Abstract
The clinical syndromes comprising urinary tract infection (UTI) continue to exert significant impact on millions of patients worldwide, most of whom are otherwise healthy women. Antibiotic therapy for acute cystitis does not prevent recurrences, which plague up to one fourth of women after an initial UTI. Rising antimicrobial resistance among uropathogenic bacteria further complicates therapeutic decisions, necessitating new approaches based on fundamental biological investigation. In this review, we highlight contemporary advances in the field of UTI pathogenesis and how these might inform both our clinical perspective and future scientific priorities.
Keywords: urinary tract infection, Escherichia coli, cystitis, pyelonephritis
A Pervasive and Persistent Problem
Urinary tract infections (UTIs) are among the most common bacterial infections, affecting 150 million people worldwide each year [1–3]. Although both men and women may become infected, UTIs are traditionally thought of as a disease of women, among whom 50% will be affected across their lifespan [2]. Approximately 25% of women presenting with a first episode of bacterial cystitis (see Glossary) go on to suffer recurrent UTI within 6 months, some having 6 or more infections in the year following the initial episode [2, 4]. Current therapeutics are suboptimal, as the prevalence of multidrug-resistant uropathogens is increasing and antibiotic treatment for acute infection does not preclude recurrences [2, 5, 6]. These recalcitrant infections can become a significant health problem and diminish quality of life for affected men and women (Box 1).
Box 1. The Clinician’s Corner.
Common uropathogenic bacteria, including Escherichia coli, multiply within the cytoplasm of bladder epithelial cells during acute cystitis.
In relevant animal models, oral antibiotic therapy for acute cystitis does not completely eradicate E. coli from bladder tissue, perhaps enabling same-strain recurrent cystitis.
New therapeutics currently in development aim to target adhesive surface factors of E. coli, such as pili; vaccine targets including pili, siderophores and toxins are also being studied.
The bladder, rather than representing a sterile environment, may in fact host a “urinary microbiome” of commensal organisms that may influence UTI and other symptomatic urinary tract conditions.
Recent laboratory advances now permit the modeling of recurrent UTI, ascending renal abscess formation, and catheter-associated UTI in mice.
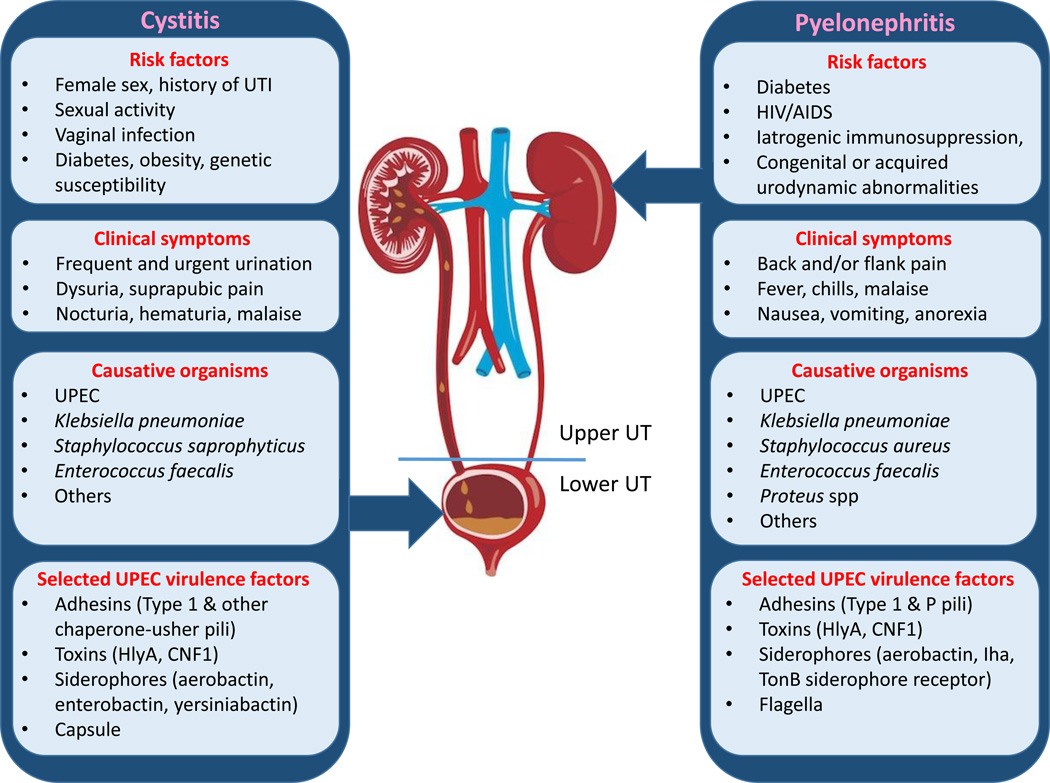
Bacterial infections of the urinary tract (UT) present clinically with a variety of signs and symptoms and may be caused by an array of organisms (see Figure 1, Key Figure). In this review, we focus primarily on uropathogenic Escherichia coli (UPEC) as the etiologic agent of UTI, as UPEC is responsible for >80% of all community-acquired infections [2]. Other etiologies include infections from Staphylococcus, Klebsiella, Enterobacter, Proteus, and Enterococcus; these organisms become particularly relevant during catheter-associated and hospital-acquired infections [7]. The pathogenic cascade of UPEC cystitis has been extensively studied in recent years, largely in cell-culture and mouse models, as mice recapitulate many facets of the bladder epithelial environment (reviewed in [8]). Through these studies, unprecedented light has been shed on the molecular and cellular basis of infection. Further, recent years have seen the advent of several new mouse models, enabling the study of complicated UTIs (pyelonephritis, renal abscess, catheter-associated UTI) and recurrent cystitis. In addition, recent data suggest that the normal, healthy bladder is not always sterile, and a picture of the urinary microbiome is emerging. Such advances promise to further illuminate molecular mechanisms of virulence in UPEC (reviewed recently in [9]) and other uropathogens, as well as the intricacies of the host immune response. With these tools, we are poised to address heretofore unanswered questions with clinical relevance to treatment and prevention.
Figure 1. Clinical Features and Virulence Mechanisms in Cystitis and Pyelonephritis.
UTIs can present clinically in a variety of ways, most often reflecting cystitis (infection of the bladder) or pyelonephritis (infection of the kidney). Uropathogenic Escherichia coli (UPEC) is the most common cause of UTI (especially among community-onset infections), among other pathogens. Selected virulence factors associated with the pathogenesis of UPEC cystitis or pyelonephritis are shown and include adhesins, siderophores, toxins, siderophores, capsule, and other systems (see text for details). UT: urinary tract.
