthermore, we wanted to determine whether titi monkeys show the increased social behavior and increased peripheral OT that is seen in rodents.
Design and Procedures
In experiment 1, four male titi monkeys in established pair bonds (but with no dependent offspring) were given each of three doses of 8-OH-DPAT (0.05, 0.1, 0.5 mg/kg) or saline vehicle in randomized order with one week separating conditions. On each test day, each subject was captured in a transport box, manually restrained using leather handling gloves, given a subcutaneous (SC) injection of 8-OH-DPAT or saline vehicle at the nape of the neck, and baseline rectal temperature was taken. The subject was returned to the transfer box, placed in a quiet corner of the animal room, and covered with a towel during drug uptake. At 15 minutes post-injection, subjects were manually restrained and a 1 ml blood sample was collected via femoral venipuncture using a heparinized needle and a second rectal temperature was taken. The subject was then released back into his home cage with his female pair-mate, and the subject’s behavior was video recorded for 30 minutes (see Behavioral Observations and Quantification). After video recording, the subject was captured in a transport box, manually restrained, a second 1 ml blood sample was collected and a third rectal temperature was taken.
Experiment 2
The purpose of experiment 2 was to examine the effect of chronic treatment with 8-OH-DPAT on behavior within the pair-bond, peripheral hormone release, and the relationship between hormone release and behavioral changes. Chronic treatment was employed to examine the effect of autoregulatory negative feedback at the 5-HT1A receptor over time.
Design and Procedures
In experiment 2, 0.1 mg/kg 8-OH-DPAT or saline vehicle was administered SC once daily for 15 days to eight male titi monkeys in established pair bonds using a within-subjects design. Each subject received both conditions with a minimum of four weeks between conditions, and condition order was counterbalanced. All testing sessions occurred between 1000 and 1200 h. Daily testing sessions followed the same procedure as those used in experiment 1, with the exception that blood was collected on days 1, 3, 8, 10, and 15. For the two subjects with preexisting offspring (Table 1), offspring were removed from the home cage after the subject was removed from the cage and placed out of visual contact for the duration of the daily test session.
Drugs
8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT) hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% isotonic saline using gentle warming below 60º C. The 8-OH-DPAT solution was filtered using a 0.2 μm sterile syringe filter and stored at −20º C until use. 8-OH-DPAT and saline vehicle were injected SC at the nape of the neck in a volume of 0.1 ml/kg.
Blood Sampling and Hormone Analysis
Animals were trained to enter 0.3m x 0.3m x 0.3m transport boxes prior to the start of the experiment. Once captured, subjects were manually restrained using leather handling gloves and a 1 ml blood sample was collected via femoral venipuncture using a heparinized needle. Baseline blood samples were collected five days prior to the start of daily dosing. Blood samples were stored on ice and subsequently centrifuged at 4ºC, the plasma fraction extracted, and stored at −80º C until assay. Plasma concentrations of OT and AVP were measured in duplicate using commercial enzyme immunoassay kits (Enzo Life Sciences, Farmingdale, NY) previously validated for titi monkeys (Bales et al., 2005). Intra- and inter-assay coefficients of variation (Jordan et al.) were 2.05% and 6.69%, respectively for OT, and 3.63% and 10.64%, respectively for AVP. Plasma cortisol concentration was estimated in duplicate using commercial radioimmunoassay kits (Siemens Healthcare, Malvern, PA) previously validated for titi monkeys (Bales et al., 2007a; Hoffman et al., 1995; Jarcho et al., 2011; Ragen et al., 2013). Intra- and inter-assay CVs were 6.4% and 6.01% respectively.
Behavioral Observations and Quantification
Following drug injection and uptake in a transfer box, the male was returned to his home cage with his female pair-mate and behavior of the male was video recorded for 30 minutes. At a later time, a coder blind to drug condition quantified each subject’s affiliative, locomotor, sexual, aggressive, and arousal behavior (see ethogram in Table 2) from the focal video recordings using Behavior Tracker 1.5 software (behaviortracker.com). Rodent-specific behaviors associated with the serotonin syndrome (e.g. limb extensions, flat body posture, forepaw treading, and head weaving) which were not observed in experiment 1 were excluded from the ethogram, while those that were observed were included (twitching).
Table 2.
Ethogram for behavioral quantification.
| Behavior* | Description |
|---|---|
| Affiliative | |
| Proximity (d) | Male’s body is within arm’s length (6 inches) of female’s body excluding the tail. |
| Contact (d) | Male’s body is in contact with female. |
| Male Grooms Female (d) | Male examines/picks through hair of female by parting fur with hands or mouth. |
| Female Grooms Male (d) | Female examines/picks through hair of male by parting fur with hands or mouth. |
| Male Approach | Male moves to within proximity (6 inches) of female and remains in proximity for 1 second. |
| Female Approach | Female moves to within proximity (6 inches) of male and remains in proximity for 1 second. |
| Male Leave | Male withdraws from proximity (6 inches) of female. |
| Female Leave | Female withdraws from proximity (6 inches) of male. |
| Tail Twine (d) | Male and female in contact wrap tails around one another forming at least one full rotation. |
| Lipsmack | Male makes rapid lip movement accompanied by smacking sound. |
| Initiate contact | Male moves any part of body into physical contact with female. |
| Withdraw from Contact | Male withdraws all body parts from contact with female. |
| Solicit Grooming | Male stretches out body or limbs to invite grooming from female. |
| Arousal | |
| Back Arch | Male raises dorsal surface of the back. May be accompanied by piloerection. |
| Tail Lash | Lateral whipping of the tail to at least 45°. |
| Sexual | |
| Anogenital Explore (d) | Male inspects female’s ano-genital region visually, orally, olfactorily, or manually. |
| Mount/Sex (d) | Engagement in copulation or behaviors that could lead to copulation including mount, thrust, or insertion. |
| Aggressive | |
| Grab/Hit/Bite | Male grabs, hits, or bites female. Low intensity display of aggression. |
| Locomotor | |
| Locomotion (d) | Male moves at least one body length. |
| Other | |
| Eat (d) | Male touches or eats food, includes time spent chewing. |
| Drink (d) | Male drinks water. Begins when mouth touches lixit and ends when drinking terminates. |
| Twitch | Male’s hands, feet, or tail make a rapid twitching movement. |
Behaviors marked (d) were measured as duration. All others were measured as frequency.
Body Temperature
In order to assess the hypothermic effects of 8-OH-DPAT, rectal body temperature was collected at three time points with a Mabis 8-Second Ultra-Premium digital thermometer (Briggs Healthcare, Waukegan, IL): immediately post-injection, 15 minutes post-injection at peak effect, and 45 minutes post-injection.
Data Analysis
Statistical analyses were carried out using generalized linear mixed models (GLMM) for both experiments in SAS 9.4 (SAS Institute, Cary, NC) utilizing backward selection to eliminate non-significant variables from the model. For both experiments, the GLMM included animal ID (identification) as a random factor. For experiment 1, the model included drug treatment (saline or 0.05, 0.1, or 0.5 mg/kg 8-OH-DPAT) in addition to animal ID. The dependent variables for both experiments were body temperature, hormones, and behavior. For behavioral analyses in experiment 2, the model included day of treatment (1–15), drug condition (saline or 0.1 mg/kg 8-OH-DPAT), a drug condition by day interaction term, and order of condition (saline first or 8-OH-DPAT first). Duration of locomotion was also included in the model as a covariate to control for motor effects of 8-OH-DPAT on behavior. Twitching did not significantly account for any additional variability and was thus not included in the model. Locomotion, order of condition, the interaction term, day of treatment, and animal ID were removed from the model if non-significant in this order. In both experiments, hormones were examined as change from baseline concentrations and change from 15 to 45 minutes post-injection to account for trait-like individual differences in OT and AVP, and to examine stress reactivity in cortisol. For these hormone analyses, drug condition, day of treatment, a drug condition by day interaction term and order of condition were included in the model. Time (referring to 15 or 45 minutes post-injection) was also included in the model, and removed if non-significant.
To examine hormonal effects on behavior in experiment 2, separate analyses were conducted including only days on which both hormones and behavior were concurrently measured (days 1, 3, 8, 10, and 15). All behaviors were analyzed using identical modeling procedures as those used for behavior mentioned above, with change in OT, AVP and cortisol concentration included in the model in this order and removed if non-significant. If data were not normally distributed, square root, quad root, or log transformations were used.
If the data could not be transformed to normality, a GLMM was still used as recommended by Feir-Walsh and Toothaker, 1974 (Feir-Walsh and Toothaker, 1974). Post-hoc analyses utilized least squares means when the omnibus test was significant. False discovery rate was used to correct for multiple comparisons (Benjamini and Hochberg, 1995). Effect size estimations utilized Cohen’s d for pairwise comparisons and η2 for within-subjects data even when analyzed using a mixed model to prevent data loss due to missing data. Effect size estimations for data analyzed in a truly mixed model utilized ηp2 to approximate relative contribution of the individual predictor, and should be interpreted carefully given the mixed design employed for these analyses. Statistical significance was set at p<.05, and all tests were two-tailed.
Results
Experiment 1
Acute 8-OH-DPAT treatment at any dose did not significantly alter involuntary motor movement or affiliative behavior, nor was there a significant effect on plasma OT or AVP concentrations compared to saline (Table 3). However, the 0.1 mg/kg dose of 8-OH-DPAT led to a 24.2% greater plasma OT concentration than saline and was thus selected to be used in experiment 2 despite statistical non-significance. Body temperature was not significantly altered by any dose of 8-OH-DPAT compared to saline.
Table 3.