Molecular Pathogenesis of UTI
Infection of the urinary tract begins when UPEC, likely introduced after colonization of the periurethral area by gastrointestinal tract flora [10–12], accesses and ascends the urethra by an undetermined mechanism. Upon reaching the urinary bladder, UPEC bind to superficial epithelial (facet) cells in a type 1 pili-dependent manner [13]. A subset of adherent bacteria are then internalized into facet cells [14, 15], a dynamic process that likely relies on the normal cycling of apical membrane segments in these cells [16]. Countering this key pathogenic activity, bladder epithelial cells undertake active expulsion of internalized UPEC. Recent data show that UPEC are capable of neutralizing the lysosome, and that this neutralization is sensed by a lysosomal membrane protein termed mucolipin TRP channel 3 (TRPML3), activating pathways that direct exocytosis of UPEC-containing lysosomes [17]. Through a distinct mechanism, activation of Toll-like receptor 4 (TLR4) by internalized UPEC leads to specific ubiquitination of TNF Receptor Associated Factor 3 (TRAF3), enabling its interaction with a guanine-nucleotide exchange factor that directs assembly of the exocyst complex, thereby accomplishing expulsion of intracellular bacteria [18].
Using incompletely defined strategies, UPEC may gain access to the bladder epithelial cell cytoplasm, thereafter developing clonal, biofilm-like masses termed intracellular bacterial communities (IBCs) [14, 19]. As part of the host response, the superficial facet cells are largely exfoliated [20], liberating IBCs into the urine and ridding the body of thousands of bacteria. Shed IBC-containing cells are observed in the urine of infected women and children, supporting their clinical relevance [21, 22]. After 16–24 h in murine UTI models, a subset of UPEC in remaining IBCs adopt a neutrophil-resistant, filamentous morphology and escape the IBCs, subsequently re-invading naïve bladder epithelial cells [23]. Some of these bacteria will go on to infect immature bladder epithelium which is exposed after exfoliation, later forming quiescent intracellular reservoirs, which avoid immune clearance and resist systemic antibiotic treatment [24–26]. These persistent UPEC may re-emerge, in response to currently undefined signals, to cause the recurrent cystitis that is so clinically common.
A significant gap in our understanding is the mechanism by which UPEC escape the initial vacuole (after internalization) to reach the cytoplasm, where the IBC is formed. Unlike other Gram-negative pathogens that escape an endosome, UPEC do not encode a type III secretion system to deliver virulence factors [27]. Further, the bottleneck imposed by IBC formation precludes classical in vivo screens, and no in vitro model for IBC formation has been wholly accepted by the field [13, 14]. As a result, surrogate methods have been used to illuminate requirements for IBC formation. For example, since IBCs exhibit many characteristics of biofilms, one group performed a transposon screen for genes necessary for in vitro biofilm formation, using polyvinyl chloride as a substrate, as well as sampling the pellicle of standing broth cultures. This screen yielded genes with functions in attachment, motility, LPS synthesis and modification, metabolism, as well as bacterial cell maintenance [28]. In other studies, murine UTI models have shown that single-gene mutants of UPEC exhibit defects in specific steps of the IBC pathogenic cascade, as in the case of OmpA, a major outer membrane porin. OmpA deletion does not inhibit UPEC binding to superficial epithelial cells or internalization; however, once within the cytoplasm of these cells, mutant ΔompA cannot complete the intracellular pathway and, as assessed by dwindling organ bacterial loads and confocal microscopy, these UPEC fail to progress past very early stages of IBC formation in mice [29]. Similarly, UPEC harboring a deletion of the small non-coding RNA Hfq cannot replicate within cultured human bladder epithelial cells, despite exhibiting normal levels of binding and invasion [30]. Defining the roles of relevant host factors (exemplified by the exocytosis studies mentioned above) will also help to elucidate the mechanism by which UPEC gains the critical cytoplasmic niche. Answering questions such as these will require collaborative and broad-based efforts involving cell biology, bacteriology, biochemistry, and optimized in vitro or ex vivo models.
Following escape into the cytoplasm, the bacteria find themselves occupying an environment very different from the nutrient-poor bladder lumen. Transcriptomic analyses of UPEC in different models (such as during murine UTI or bacterial growth in urine) have suggested that various metabolic pathways are essential for pathogenesis; these include sialic acid transport/metabolism, gluconeogenesis, the tricarboxylic acid (TCA) cycle, iron uptake, ethanolamine and phosphate metabolism, as well as amino acid metabolism [31–34] (reviewed in [35]). Although this work has provided broad insight into the metabolic activities required to cause UTI, we are on the verge of being able to specifically interrogate UPEC populations in defined niches and times during infection. UPEC survival and growth at distinct spatiotemporal points during infection could rely on very different metabolic sources. Intracellular survival presumably requires a unique set of metabolic capabilities, but the precise needs are incompletely defined. Metabolism of a chromogenic substrate during cystitis provides circumstantial evidence that UPEC can utilize β-galactosidase, perhaps reflecting a glucoselimited milieu during this intracellular step [36]. Transcriptional profiling from whole mouse bladders 6 h post infection with UPEC strain UTI89 was posited to reflect mostly bacteria that are internalized and actively forming IBCs [37]. This analysis found that 2.3% of the UPEC genome was differentially regulated within the bladder at this time point (6 h), when compared to the statically grown UPEC broth culture that was used as inoculum. Genes associated with alternative carbon source utilization pathways, such as lacZ and srlA for galactose and sorbitol utilization, respectively, were upregulated; deletion of lacZ was subsequently found to impair virulence [37]. Genes associated with iron acquisition were also highly expressed, including siderophores (secreted bacterial proteins that chelate extracellular iron and return it to the bacterial cell). In contrast, tryptophan and cysteine synthetic genes were downregulated, reflecting an abundance of these amino acids within the IBC niche [37]. A more specific understanding of bacterial metabolism within pathogenic niches could reveal points of potential intervention, halt infection, and/or eliminate reservoirs that seed recurrent UTIs. Of note, the central metabolic pathways in E. coli do not necessarily represent all uropathogenic species; other pathogens with distinct metabolism may respond to different nutritional cues during infection [31].
Comparatively less is known about the molecular pathogenesis of infection in the kidney. In traditional mouse models, severe kidney infection (including renal abscess formation) is uncommon, hampering the study of this entity. Attenuation in mouse kidney infections has been observed with UPEC mutants lacking specific virulence factors, such as type 1 pili, P pili, flagella, α-hemolysin, and cytotoxic necrotizing factor 1 (CNF1) [3, 9]. Further, genetics appear to play a role in host susceptibility to acute pyelonephritis. For example, increased risk of acute pyelonephritis and renal scarring have been linked to polymorphisms that reduce Interferon Regulatory Factor 3 (IRF3) or CXCR1 (encoding the IL-8 receptor) gene expression in certain UTI-prone patient populations [38]. Compared to bacterial cystitis, the understanding of pyelonephritis remains limited and, consequently, represents a fertile area of study.