Results of Experiment 1.
| Behavior | Condition
|
DF | F-statistic | P-value | |||
|---|---|---|---|---|---|---|---|
| Saline | 0.05 mg/kg | 0.1 mg/kg | 0.5 mg/kg | ||||
| Proximity | 567.3±426.3 | 606.0±428.1 | 450.0±282.1 | 398.5±158.8 | 3,9 | 0.08 | 0.97 |
| Contact (d) | 541.3±259.5 | 144.3±48.4 | 248.3±32.2 | 270.0±100.7 | 3,9 | 1.60 | 0.26 |
| Male Grooms Female (d) | 4.8±3.8 | 11.3±11.3 | 3.8±3.8 | 0±0 | 3,9 | 0.98 | 0.45 |
| Female Grooms Male (d) | 49.0±15.7 | 17.3±12.4 | 44.0±20.1 | 38.8±10.4 | 3,9 | 1.01 | 0.33 |
| Male Approach | 7.5±3.6 | 9.0±5.0 | 12.8±3.9 | 4.8±3.8 | 3,9 | 0.57 | 0.65 |
| Female Approach | 11.8±0.9 | 14.5±5.2 | 27.8±6.4 | 17.5±6.6 | 3,11 | 0.90 | 0.47 |
| Male Leave | 7.8±2.5 | 7.3±3.7 | 17.0±6.1 | 5.5±5.5 | 3,11 | 0.26 | 0.85 |
| Female Leave | 11.0±2.7 | 16.5±3.3 | 22.0±5.6 | 16.3±4.7 | 3,11 | 0.73 | 0.55 |
| Tail Twine (d) | 504.8±216.4 | 324.5±190.9 | 419.0±205.2 | 280.3±276.3 | 3,9 | 0.41 | 0.75 |
| Lipsmack | 1.0±0.7 | 0±0 | 1.8±0.6 | 0.5±0.5 | 3,9 | 2.21 | 0.16 |
| Solicit Grooming | 0.3±0.3 | 0.5±0.5 | 0±0 | 0±0 | 3,11 | 0.63 | 0.61 |
| Back Arch | 0.3±0.3 | 0±0 | 0.3±0.3 | 0±0 | 3,11 | 0.72 | 0.56 |
| Tail Lash | 0.3±0.3 | 0.3±0.3 | 0±0 | 0±0 | 3,12 | 0.67 | 0.59 |
| Grab/Hit/Bite | 0.5±0.3 | 0±0 | 1.0±1.0 | 0±0 | 3,12 | 0.85 | 0.49 |
| Locomotion (d) | 104.5±42.2 | 63.0±32.0 | 389.3±311.8 | 52.8±50.4 | 3,9 | 0.98 | 0.44 |
| Twitch | 21.8±19.8 | 20.3±18.9 | 74.3±58.1 | 24.8±16.0 | 3,9 | 0.61 | 0.62 |
| Hormone Concentration | |||||||
| Oxytocin | 923.7±196.6 | 941.3±209.3 | 949.5±193.2 | 789.0±107.4 | 2.9 | 0.24 | 0.79 |
| Vasopressin | 450.2±132.9 | 345.5±94.5 | 374.9±77.6 | 601.3±115.4 | 2,9 | 3.71 | 0.07 |
| Cortisol | 37.0±1.7 | 59.9±8.2 | 42.2±4.1 | 55.3±7.2 | 2,9 | 1.79 | 0.22 |
(d) behavior measured in duration in seconds
Experiment 2
Indicators of the Serotonin Syndrome
We examined body temperature and behavioral changes caused by treatment with 8-OH-DPAT indicative of the serotonin syndrome. High levels of 5-HT activity can alter an individual’s ability to move, thus we wanted to make sure that social behavior was not reduced due to locomotor impairment or limb twitching. The frequency of twitching of the hands, feet, and tail was greater in the 8-OH-DPAT group on day one (mean ± SEM = 349.38 ± 226.86) compared to the saline condition on day one (mean ± SEM = 6.38±5.68), and on day one in the 8-OH-DPAT condition compared to all other days (F1,201=16.67, p<.0001, ηp2=0.06). Other effects of 5-HT1A stimulated serotonin syndrome typically seen in rodents, such as limb extensions, flat body posture, forepaw treading, or head weaving, were not seen during the course the experiment.
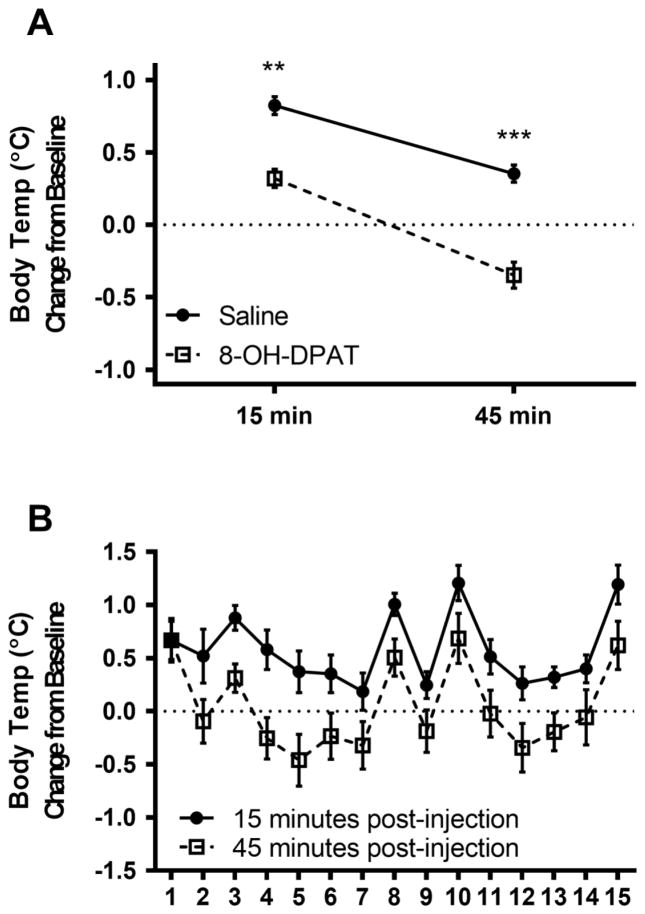
Body temperature was increased from baseline at 15 minutes post-injection, but more so in the saline condition than 8-OH-DPAT condition (F1,219=35.94, p<.001, η2=0.10; Figure 1). Body temperature was significantly altered by condition at 45 minutes post-injection (F1,219=51.40, p<.0001, η2=0.13), with a decrease in the 8-OH-DPAT condition and an increase in the saline condition compared to baseline. There was a significant reduction in body temperature from 15 to 45 minutes post-injection in both the 8-OH-DPAT condition (t=9.66, p<.0001, d=0.53) and the saline condition (t=8.36, p<.0001, d=0.53), but this reduction was significantly greater in the 8-OH-DPAT condition than the saline condition (F1,219=4.56, p<.05, η2=0.02).
Figure 1.
A) Mean (±SEM) body temperature change from baseline at 15 and 45 minutes post-injection comparing saline and 8-0H-DPAT treatment. B) Mean (±SEM) body temperature across 15 days of treatment. **p<.01, ***p<.0001.
There was a significant effect of day of treatment on body temperature 15 minutes post-injection (F14, 219=5.45, p<.001, η2=0.22) and 45 minutes post-injection (F14,219=4.72, p<.001, η2=0.17), but did not interact with drug condition (Figure 1B).
Behavior
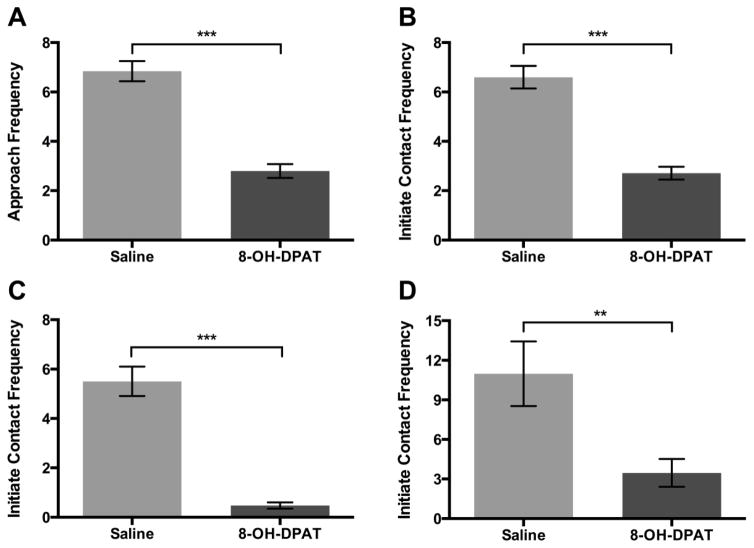
Drug condition significantly altered locomotor and affiliative behaviors. 8-OH-DPAT decreased duration of locomotion compared to saline (F1,211=8.62, p<.01, η2=0.18). Thus, locomotion was included in the subsequent models in order to control for sedative effects of 8-OH-DPAT on other behavior. Subjects approached their pair-mate less frequently in the 8-OH-DPAT condition compared to saline (F1,197 = 79.11, p<.0001, η2=0.22, Figure 2A). There was no significant interaction of day by condition for subjects approaching the pair-mate (F14,183= 0.99, p=.46). Subjects initiated physical contact with their pair-mate less frequently in the 8-OH-DPAT condition compared to saline (F1,187 = 59.94, p<.0001, η2=0.19 Figure 2B). Subjects also lipsmacked less frequently in the 8-OH-DPAT condition (F1,211 = 72.24, p<.0001, η2=0.21, Figure 2C) and groomed their pair-mate for a shorter duration (F1,218 = 7.46, p<.01, η2=0.03 Figure 1D) compared to saline. There was no significant effect of drug condition on any other affiliative behaviors measured.
Figure 2.
A) Mean (±SEM) frequency of subject approach to female pair-mate comparing saline and 8-OH-DPAT treatment. B) Mean (±SEM) frequency of subject initiation of physical contact with pair-mate comparing saline and 8-OH-DPAT treatment. C) Mean (±SEM) frequency of subject lipsmack comparing saline and 8-OH-DPAT treatment. D) Mean (±SEM) duration in seconds of subject grooming female pair-mate comparing saline and 8-OH-DPAT treatment. **p<.01, ***p<.0001.
Female approach frequency was not altered by their pair-mates’ drug condition (F1,203 = 2.17, p=.14), nor did subjects’ approach frequency alter the rate at which females approached their pair-mate (F1,202 = 0.01, p=.92). Females’ leave frequency was altered by subjects drug condition (F1,208 = 3.80, p=.05, η2<.01) with females leaving proximity of their pair-mate more often in the 8-OH-DPAT condition.