Immune Control and Pathogen Evasion
After ascending the urethra, bacterial pathogens are challenged by innate defenses within the bladder. Arrival in the bladder triggers a TLR4-dependent, lipopolysaccharide (LPS)-stimulated inflammatory response from bladder epithelial cells and resident leukocytes, culminating in the activation of the NF-κB pathway, which in turn promotes the expression of inflammatory cytokines and neutrophil chemoattractants [39]. This inflammatory milieu engenders massive neutrophil influx into the bladder tissue and lumen, correlating with a diagnostic hallmark of UTI. The importance of this neutrophil influx in controlling UPEC infection has been well established (e.g., [40–43]). Production of polysaccharide capsule antigens by UPEC, particularly of the K2 or K1 serotype, may provide some protection against UPEC eradication by the host [44]. Further, many other soluble factors (e.g., antimicrobial peptides, complement, lipocalin 2, lysozyme, lactoferrin) are also released by host cells into the bladder lumen, potentially creating a less hospitable environment for arriving bacteria [45, 46]. Antimicrobial peptides likely protecting the urinary tract include defensins, the human cathelicidin LL-37, and ribonuclease 7 [47–50]. These molecules may exert direct antimicrobial activity, augment innate cellular recruitment, or function to alter the environmental niche to make it less favorable for uropathogens (e.g., by sequestering siderophores and critical nutrients such as iron, from the bacteria) [51]. Other host transcriptional regulators such as hypoxiainducible factor 1α (HIF-1α) are also expressed in response to bacteria, potentially boosting innate defense components such as nitric oxide, cathelicidin, and β-defensin 2 [51, 52]. Recently, the humoral pattern recognition molecule pentraxin 3 (PTX3) was shown to help control UTI by serving as an opsonin and promoting bacterial uptake by neutrophils; UTI-prone children and adult cystitis patients who had suffered recurrent UTI as children exhibited polymorphisms in PTX3 [53], suggesting that the cellular and soluble components of innate immunity can influence disease outcomes.
The formation of IBCs is a key means by which bacteria subvert neutrophil activity, as arriving neutrophils accurately locate IBC-bearing facet cells but cannot access the bacteria within [39, 54]. UPEC can subvert and delay the innate immune response in multiple ways (reviewed in [39]). For example, secretion of proteins such as UPEC YbcL can lead to a measurable dampening of neutrophil infiltration into the bladder [55–57]. Further, UPEC induces host expression of genes such as IDO, which, via generation of kynurenine metabolites, can cause decreased neutrophil migration across infected bladder epithelia, as evidenced from in vitro Transwell systems, as well as in mice [58, 59]. Some UPEC strains, such as CFT073, can also disrupt host signaling by producing TIR domain-containing proteins such as TcpC; this virulence factor interacts with the host adaptor MyD88 to disrupt TLR4 signaling, while also reducing urinary IL-1β in mice and inhibiting the NLRP3 inflammasome in macrophages [60, 61]. While robust innate defenses are able to repel most bacterial challenges, this inflammatory response may represent a double-edged sword. In murine cystitis, excessive inflammation and resulting bladder tissue damage predisposes the host to worse infection outcomes, including chronic cystitis [62, 63].
As mucosal barriers such as the bladder epithelium are repeatedly assaulted with bacteria, they are generally tolerant to a transient microbial presence, and innate defenses are key to preventing infection. However, clinical syndromes such as recurrent UTI raise questions about the importance of adaptive immunity in bladder protection. Pro-inflammatory cytokines that also elicit adaptive immune effects, such as IL-17, are prominently secreted during the acute phase of murine experimental UTI [64, 65]. CD8+ T cells are recruited to the bladder as early as 24 h post infection, but the precise roles of these and other adaptive immune cell populations are unknown [66]. Regarding humoral immunity, the prevalence of recurrent UTI in the female population suggests that a lasting protective immune response is not established following cystitis, at least in this subpopulation of women [67]. Upper-tract UTI (pyelonephritis) may generate a more robust serological response, although it is not clear if elicited antibodies would subsequently reach the bladder to provide protection against future cystitis. In total, the importance of adaptive immunity in controlling UPEC infection is substantially understudied in comparison with the innate immune system. Understanding the basis of functional adaptive immunity against UTI could have major implications for recurrent UTIs and vaccine development, as further discussed below.
Next-generation Therapeutics
Put simply, UTI therapies are in need of innovation. For decades, finite courses of antibiotics have been prescribed for women with UTIs, often in the absence of bacterial culture data; such empiric treatment is effective at resolving acute symptoms, but clearly fails to eliminate a recurrence risk [2]. In addition, the rise of multidrug-resistant uropathogens (e.g., [68]) mandates therapeutic selection based on actual patient bacterial cultures, susceptibility results and/or local as well as institutional antibiograms. As the pace of resistance development (especially among Gram-negative uropathogens) has overtaken the pace of new antibiotic development, fundamentally new approaches are needed [69]. Further, prophylactic antibiotics are incompletely effective in preventing infection [70], and in one mouse study, subtherapeutic levels of ciprofloxacin were shown to augment murine UTI [71]. To move forward in the therapeutic realm, we must extend our molecular understanding of both the pathogen and the host. Contemporary development of novel UTI therapeutics has focused on interfering with pathogen binding to bladder epithelium or other key pathogen processes, the development of vaccines based on bacterial components, as well as the modulation of host responses -- specifically those promoting exfoliation to eradicate chronically resident bacteria from the bladder.
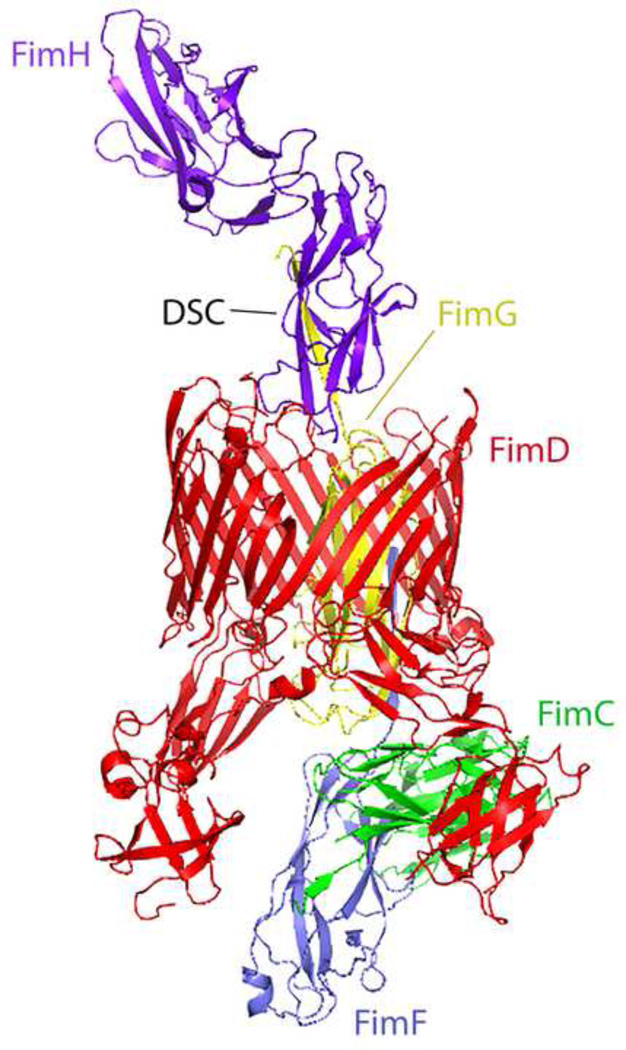
An emerging example in which basic biology of the host-pathogen interaction has informed therapeutics development is that of mannosides and pilicides, compound families which target the crucial step of bacterial adherence to host cells in distinct ways. Pilicides interfere with the chaperone-usher pathway for assembly of adhesive type 1 pili, preventing their presentation on the bacterial surface and thereby abolishing epithelial binding [72, 73]. In contrast, mannosides serve as competitive inhibitors, occupying the binding pocket of the type 1 pilus adhesin FimH, with affinities that are orders of magnitude higher than those of the mannosylated uroplakins decorating the bladder epithelial surface [74]. The oral bioavailability and efficacy of mannosides in preventing UTI in mice portend substantial potential utility in the clinic [75, 76]. Beyond uncomplicated cystitis, mannosides have also shown efficacy in mouse models for prevention of catheter-associated UTI (as reflected by diminished bladder and catheter colonization) [77]. Mannosides are being rationally optimized to exhibit more drug-like pharmacokinetic properties, such as improved metabolic stability and bioavailability [74, 78]. Agents such as the so-called “anti-virulence” compounds that block specific molecular steps in pathogenesis, apply much less selective pressure on pathogenic bacteria, thereby reducing the rapidity of resistance development [79]. Further, due to their known mechanism of action, such agents can be used as tools to further probe the biology of host-pathogen interactions [80]. Recent structural “snapshots” of bacterial pilus assembly via the chaperone-usher pathway (see Figure 2) may illuminate additional routes to inhibition [81–84], with potentially much broader impact, as this bacterial secretion pathway also underlies virulence factor production by diverse bacterial pathogens (e.g., Yersinia pestis). Direct application to the bladder luminal surface of nanoparticles, perhaps coated with the FimH adhesin [85], has also been explored in mice as a means to accomplish targeted delivery of novel therapeutics to the host [86].
Figure 2. Ribbon Representation of the Chaperone-Adhesin-Usher Complex for Assembly of Type 1 Pili from Escherichia coli.
The periplasmic chaperone FimC (green) delivers structural subunits to the outer membrane usher (FimD, red) for assembly. Subunits shown represent the pilus tip structure and include the adhesin FimH (purple) and adapters FimG (yellow, within the barrel of FimD) and FimF (gray). Each subunit has its immunoglobulin-like fold completed by a strand provided by the next subunit, in a process called donor-strand complementation (DSC). The energetic favorability provided by this final structure drives assembly on the periplasmic side of the usher, as the periplasm is devoid of ATP. Protein Database PDB# 4J3O; adapted from [82].
Successful vaccination against UPEC and other uropathogens could have monumental impact on the lives of those at risk for complicated UTIs or who suffer from recurring episodes. Multiple groups have worked to identify specific UPEC factors for potential use as vaccine antigens. Candidate antigens include the FimH adhesin, siderophores such as yersiniabactin [87], and other immunodominant proteins identified in mouse models [88, 89] (reviewed in [3, 90]). Two important considerations may hinder the effectiveness of vaccine candidates against UTI. First, as strains of E. coli (expressing type 1 pili, iron acquisition systems, and other factors) are present in the normal gut microbiota, vaccination could potentially alter the populations of proteobacteria in the gut. Second, as noted above, it is not clear how much antibody (IgG) in the healthy urinary tract should reach the bladder lumen. Therefore, elicitation of serum antibodies against UPEC antigens may be more effective in preventing pyelonephritis, where antibodies are more readily delivered. Further studies into the correlates of adaptive immunity in both the upper and lower urinary tract are thus needed to advance these efforts.
Another strategy for the management of acute or recurrent UTI may be to modulate or enhance host responses to UTI. As noted earlier, an exuberant inflammatory response predisposes women to chronic cystitis [62]. In fact, in a mouse UTI model, inhibiting this response using an oral anti-inflammatory COX-2 inhibitor yielded better outcomes without actually targeting the bacteria (and thereby applying no selective pressure). These findings corroborated small clinical trials in women receiving ibuprofen, in which symptomatic improvement at 4 and 7 days with ibuprofen treatment alone was equivalent to using oral antibiotics [91, 92]. Further, as the bladder exfoliation accompanying acute UPEC cystitis is not complete, bacteria within quiescent reservoirs may re-emerge to seed recurrent infection. Advanced, more efficacious exfoliants are being designed to unearth these quiescent reservoirs [93, 94]. Once these bacteria are forced to emerge, they may be more susceptible to the actions of standard antibiotics. Therefore, combined exfoliant-antimicrobial strategies might rid the host of the UPEC reservoirs that underlie some recurrent UTIs [94].
Finally, with regard to updated UTI therapeutics, one must consider an impending paradigm shift regarding the “normal” state of the bladder – which has long been assumed to be sterile [95]. Enhanced culture techniques, as well as metagenomics on catheter-collected samples, have detected urinary bacteria in healthy and asymptomatic women [96]. Interactions between these apparent commensals and soluble mediators such as antimicrobial peptides might alter susceptibility to UTI [97]. Moreover, specific microbiome structures might also be related to conditions traditionally thought to be non-infectious, such as stress or urgency incontinence [98, 99] and interstitial cystitis/chronic bladder pain. As the urinary microbiome is more extensively defined, we will have to account for it when considering the pathogenesis of UTI, as well as when choosing therapies for symptomatic patients.
Emerging, Clinically Relevant Models for UTI
Although many uncomplicated UTIs can resolve spontaneously or with antibiotic treatment, more complicated forms of UTI have not, until recently, been reflected in animal models. The majority of preclinical work in the last two decades on cystitis and pyelonephritis has relied on transurethral inoculation of UPEC into the bladder of female mice [100, 101]. Emerging mouse models may enable additional clinically relevant questions to be addressed.
Catheter-associated UTI (CAUTI)
Prolonged urinary catheter usage is a risk factor for UTI, due largely to the ability of bacteria to establish a biofilm on the catheter that resists clearance by host defense and antibiotics. CAUTIs represent the most common nosocomial infections and are associated with increased hospital length of stay, morbidity, and mortality [102, 103]. As UPEC are less prominent in the epidemiology of CAUTI, other organisms such as Enterococcus faecalis have emerged as model organisms for study [7]. Insertion of a urinary catheter elicits an inflammatory environment in the bladder, which is manifested histologically as exfoliation, edema of the lamina propria and submucosa, urothelial thinning, and mucosal lesions [7]. Damaged mucosa and the catheter itself offer surfaces for bacterial adhesion [104]. Recent data indicate that enterococcal adherence to urinary catheter material is mediated by fibrinogen, a host protein that is released into the bladder lumen and deposited on the catheter following insertion. E. faecalis then binds fibrinogen via the pilus tip adhesin EbpA, subsequently forming a biofilm on the catheter [105, 106]. These pathogenic events can be modeled in C57BL/6 mice in which a short length of silicone catheter material is transurethrally deposited in the bladder, followed by introduction of E. faecalis [104, 107]. A structural understanding of bacterial pilus association with catheter material and proteinaceous deposits may enable the design of new strategies to counteract catheter-associated UTI.