Subjects displayed fewer behaviors indicative of arousal with 5-HT1A agonism. Subjects did significantly fewer back-arches (F1,197 = 7.22, p<.01, η2=0.03) and tail lashes (F1,211 = 27.14, p<.0001, η2=0.09) in the 8-OH-DPAT condition compared to the saline condition. Subjects also showed decreased sexual behavior, spending less time inspecting the anogenital region of their pair-mate (F1,211 = 16.62, p<.0001, η2=0.06), and displayed fewer mounting attempts/copulations (F1,211 = 12.78, p<.001, η2=0.05) with 8-OH-DPAT treatment. There was no significant effect of drug condition on aggressive behavior.
Day of treatment altered subjects’ frequency of approach to the pair-mate (F14,197 = 2.97, p<.001, η2=0.11), initiation of physical contact (F1,197 = 2.13, p=.01, η2=0.09), and back-arching (F14,197 = 1.98, p<.05, η2=0.10) such that these behaviors were more frequent at the beginning of daily testing and then stabilized at lower levels. Other social, arousal, or sexual behaviors were not significantly impacted by day of treatment. There was no significant interaction of drug treatment and day of treatment on any behaviors examined.
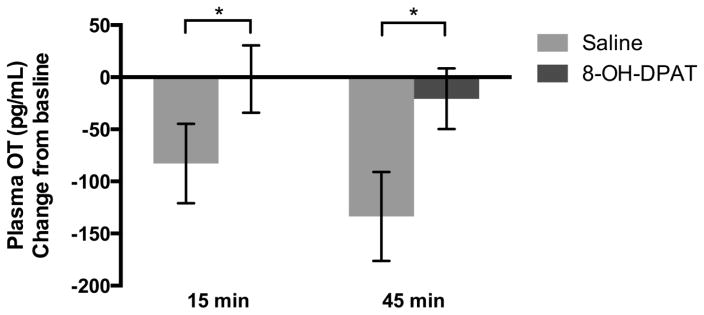
Oxytocin
Mean change in OT concentration (Figure 3) from baseline to 15 minutes post-injection was lower in the saline condition compared to the 8-OH-DPAT condition (F1,61 =4.49, p<.05, ηp2=0.07). In the saline condition, mean change in plasma OT was negative because it was lower at 15 minutes post-injection compared to baseline. In the 8-OH-DPAT condition, mean plasma OT was not different from baseline. Mean change in OT concentration from baseline to 45 minutes post-injection was also lower in the saline condition compared to the 8-OH-DPAT condition (F1,61=11.86, p=.001, ηp2=0.16).
Figure 3.
Mean (±SEM) plasma oxytocin concentration change from baseline to 15 and 45 minutes post-injection comparing saline and 8-OH-DPAT treatment.*p<.05.
Order of drug treatment altered mean OT concentration change from baseline 15 minutes post-injection. Subjects treated with 8-OH-DPAT prior to saline had lower OT concentration than subjects treated with saline first (F1,61=5.03, p<.05, ηp2=0.08).
When we compared OT concentration change from 15 min to 45 min, we observed no significant effect of drug condition. There was no significant effect of day of treatment (F4,58 = 1.10, p=.36) or an interaction of drug condition and day of treatment on change in OT concentrations (F4,58 = 1.10, p=.36).
Vasopressin
Mean AVP concentration change from baseline to 15 min post-injection was not different with 8-OH-DPAT compared to saline treatment (F1,65 = 1.78, p=.19). There was no significant effect of day of treatment at 15 minutes post-injection (F4,58=1.16, p=.34) or 45 minutes post-injection (F1,65=.06, p=.08), nor was there an interaction of drug condition and day of treatment at 15 minutes post-injection (F4,58=.40, p=.80) or 45 minutes post-injection (F4,58=1.27, p=.29).
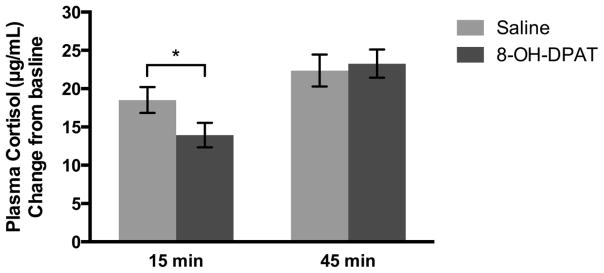
Cortisol
We found a time by condition interaction (F1,131 = 4.28, p<.05, ηp2=0.03, Figure 4) such that the increase in cortisol was greater in the saline condition at 15 minutes post-injection compared to 8-OH-DPAT, while there was no difference between saline and 8-OH-DPAT at 45 minutes post-injection (F1,131 = 0.78, p=.38). However, there was a significant increase in cortisol from 15 to 45 minutes post-injection (F1,135=23.90, p<.0001, ηp2=0.08) which was greater with 8-OH-DPAT treatment compared to saline (t=3.49, p<.001, d=0.47). Day of treatment had no effect on plasma cortisol (F4,135 = 0.42, p<.79).
Figure 4.
Mean (±SEM) plasma cortisol concentration change from baseline to 15 and 45 minutes post-injection comparing saline and 8-OH-DPAT treatment. *p<.05.
Increased mean change in cortisol concentration from baseline to 15 minutes significantly increased frequency of male approach (F1,60=11.96, p=.001, ηp2=0.17) and decreased duration of mounting attempts/copulation (F1,64=6.54, p=.01, ηp2=0.09).