Recurrent UTI
Approximately 20–30% of women with acute cystitis go on to develop recurrent UTI (rUTI), and those who do suffer on average 2–3 additional UTIs in the year following an initial episode [2]. The subsequent UTI might arise from reinoculation of the urethra with flora from the gastrointestinal tract, or from re-emergence of a bladder epithelial reservoir. In a recent study, isolates from four patients with rUTI were analyzed by whole-genome sequencing [10]. In two patients, the same UPEC clone dominated both gut and urinary tract habitats at the initial and subsequent infection; in the other two, a new clone had established dominance in both habitats at the time of recurrent UTI. Further, isolates causing subsequent UTI in these patients, when introduced into mice and compared with their initial infecting strain, exhibited increased fitness in both the gut and the urinary tract, demonstrating that fitness in these two important niches is not mutually exclusive [10].
In a newly developed mouse model of rUTI, the C3H mouse strain – known to have increased vesicoureteral reflux compared to C57BL/6 mice [108] – can be sensitized to later infection. Following an initial infection (experimentally resolved by treatment with antibiotics) and upon subsequent re-challenge with a later infection, these “sensitized” mice were more likely than naïve mice to suffer persistent bacteriuria and chronic cystitis [62]. A leading hypothesis for recurrent UTIs is that an exuberant inflammatory response to initial infection causes bladder remodeling that somehow predisposes the host to recurrent infection or more inflammatory outcomes [4, 62, 103]. This model may enable a mechanistic understanding of apparent predisposition to recurrent infection, in turn informing therapies that could interfere with or dampen this process.
Male and Complicated UTI
The higher prevalence of UTI in females is chiefly attributed to anatomic factors in women, such as shorter urethral length, shorter distance from the anus to urethral meatus, and permissiveness of the vaginal and perineal environments to microbial colonization [12, 103]. However, males at both ends of the age spectrum (mainly infants <1 year of age and elderly men with prostatic hypertrophy) exhibit a higher incidence of UTI, and other conditions in males (diabetes, spinal cord injury, catheter use) also promote UTI [109]. Among individuals with upper-tract UTI (pyelonephritis), males exhibit greater morbidity and mortality than females [110], suggesting that non-anatomical differences may be at work in these more severe infections.
Until recently, essentially all cystitis and pyelonephritis studies have been performed in female mice, as the male mouse bladder is not reliably accessible by catheter. Of note, instillation of uropathogens into the urethra of male mice elicits prostatic infection [111, 112]. In a recently developed, new model of UTI, a small abdominal incision is made and bacteria are inoculated via needle into the bladders of male and female mice, permitting direct sex comparisons [113]. This inoculation method recapitulates the IBC cascade of acute cystitis established in studies with catheter-infected females. Interestingly, once anatomic barriers are bypassed in this way, male mice experience more severe infection than females, mirroring epidemiologic data observed clinically in men; indeed, male C3H mice uniformly develop severe pyelonephritis and renal abscesses that are seen much less frequently in female mice [113]. This new model opens doors to study sex differences in UTI pathogenesis and host response, as well as sequelae of severe pyelonephritis and abscess formation; these latter phenotypes are relevant to febrile UTI in children, following which renal scarring is a common complication.
Concluding Remarks
Urinary tract infections continue to be among the most common bacterial infections in humans, drawing millions of antibiotic prescriptions annually. Available therapies have not evolved significantly in recent years, do not prevent recurrences, and are challenged by rising antibiotic resistance. Creative approaches to treatment, including the development of antivirulence therapeutics, should be prioritized (see Outstanding Questions and Box 2). In addition, the field lacks a thorough understanding of protective host immunity related to UTI, if such is generated after natural infection (especially pyelonephritis) or can be elicited via vaccination. Given the broad range of organisms that can cause UTI and the unavoidable nature of some risk factors (e.g., urinary catheters), even highly effective novel interventions will not completely mitigate the impact of these infections on human health. However, the common pathogenic themes in Gram-negative community-onset UTI make this subset of infections a particularly important epidemiologic target.
Outstanding Questions Box.
How does UPEC, upon internalization into the superficial epithelial cell in the bladder, escape from the endocytic vesicle into the cytoplasm to form the IBC? A molecular understanding of this apparently critical step in acute cystitis might illuminate a novel bacterial strategy for intracellular pathogenesis, as well as informing new targets for intervention.
Do antimicrobial peptides provide primarily an antimicrobial or immunemodulating role during UTI? Many of these peptide species are secreted into the urinary space, especially upon infection; the immunostimulatory effects of these peptides may be more important than their direct antibacterial activity.
What elicits adaptive immunity to uropathogenic bacteria, and can such immunity help to protect the bladder? Highly expressed bacterial targets such as pili and siderophores are enticing vaccine candidates, but a larger question is whether traditional humoral immunity has a significant role in protecting the bladder lumen.
How does biological sex and associated hormonal milieu influence the outcomes of infection? UTIs are considered a disease of women, but significant male populations are susceptible and may exhibit higher morbidity. Male UTI has been largely ignored in preclinical studies but can now be addressed with updated models.
How can we better understand the biological basis of susceptibility to recurrent cystitis? This is perhaps the most frustrating clinical problem, affecting millions of otherwise healthy women, and remains unresolved with current treatments and lifestyle changes.
What can be done therapeutically in the face of emerging multidrug-resistant UPEC isolates? Advanced diagnostics with improved performance characteristics, and available at the point of care, will allow for more accurate selection of empiric therapy (when indicated). Molecular detection methods may allow earlier identification of multidrug-resistant isolates that may require parenteral or inpatient treatment.
Box 2. The Future of UTI Diagnostics.
For decades, the diagnosis of UTI has relied on culturing urine samples and looking under the microscope for the presence of white blood cells. Providers also utilize point-of-care dipstick tests to search for the presence of leukocyte esterase, nitrites, and other compounds. Even in combination with careful symptom history and risk factor ascertainment, these tests offer only 50–85% sensitivity and 80–90% specificity [114]. Further, community diagnosis of UTI is typically made on clean-catch urine samples, raising the possibility of contamination and rendering some positive cultures difficult to interpret (including “false-positives”). In the age of “omics,” widespread mass spectroscopy, point-of-care molecular detection, bacterial genomic sequencing, and other tools, the time is right to move towards better UTI diagnostics. These might rely on a combination of host immune and metabolic markers, as well as on the detection of uropathogens and their components (DNA, proteins, etc.). For example, if sample preparation challenges could be circumvented, direct mass spectrometry on infected urine might be useful, detecting bacteria promptly in urine without the need to wait for growth on solid media [115]. Alternatively, rapid molecular identification of E. coli at the substrain level, as well as prediction of antibiotic resistances, might enable more efficient selection of antibiotics for treatment [116, 117]. Ultimately, improved and accurate diagnostics for UTI should translate into more satisfying care for patients, less frustration and speculation on the part of providers, and an overall reduction in antibiotic use.
Trends Box.
Mouse and human studies have revealed that during acute cystitis, Escherichia coli and other Gram-negative uropathogens can occupy the cytoplasm of bladder epithelial cells, using this niche as a haven for replication while protected from infiltrating neutrophils.
Novel therapeutics for UTI are being explored, based on detailed molecular and structural information of bacterial virulence factor expression, as well as patterns of bacterial binding to urinary epithelium, iron acquisition, and other pathogenic processes.
Highly expressed and immunogenic bacterial factors, including siderophores, have been identified in rodent models, potentially informing the development of vaccines and immunotherapies for UTI. However, the putative role of adaptive immunity in control of lower urinary tract infection remains unclear.
Though the urinary tract is traditionally considered to be sterile, advances in metagenomics and other technologies have enabled the first definitions of a “urinary microbiome,” which may alter the way in which we think about UTI (e.g., as dysbiosis, rather than simply introduction of one pathogenic species).
Technical advances in mouse models now permit detailed modeling of complicated UTI syndromes common in humans – recurrent UTI, catheter-associated UTI, UTI in the male host, and ascending renal abscess formation.
Acknowledgments
This work was supported by the National Institutes of Health (P50-DK064540) and by the Mr. and Mrs. Spencer T. Olin Fellowship for Women in Graduate Study (to L.K.M.)
GLOSSARY
- Cathelicidin
A class of antimicrobial peptide; there is a single cathelicidin encoded in the human and mouse genomes
- Chaperone-usher pathway
A broadly conserved molecular paradigm for Gram-negative bacterial secretion of polymeric surface structures, including pili
- Cyclooxygenase-2 (COX-2)
A mammalian enzyme expressed in many cell types that promotes generation of immunostimulatory molecules including prostaglandins
- Cystitis
Bacterial infection of the urinary bladder
- Cytotoxic necrotizing factor 1 (CNF1)
A secreted UPEC toxin that causes cell death to neutrophils and other leukocytes
- Defensins
A broad class of antimicrobial peptides, some of which are also secreted in the urinary tract, especially during infection
- Exfoliation
Shedding of the superficial epithelial layer of the bladder
- Flagella
Whiplike surface structures, produced by many UPEC, that propel the organism in swimming motility
- Gram-negative
A large subset of bacteria, including pathogenic and nonpathogenic species, possessing an outer membrane and periplasmic space outside of the cell membrane; so called because they do not retain the purple crystal violet during the Gram staining procedure
- α-Hemolysin
A multifunctional secreted toxin of UPEC and other pathogenic bacteria
- Intracellular bacterial communities (IBCs)
Biofilm-like collections of UPEC residing within superficial epithelial cells of the bladder
- Mannoside
A small molecule derived from mannose that serves as a high-affinity ligand for FimH, the adhesive subunit of type 1 pili
- Microbiome
An ecological community of commensal, symbiotic, and pathogenic organisms occupying a body space
- NF-κB pathway
A major transcriptional pathway regulating inflammation and apoptosis, stimulated by activation of Toll-like receptors and other host cell sensors
- P pili
Heteropolymeric surface structures expressed by some uropathogenic Escherichia coli strains and associated with adherence to kidney epithelium in some hosts
- Pilicide
A small molecule designed to interrupt the function of the chaperone promoting pilus assembly
- Pyelonephritis
Bacterial infection of the kidney(s)
- Quiescent intracellular reservoir
Chronically resident UPEC that persist in bladder tissue following resolution of acute cystitis, and may represent a seed for recurrent cystitis
- Renal abscess
A large collection of neutrophilic pus surrounding a nidus of bacterial infection in the kidney parenchyma
- Siderophore
A bacterial protein with high affinity for iron; secreted from bacteria and re-internalized once it captures iron from the host
- Type 1 pili
Hairlike, adhesive, heteropolymeric surface structures expressed by uropathogenic Escherichia coli that mediate binding to bladder epithelium
- Type III secretion system
A specialized, multi-component protein complex assembled by certain pathogenic Gram-negative bacteria (e.g., Salmonella) to accomplish delivery of effector proteins directly into host cells
- UPEC
Uropathogenic Escherichia coli, the most common bacterial cause of urinary tract infection
- Uroplakins
Mannosylated proteins decorating the apical surfaces of superficial bladder epithelial cells, providing a permeability barrier but also offering binding sites for UPEC and other uropathogens
- UTI
Urinary tract infections, comprising cystitis, pyelonephritis, renal abscess, urethritis, and prostatitis
- Vesicoureteral reflux
Movement of urine in a retrograde direction from the bladder to the renal pelvis and collecting system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
D.A.H. serves on the Board of Directors of BioVersys AG, Basel, Switzerland.
REFERENCES
- 1.Harding GK, Ronald AR. The management of urinary infections: what have we learned in the past decade? Int J Antimicrob Agents. 1994;4:83–88. doi: 10.1016/0924-8579(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien VP, et al. Drug and vaccine development for the treatment and prevention of urinary tract infections. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.UTI-0013-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien VP, et al. Are you experienced? Understanding bladder innate immunity in the context of recurrent urinary tract infection. Curr Opin Infect Dis. 2015;28:97–105. doi: 10.1097/QCO.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta K, et al. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 6.Al-Badr A, Al-Shaikh G. Recurrent urinary tract infections management in women: a review. Sultan Qaboos Univ Med J. 2013;13:359–367. doi: 10.12816/0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flores-Mireles AL, et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber AE, et al. Strengths and limitations of model systems for the study of urinary tract infections and related pathologies. Microbiol Mol Biol Rev. 2016;80:351–367. doi: 10.1128/MMBR.00067-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luthje P, Brauner A. Virulence factors of uropathogenic E. coli and their interaction with the host. Adv Microb Physiol. 2014;65:337–372. doi: 10.1016/bs.ampbs.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Chen SL, et al. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci Transl Med. 2013;5:184ra160. doi: 10.1126/scitranslmed.3005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto S, et al. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol. 1997;157:1127–1129. [PubMed] [Google Scholar]
- 12.Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17:259–268. doi: 10.1016/s0924-8579(00)00350-2. [DOI] [PubMed] [Google Scholar]
- 13.Hannan TJ, et al. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev. 2012;36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz DJ, et al. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun. 2011;79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez JJ, et al. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishop BL, et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 17.Miao Y, et al. A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell. 2015;161:1306–1319. doi: 10.1016/j.cell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao Y, et al. Ubiquitination of innate immune regulator TRAF3 orchestrates expulsion of intracellular bacteria by exocyst complex. Immunity. 2016;45:94–105. doi: 10.1016/j.immuni.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson GG, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 20.Mulvey MA, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 21.Robino L, et al. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin Infect Dis. 2014;59:e158–e164. doi: 10.1093/cid/ciu634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen DA, et al. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Justice SS, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvey MA, et al. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schilling JD, et al. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002;70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch RA, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadjifrangiskou M, et al. Transposon mutagenesis identifies uropathogenic Escherichia coli biofilm factors. J Bacteriol. 