Discussion
The present study investigated whether chronic 5-HT1A agonism altered social behavior in pair-bonded adult male titi monkeys. As this was the first study to examine serotonergic manipulation in titi monkeys, we sought to confirm the activity of 8-OH-DPAT by evaluating measures indicative of the serotonin syndrome. In experiment 2, we found greater twitching on day 1 with 8-OH-DPAT treatment compared to saline and all other days of 8-OH-DPAT treatment, as well as lower body temperature with 8-OH-DPAT treatment compared to saline at both 15 and 45 minutes post-injection. Together these indicate that 8-OH-DPAT worked as predicted in titi monkeys, acutely inducing both spontaneous motor behavior and hypothermia characteristic of the serotonin syndrome.
In rodents, 5-HT1A agonists acutely increase social behavior and peripheral OT (Bagdy and Kalogeras, 1993; Bell and Hobson, 1994; Jorgensen et al., 2003; Picazo et al., 1995; Thompson et al., 2007), but here we found that treatment with 8-OH-DPAT in the titi monkey does not increase peripheral OT, nor does it increase social behavior acutely. Rather, it had the opposite effect, inhibiting affiliative behavior directed at the pair-mate in pair-bonded males. This result suggests substantial differences between rodents and primates with regard to the 5-HT1A receptor. Indeed, in female marmosets, chronic 8-OH-DPAT treatment induces social rejection (Aubert et al., 2013), suggesting the effect of 5-HT1A agonism is consistent across new world primates and indicating the utility of primate species as translational models for humans in this area (Phillips et al., 2014).
Interestingly, day of treatment had minimal impact on behavior or hormones across 15 days of chronic treatment. Autoregulatory negative feedback caused by chronic activity at the 5-HT1A receptor is thought to decrease serotonergic activity (McDevitt and Neumaier, 2011) and synthesis (Brambilla et al., 1999), ultimately leading to a reduction in sociality. Thus we expected that, like is seen in rodents, 8-OH-DPAT would cause an acute increase in social behavior, followed by decreasing sociality with adaptation to reduced serotonergic activity consequent to negative feedback. We did not find significant interactions of day of treatment and drug condition in any behaviors or peripheral hormone concentrations examined here, indicating that the suppression of sociality seen was not due to adaptation consequent to negative feedback.
In addition to suppression of social behavior, we found that arousal and sexual behavior were decreased by 8-OH-DPAT treatment. 8-OH-DPAT functions as an agonist at both pre- and postsynaptic 5-HT1A receptors, however both sexual behavior and arousal are postulated to be influenced specifically by presynaptic 5-HT1A receptor activity (De Vry, 1995). 8-OH-DPAT has higher selectivity for presynaptic than postsynaptic receptors, making a presynaptic mechanism likely to have been responsible for these changes. In addition, 8-OH-DPAT may be active at both postsynaptic 5-HT1A and 5-HT7 receptors (Bard et al., 1993). Although it has higher affinity for 5-HT1A compared to 5-HT7, the action of 8-OH-DPAT may be more complex than intended. Future work should utilize pharmacological agents with more specific activity, or concurrently block activity at 5-HT7 receptors to more clearly elucidate the mechanism of action responsible for changes to behavior.
We examined cortisol and AVP because they are stress responsive and interact with the serotonin system. The lack of a change in AVP is not surprising given that the 5-HT1A receptor does not directly act to stimulate AVP release (Jorgensen et al., 2003). In addition to being stress responsive, it is also closely related to oxytocin activity, thus we chose to examine it nonetheless, but did not find any alteration dependent on drug condition. In rodents, 8-OH-DPAT increases plasma corticosterone in a dose-dependent manner (Fuller and Snoddy, 1990; Gehlert and Shaw, 2014; Przegalinski et al., 1989; Vicentic et al., 1998). Here we found that the increased cortisol concentration from baseline to 15 minutes was greater with saline treatment than with 8-OH-DPAT, but that the increase from 15 to 45 minutes post-injection was greater with 8-OH-DPAT treatment. The 15-minute post-injection time point may have been too early for plasma cortisol concentration to reflect changes in hypothalamic-pituitary-adrenal (HPA) activity subsequent to the initial stressor, while the 45-minute time point concentration likely reflected response to the multiple and repeated stressors employed in our testing procedure, including a social stressor (separation from the pair-mate) and physical stressors (capture, restraint, injection, blood draw, rectal temperature) across multiple time points. Thus the increased cortisol concentration from 15 to 45 minutes post-injection with 8-OH-DPAT treatment does not reflect response to any single stressor, nor can it be tied to a specific time point as its origin. The greater increase from 15 to 45 minutes post-injection with 8-OH-DPAT suggests that 5-HT1A agonism alters hypothalamic-pituitary-adrenal axis activity in a manner consistent with the response in rodents. However, it is also possible that the alterations to social behavior found with 8-OH-DPAT treatment precluded the social buffering of HPA activity typically found in the presence of the pair-mate in titi monkeys (Hennessy et al., 1995; Ragen et al., 2013).
This study was the first to examine the effects of 5-HT1A agonism on pair-bonding behaviors in a monogamous primate. We found a reduction across multiple social behaviors in 8-OH-DPAT treated males, indicating that 5-HT1A activity is involved in the regulation of behaviors expressed in the pair-bond. Greater understanding of the mechanisms underlying serotonergic effects on sociality is needed to more fully elucidate how it is involved in pair-bonding, and in sociality more generally. This in turn, will be important to understanding the neurobiological basis of adult attachment relationships and their impacts on health.