2012;194:6195–6205. doi: 10.1128/JB.01012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson TF, et al. OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis. Infect Immun. 2009;77:5245–5251. doi: 10.1128/IAI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulesus RR, et al. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect Immun. 2008;76:3019–3026. doi: 10.1128/IAI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alteri CJ, et al. Preferential use of central metabolism in vivo reveals a nutritional basis for polymicrobial infection. PLoS Pathog. 2015;11:e1004601. doi: 10.1371/journal.ppat.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subashchandrabose S, et al. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A. 2014;111:18327–18332. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alteri CJ, et al. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009;5:e1000586. doi: 10.1371/journal.ppat.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alteri CJ, et al. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009;5:e1000448. doi: 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alteri CJ, Mobley HL. Metabolism and Fitness of Urinary Tract Pathogens. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.MBP-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Justice SS, et al. Maturation of intracellular Escherichia coli communities requires SurA. Infect Immun. 2006;74:4793–4800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conover MS, et al. Metabolic requirements of Escherichia coli in intracellular bacterial communities during urinary tract infection pathogenesis. mBio. 2016;7:e00104–e00116. doi: 10.1128/mBio.00104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragnarsdottir B, et al. Genetics of innate immunity and UTI susceptibility. Nat Rev Urol. 2011;8:449–468. doi: 10.1038/nrurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 39.Olson PD, Hunstad DA. Subversion of host innate immunity by uropathogenic Escherichia coli. Pathogens. 2016;5 doi: 10.3390/pathogens5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haraoka M, et al. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180:1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 41.Artifoni L, et al. Interleukin-8 and CXCR1 receptor functional polymorphisms and susceptibility to acute pyelonephritis. J Urol. 2007;177:1102–1106. doi: 10.1016/j.juro.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 42.Ingersoll MA, et al. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell Microbiol. 2008;10:2568–2578. doi: 10.1111/j.1462-5822.2008.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svensson M, et al. Effects of epithelial and neutrophil CXCR2 on innate immunity and resistance to kidney infection. Kidney Int. 2008;74:81–90. doi: 10.1038/ki.2008.105. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar S, et al. Role of capsule and O antigen in the virulence of uropathogenic Escherichia coli. PLoS One. 2014;9:e94786. doi: 10.1371/journal.pone.0094786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weichhart T, et al. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur J Clin Invest. 2008;38(Suppl 2):29–38. doi: 10.1111/j.1365-2362.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- 46.Danka ES, Hunstad DA. Cathelicidin augments epithelial receptivity and pathogenesis in experimental Escherichia coli cystitis. J Infect Dis. 2015;211:1164–1173. doi: 10.1093/infdis/jiu577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durr UH, et al. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen KL, et al. Role of urinary cathelicidin LL-37 and human beta-defensin 1 in uncomplicated Escherichia coli urinary tract infections. Infect Immun. 2014;82:1572–1578. doi: 10.1128/IAI.01393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becknell B, et al. Expression and antimicrobial function of beta-defensin 1 in the lower urinary tract. PLoS One. 2013;8:e77714. doi: 10.1371/journal.pone.0077714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer JD, et al. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 2013;83:615–625. doi: 10.1038/ki.2012.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steigedal M, et al. Lipocalin 2 imparts selective pressure on bacterial growth in the bladder and is elevated in women with urinary tract infection. J Immunol. 2014;193:6081–6089. doi: 10.4049/jimmunol.1401528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin AE, et al. Role of hypoxia inducible factor-1α in innate defense against uropathogenic Escherichia coli infection. PLoS Pathog. 2015;11:e1004818. doi: 10.1371/journal.ppat.1004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaillon S, et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity. 2014;40:621–632. doi: 10.1016/j.immuni.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Justice SS, et al. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc Natl Acad Sci U S A. 2006;103:19884–19889. doi: 10.1073/pnas.0606329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau ME, et al. YbcL of uropathogenic Escherichia coli suppresses transepithelial neutrophil migration. Infect Immun. 2012;80:4123–4132. doi: 10.1128/IAI.00801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loughman JA, Hunstad DA. Attenuation of human neutrophil migration and function by uropathogenic bacteria. Microbes Infect. 2011;13:555–565. doi: 10.1016/j.micinf.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lau ME, et al. Bacterial lysis liberates the neutrophil migration suppressor YbcL from the periplasm of uropathogenic Escherichia coli. Infect Immun. 2014;82:4921–4930. doi: 10.1128/IAI.01838-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loughman JA, Hunstad DA. Induction of indoleamine 2,3-dioxygenase by uropathogenic bacteria attenuates innate responses to epithelial infection. J Infect Dis. 2012;205:1830–1839. doi: 10.1093/infdis/jis280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loughman JA, et al. Local generation of kynurenines mediates inhibition of neutrophil chemotaxis by uropathogenic Escherichia coli. Infect Immun. 2016;84:1176–1183. doi: 10.1128/IAI.01202-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waldhuber A, et al. Uropathogenic Escherichia coli strain CFT073 disrupts NLRP3 inflammasome activation. J Clin Invest. 2016;126:2425–2436. doi: 10.1172/JCI81916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cirl C, et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008;14:399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 62.Hannan TJ, et al. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz DJ, et al. Uropathogenic Escherichia coli superinfection enhances the severity of mouse bladder infection. PLoS Pathog. 2015;11:e1004599. doi: 10.1371/journal.ppat.1004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immun. 2010;78:32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sivick KE, et al. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J Immunol. 2010;184:2065–2075. doi: 10.4049/jimmunol.0902386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sivick KE, Mobley HL. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun. 2010;78:568–585. doi: 10.1128/IAI.01000-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kantele A, et al. Antibody-secreting cells in acute urinary tract infection as indicators of local immune response. J Infect Dis. 1994;169:1023–1028. doi: 10.1093/infdis/169.5.1023. [DOI] [PubMed] [Google Scholar]