Highlights.
Agonism of 5-HT1A receptors decreases adult social behavior in titi monkeys.
Serotonin activity plays an important role in social behavior within the pair-bond.
Effects of 5-HT1A agonism on social behavior differ between rodents and primates.
Acknowledgments
We would like give thanks to the animal care and veterinary staff at the California National Primate Research Center, especially Dr. Angela Colagross-Schouten. Also special thanks to Emily Rothwell, Sarah Carp, Thomas Schaefer, Carlos Almeida, and Luana Griffin. Funding: NICHD HD053555, Good Nature Institute, Autism Science Foundation, and NIH P51OD011107 to the California National Primate Research Center.
Footnotes
Financial Disclosures
We have no disclosures or conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aragona BJ, Wang Z. The prairie vole (Microtus ochrogaster): An animal model for behavioral neuroendocrine research on pair bonding. ILAR Journal. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Frontiers in behavioral neuroscience. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert Y, Allers KA, Sommer B, de Kloet ER, Abbott DH, Datson NA. Brain region-specific transcriptomic markers of serotonin-1A receptor agonist action mediating sexual rejection and aggression in female marmoset monkeys. The journal of sexual medicine. 2013;10:1461–1475. doi: 10.1111/jsm.12131. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Kalogeras KT. Stimulation of 5-HT1A and 5-HT2/5-HT1C receptors induce oxytocin release in the male rat. Brain research. 1993;611:330–332. doi: 10.1016/0006-8993(93)90521-n. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Hostetler CM, Capitanio JP, Mendoza SP. Validation of oxytocin and vasopressin plasma assays for primate: what can blood tell us? American journal of primatology. 2005;66:73. [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain research. 2007a;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Hormones and behavior. 2007b;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. The Journal of biological chemistry. 1993;268:23422–23426. [PubMed] [Google Scholar]
- Bell R, Hobson H. 5-HT1A receptor influences on rodent social and agonistic behavior: a review and empirical study. Neuroscience and biobehavioral reviews. 1994;18:325–338. doi: 10.1016/0149-7634(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Brambilla A, Baschirotto A, Grippa N, Borsini F. Effect of flibanserin (BIMT 17), fluoxetine, 8-OH-DPAT and buspirone on serotonin synthesis in rat brain. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1999;10:63–67. doi: 10.1016/s0924-977x(99)00056-5. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of mu-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E, Bales KL. Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus) American journal of primatology. 2015 doi: 10.1002/ajp.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Tucci S, Sandhu J, Williams AR, Rupniak NMJ, File SE. Anxiolytic actions of the substance P (NK1) receptor antagonist L-760735 and the 5-HT1A agonist 8-OH-DPAT in the social interaction test in gerbils. Brain research. 2001;915:170–175. doi: 10.1016/s0006-8993(01)02846-3. [DOI] [PubMed] [Google Scholar]
- De Vry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology. 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- Eaton JL, Roache L, Nguyen KN, Cushing BS, Troyer E, Papademetriou E, Raghanti MA. Organizational effects of oxytocin on serotonin innervation. Developmental psychobiology. 2012;54:92–97. doi: 10.1002/dev.20566. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Boyer WF. Serotonin-1A anxiolytics: an overview. Psychopathology. 1989;22:21–26. doi: 10.1159/000284623. [DOI] [PubMed] [Google Scholar]
- Feir-Walsh BJ, Toothaker LE. An empirical comparison of the ANOVA F-test, normal scores test, and Kruskal Wallis test under violation of assumptions. Educational Psychological Measures. 1974:789–799. [Google Scholar]
- Fuller RW, Snoddy HD. Serotonin receptor subtypes involved in the elevation of serum corticosterone concentration in rats by direct- and indirect-acting serotonin agonists. Neuroendocrinology. 1990;52:206–211. doi: 10.1159/000125586. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Shaw J. 5-Hydroxytryptamine 1A (5HT1A) receptors mediate increases in plasma glucose independent of corticosterone. European journal of pharmacology. 2014;745:91–97. doi: 10.1016/j.ejphar.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Hazan C, Shaver P. Romantic love conceptualized as an attachment process. Journal of personality and social psychology. 1987;52:511. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Hypothalamic-Pituitary-Adrenal Responses to Brief Social Separation. Neuroscience & Biobehavioral Reviews. 1997;21:11–29. doi: 10.1016/s0149-7634(96)00013-9. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Mendoza SP, Mason WA, Moberg GP. Endocrine sensitivity to novelty in squirrel monkeys and titi monkeys: species differences in characteristic modes of responding to the environment. Physiology & behavior. 1995;57:331–338. doi: 10.1016/0031-9384(94)00250-9. [DOI] [PubMed] [Google Scholar]
- Hoffman KA, Mendoza SP, Hennessy MB, Mason WA. Responses of infant titi monkeys, Callicebus moloch, to removal of one or both parents: evidence for paternal attachment. Developmental psychobiology. 1995;28:399–407. doi: 10.1002/dev.420280705. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Harkey SL, Krzywosinski TB, Aragona BJ, Bales KL. Neonatal exposure to the D1 agonist SKF38393 inhibits pair bonding in the adult prairie vole. Behavioural pharmacology. 2011;22:703–710. doi: 10.1097/FBP.0b013e32834affd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Wang Z, Young LJ. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Advances in experimental medicine and biology. 1998;449:215–224. doi: 10.1007/978-1-4615-4871-3_28. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes, brain, and behavior. 2011;10:375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Cvejic S, Devi LA. Opioids and their complicated receptor complexes. Neuropsychopharmacology. 2000;23:S5–S18. doi: 10.1016/S0893-133X(00)00143-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Riis M, Knigge U, Kjaer A, Warberg J. Serotonin receptors involved in vasopressin and oxytocin secretion. Journal of neuroendocrinology. 2003;15:242–249. doi: 10.1046/j.1365-2826.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Social neuroscience. 2015;10:561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri N, Simpson Kl, Lin RCS, Paul IA. Lasting neurobehavioral abnormalities in rats after neonatal activation of serotonin 1A and 1B receptors: possible mechanisms for serotonin dysfunction in autistic spectrum disorders. 2014 doi: 10.1007/s00213-013-3242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser D, SteemerS B, Branchi I, Homberg JR. The reciprocal interaction between serotonin and social behaviour. Neuroscience & Biobehavioral Reviews. 2012;36:786–798. doi: 10.1016/j.neubiorev.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Lim MM, Hammock EA, Young LJ. The role of vasopressin in the genetic and neural regulation of monogamy. Journal of neuroendocrinology. 2004;16:325–332. doi: 10.1111/j.0953-8194.2004.01162.x. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Martin MM, Liu Y, Wang Z. Developmental exposure to a serotonin agonist produces subsequent behavioral and neurochemical changes in the adult male prairie vole. Physiology & behavior. 2012;105:529–535. doi: 10.1016/j.physbeh.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA. Social organization of the south American monkey, Callicebus Moloch: a preliminary report. Tulane Studies in Zoology. 1966:5. [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology. 1998;23:765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Neumaier JF. Regulation of dorsal raphe nucleus function by serotonin autoreceptors: A behavioral perspective. Journal of Chemical Neuroanatomy. 2011;41:234–246. doi: 10.1016/j.jchemneu.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B. Serotonin: A never-ending story. European journal of pharmacology. 2015;753:2–18. doi: 10.1016/j.ejphar.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van der Heyden J, Hartog J. Serotonergic modulation of social interactions in isolated male mice. Psychopharmacology. 1989;97:154–156. doi: 10.1007/BF00442239. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, James A, Crane J, Scrogin KE. 5-Hydroxytryptamine 1A receptors in the paraventricular nucleus of the hypothalamus mediate oxytocin and adrenocorticotropin hormone release and some behavioral components of the serotonin syndrome. The Journal of pharmacology and experimental therapeutics. 2005;313:1324–1330. doi: 10.1124/jpet.104.082073. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. Why primate models matter. American journal of primatology. 2014 doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picazo O, Lopez-Rubalcava C, Fernandez-Guasti A. Anxiolytic effect of the 5-HT1A compounds 8-hydroxy-2-(di-n-propylamino) tetralin and ipsapirone in the social interaction paradigm: evidence of a presynaptic action. Brain research bulletin. 1995;37:169–175. doi: 10.1016/0361-9230(94)00273-4. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegalinski E, Budziszewska B, Warchol-Kania A, Blaszczynska E. Stimulation of corticosterone secretion by the selective 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the rat. Pharmacology, biochemistry, and behavior. 1989;33:329–334. doi: 10.1016/0091-3057(89)90509-1. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus) Psychoneuroendocrinology. 2013;38:2448–2461. doi: 10.1016/j.psyneuen.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA, Aragona BJ. Mu-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. The Journal of neuroscience. 2013;33:9140–9149. doi: 10.1523/JNEUROSCI.4123-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Eidson L, Porter-Stransky KA, Nevarez N, McLean JW, Kuhnmuench MA, Murphy AZ, Mathews TA, Aragona BJ. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife. 2016:5. doi: 10.7554/eLife.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. kappa-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Hillegaart V, Alster P, Ahlenius S. Effects of 5-HT agonists, selective for different receptor subtypes, on oxytocin, CCK, gastrin and somatostatin plasma levels in the rat. Neuropharmacology. 1996;35:1635–1640. doi: 10.1016/s0028-3908(96)00078-0. [DOI] [PubMed] [Google Scholar]
- Valeggia CR, Mendoza SP, Fernandez-Duque E, Mason WA, Lasley B. Reproductive biology of female titi monkeys (Callicebus moloch) in captivity. American journal of primatology. 1999;47:183–195. doi: 10.1002/(SICI)1098-2345(1999)47:3<183::AID-AJP1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Li Q, Battaglia G, Van de Kar LD. WAY-100635 inhibits 8-OH-DPAT-stimulated oxytocin, ACTH and corticosterone, but not prolactin secretion. European journal of pharmacology. 1998;346:261–266. doi: 10.1016/s0014-2999(97)01607-5. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Journal of neuroendocrinology. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Frontiers in neuroendocrinology. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Hormones and behavior. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]