- 68.Kudinha T, et al. Escherichia coli sequence type 131 as a prominent cause of antibiotic resistance among urinary Escherichia coli isolates from reproductive-age women. J Clin Microbiol. 2013;51:3270–3276. doi: 10.1128/JCM.01315-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spellberg B, et al. Novel approaches are needed to develop tomorrow's antibacterial therapies. Am J Respir Crit Care Med. 2015;191:135–140. doi: 10.1164/rccm.201410-1894OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoberman A, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. New Engl J Med. 2014;370:2367–2376. doi: 10.1056/NEJMoa1401811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goneau LW, et al. Subinhibitory antibiotic therapy alters recurrent urinary tract infection pathogenesis through modulation of bacterial virulence and host immunity. mBio. 2015;6 doi: 10.1128/mBio.00356-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greene SE, et al. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio. 2014;5:e02038. doi: 10.1128/mBio.02038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dang HT, et al. Syntheses and biological evaluation of 2-amino-3-acyltetrahydrobenzothiophene derivatives; antibacterial agents with antivirulence activity. Org Biomol Chem. 2014;12:1942–1956. doi: 10.1039/c3ob42478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han Z, et al. Lead optimization studies on FimH antagonists: discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J Med Chem. 2012;55:3945–3959. doi: 10.1021/jm300165m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cusumano CK, et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med. 2011;3:109ra115. doi: 10.1126/scitranslmed.3003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Totsika M, et al. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis. 2013;208:921–928. doi: 10.1093/infdis/jit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guiton PS, et al. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob Agents Chemother. 2012;56:4738–4745. doi: 10.1128/AAC.00447-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jarvis C, et al. Antivirulence isoquinolone mannosides: optimization of the biaryl aglycone for FimH lectin binding affinity and efficacy in the treatment of chronic UTI. Chem Med Chem. 2016;11:367–373. doi: 10.1002/cmdc.201600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mühlen S, Dersch P. Anti-virulence strategies to target bacterial infections. Curr Top Microbiol Immunol. 2016 doi: 10.1007/82_2015_490. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Chorell E, et al. Design and synthesis of fluorescent pilicides and curlicides: bioactive tools to study bacterial virulence mechanisms. Chemistry. 2012;18:4522–4532. doi: 10.1002/chem.201103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Volkan E, et al. Molecular basis of usher pore gating in Escherichia coli pilus biogenesis. Proc Natl Acad Sci U S A. 2013;110:20741–20746. doi: 10.1073/pnas.1320528110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geibel S, et al. Structural and energetic basis of folded-protein transport by the FimD usher. Nature. 2013;496:243–246. doi: 10.1038/nature12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sarowar S, et al. The Escherichia coli P and type 1 pilus assembly chaperones PapD and FimC are monomeric in solution. J Bacteriol. 2016;198:2360–2369. doi: 10.1128/JB.00366-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hospenthal MK, et al. Structure of a chaperone-usher pilus reveals the molecular basis of rod uncoiling. Cell. 2016;164:269–278. doi: 10.1016/j.cell.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin LY, et al. Synthetic polymer nanoparticles conjugated with FimHA from E. coli pili to emulate the bacterial mode of epithelial internalization. J Am Chem Soc. 2012;134:3938–3941. doi: 10.1021/ja2091917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim YH, et al. Preparation and in vitro antimicrobial activity of silver-bearing degradable polymeric nanoparticles of polyphosphoester-block-poly(L-lactide) ACS Nano. 2015;9:1995–2008. doi: 10.1021/nn507046h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brumbaugh AR, et al. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect Immun. 2013;81:3309–3316. doi: 10.1128/IAI.00470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nesta B, et al. FdeC, a novel broadly conserved Escherichia coli adhesin eliciting protection against urinary tract infections. mBio. 2012;3 doi: 10.1128/mBio.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Langermann S, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 90.Spaulding CN, Hultgren SJ. Adhesive pili in UTI pathogenesis and drug development. Pathogens. 2016;5 doi: 10.3390/pathogens5010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hannan TJ, et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. E Bio Medicine. 2014;1:46–57. doi: 10.1016/j.ebiom.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gágyor I, et al. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection: randomised controlled trial. BMJ. 2015;351:h6544. doi: 10.1136/bmj.h6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wagers PO, et al. Imidazolium salts as small-molecule urinary bladder exfoliants in a murine model. Antimicrob Agents Chemother. 2015;59:5494–5502. doi: 10.1128/AAC.00881-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blango MG, et al. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS One. 2014;9:e93327. doi: 10.1371/journal.pone.0093327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brubaker L, Wolfe A. The urinary microbiota: a paradigm shift for bladder disorders? Curr Opin Obstet Gynecol. 2016;28:407–412. doi: 10.1097/GCO.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hilt EE, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52:871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nienhouse V, et al. Interplay between bladder microbiota and urinary antimicrobial peptides: mechanisms for human urinary tract infection risk and symptom severity. PLoS One. 2014;9:e114185. doi: 10.1371/journal.pone.0114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pearce MM, et al. The female urinary microbiome in urgency urinary incontinence. Am J Obstet Gynecol. 2015;213:347.e341–347.e311. doi: 10.1016/j.ajog.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thomas-White KJ, et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am J Obstet Gyn. 2016 doi: 10.1016/j.ajog.2016.07.049. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hung CS, et al. A murine model of urinary tract infection. Nat Protoc. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hannan TJ, Hunstad DA. A murine model for Escherichia coli urinary tract infection. Methods Mol Biol. 2016;1333:159–175. doi: 10.1007/978-1-4939-2854-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lo E, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464–479. doi: 10.1086/675718. [DOI] [PubMed] [Google Scholar]
- 103.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 104.Guiton PS, et al. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect Immun. 2010;78:4166–4175. doi: 10.1128/IAI.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Flores-Mireles AL, et al. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci Transl Med. 2014;6:254ra127. doi: 10.1126/scitranslmed.3009384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flores-Mireles AL, et al. Fibrinogen release and deposition on urinary catheters placed during urological procedures. J Urol. 2016;196:416–421. doi: 10.1016/j.juro.2016.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conover MS, et al. Establishment and characterization of UTI and CAUTI in a mouse model. J Vis Exp. 2015;2015:e52892. doi: 10.3791/52892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murawski IJ, et al. Vesico-ureteric reflux: using mouse models to understand a common congenital urinary tract defect. Pediatr Nephrol. 2011;26:1513–1522. doi: 10.1007/s00467-011-1821-1. [DOI] [PubMed] [Google Scholar]
- 109.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 110.Foxman B, et al. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol. 2003;13:144–150. doi: 10.1016/s1047-2797(02)00272-7. [DOI] [PubMed] [Google Scholar]
- 111.Boehm BJ, et al. Acute bacterial inflammation of the mouse prostate. The Prostate. 2012;72:307–317. doi: 10.1002/pros.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rudick CN, et al. Uropathogenic Escherichia coli induces chronic pelvic pain. Infect Immun. 2011;79:628–635. doi: 10.1128/IAI.00910-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olson PD, et al. Androgens enhance male urinary tract infection severity in a new model. J Am Soc Nephrol. 2016;27:1625–1634. doi: 10.1681/ASN.2015030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 115.Demarco ML, Burnham CA. Diafiltration MALDI-TOF mass spectrometry method for culture-independent detection and identification of pathogens directly from urine specimens. Am J Clin Pathol. 2014;141:204–212. doi: 10.1309/AJCPQYW3B6JLKILC. [DOI] [PubMed] [Google Scholar]
- 116.Johnson JR, et al. Rapid and specific detection, molecular epidemiology, and experimental virulence of the O16 subgroup within Escherichia coli sequence type 131. J Clin Microbiol. 2014;52:1358–1365. doi: 10.1128/JCM.03502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tchesnokova VL, et al. Rapid identification of rectal multidrug-resistant Escherichia coli before transrectal prostate biopsy. Urology. 2015;86:1200–1205. doi: 10.1016/j.urology.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]