Abstract
Background and Aims
Colonoscopy provides incomplete protection from colorectal cancer (CRC), but determinants of post-colonoscopy CRC are not well understood. We compared clinical features and molecular characteristics of CRCs diagnosed at different time intervals after a previous colonoscopy.
Methods
We performed a population-based, cross-sectional study of incident CRC cases in Denmark (2007–2011), categorized as post-colonoscopy or detected during diagnostic colonoscopy (in patients with no prior colonoscopy). We compared prevalence of proximal location and DNA mismatch repair deficiency (dMMR) in CRC tumors, relative to time since previous colonoscopy, using logistic regression and cubic splines to assess temporal variation.
Results
Of 10,365 incident CRCs, 725 occurred after colonoscopy examinations (7.0%). These were more often located in the proximal colon (odds ratio [OR], 2.34; 95% CI, 1.90–2.89) and were more likely to have dMMR (OR, 1.26; 95% CI, 1.00–1.59), but were less likely to be metastatic at presentation (OR, 0.65; 95% CI, 0.48–0.89) compared with CRCs diagnosed in patients with no prior colonoscopy. The highest proportions of proximal and/or dMMR tumors were observed in CRCs diagnosed 3–6 years after colonoscopy, but these features were still more frequent among cancers diagnosed up to 10 years after colonoscopy. The relative excess of dMMR tumors was most pronounced in distal cancers. In an analysis of 85 cases detected after colonoscopy, we found BRAF mutations in 23% of tumors and that 7% of cases had features of Lynch syndrome. Colonoscopy exams were incomplete in a higher proportion of cases diagnosed within less than 1 year (in 38%) than in those diagnosed within 1–10 years after colonoscopy (16%).
Conclusion
In a study of incident CRC cases in Denmark, we observed that tumors found in patients who have undergone colonoscopy are more often proximal and have dMMR, compared to CRCs detected in patients without previous colonoscopies. The excess of right-sided tumors and the modest independent effects of dMMR reinforce the importance of proper colonoscopic examination of the proximal large bowel.
Keywords: colorectal neoplasm, colon cancer, interval cancer, surveillance
Introduction
Colonoscopy with polypectomy reduces risk of subsequent colorectal cancer (CRC), and a negative examination portends a reduced risk as well.1,2 However, CRC diagnoses following a negative or clearing colonoscopy suggest that the protective effect of colonoscopy is weaker than originally estimated.3-8 Understanding how these cancers occur would inform interventions to optimize colonoscopy for CRC screening and prevention.
Colonoscopy quality is clearly implicated in post-colonoscopy or “interval” CRC, since risk has been associated with both endoscopist characteristics (e.g. specialty training and adenoma detection rates)6,9 and indicators of examination quality (e.g. preparation quality and completeness of examination).10,11 In addition, certain clinical characteristics have been found to be more common among post-colonoscopy CRCs, including older age at diagnosis, proximal tumor location, family history of CRC, and prior polypectomy.7,12,13 To date only a few studies have investigated the molecular characteristics of post-colonoscopy tumors, finding a relatively high prevalence of DNA microsatellite instability (MSI),5,14,15 CpG island methylator phenotypes (CIMP),16 and somatic BRAF mutations.17
Published data regarding molecular characteristics of post-colonoscopy CRC leave several questions unanswered, since relevant studies were not population-based and included only limited numbers of cases (167 in total).5,14-17 To date, all investigations have examined CRCs diagnosed at arbitrary intervals (e.g. 5 or 10 years) after colonoscopy, with little attention to possible interplay among molecular characteristics of post-colonoscopy cancers and clinical factors. The independent associations of each characteristic with risk of cancer at different time intervals after colonoscopy remain unclear.
To examine the clinical and molecular features of post-colonoscopy CRC, we conducted a population-based study comparing clinical characteristics, tumor location, and mismatch repair deficiency (dMMR) between cases diagnosed at first colonoscopy and those diagnosed at different time intervals after a colonoscopy. We also conducted a detailed analysis of the molecular characteristics of a subset of post-colonoscopy CRC cases and the quality of the colonoscopy that preceded them.
Methods
Study design
Using Danish medical registries, we conducted a population-based nationwide study of all CRCs diagnosed during 2007-2011. We subsequently characterized subjects with incident CRC as “diagnostic colonoscopy only” if their only prior colonoscopic exam was within 180 days of CRC diagnosis, or post-colonoscopy if they had at least 1 prior colonoscopy >180 days prior to diagnosis. The latest colonoscopy performed >180 days prior to CRC diagnosis was defined as the “index” examination.
Databases
Each Danish resident is assigned a unique civil registration number, facilitating linkage of individual-level data among registries.18 The Danish National Patient Registry (DNPR) has recorded all inpatient hospital encounters since 1977 as well as outpatient hospital encounters since 1995,19 covering essentially all colonoscopy in the country.20 The Danish Cancer Registry (DCR) records all incident cancers diagnosed nationwide, coded using the International Classification of Diseases, Tenth Revision (ICD-10 version), with diagnosis date, tumor location, and stage.21 The National Pathology Registry archives pathology results from all specimens examined since 1997, using International Systematized Nomenclature of Medicine (SNOMED) codes.22
Nationwide incident CRC cases
We queried the DCR to identify all individuals newly diagnosed with CRC during the 5-year study period. Their records were linked to the DNPR to identify individuals who underwent one or more colonoscopies before their CRC diagnosis. We defined post-colonoscopy cases as those with one or more examinations >180 days prior to their CRC diagnosis in order to minimize contamination with diagnostic examinations, as individual chart reviews were not performed in the nationwide analysis.
Data abstracted included demographics, tumor location (classified as proximal: cecum through transverse colon; distal: splenic flexure through rectum; or unspecified), CRC stage at diagnosis (classified as localized, regional, metastatic, or unknown), histopathologic features, history of inflammatory bowel disease (IBD), and tumor DNA mismatch repair (MMR) protein expression. Testing for dMMR has been performed routinely as part of the clinical histopathologic evaluation of CRC resection specimens since 2007, with results recorded in the Pathology Registry. Chart reviews conducted among the 19 Danish pathology institutes demonstrated that 12 had recorded dMMR status for >75% of CRC cases, whereas 7 recorded results less consistently (range: 0-64% of cases). To minimize potential bias, we restricted our study population to CRC cases evaluated at the 12 institutes performing dMMR testing regularly, representing 69% of CRC cases nationwide (Figure 1). dMMR status in CRC was assessed on the basis of immunohistochemistry (IHC) for MMR proteins with MLH1 and MSH2 in all cases, and for MSH6 and PMS2 in 74% and 45% of cases, respectively. Tumors exhibiting absent expression of one or more MMR proteins were classified as having dMMR and therefore microsatellite instability (MSI).
Figure 1.
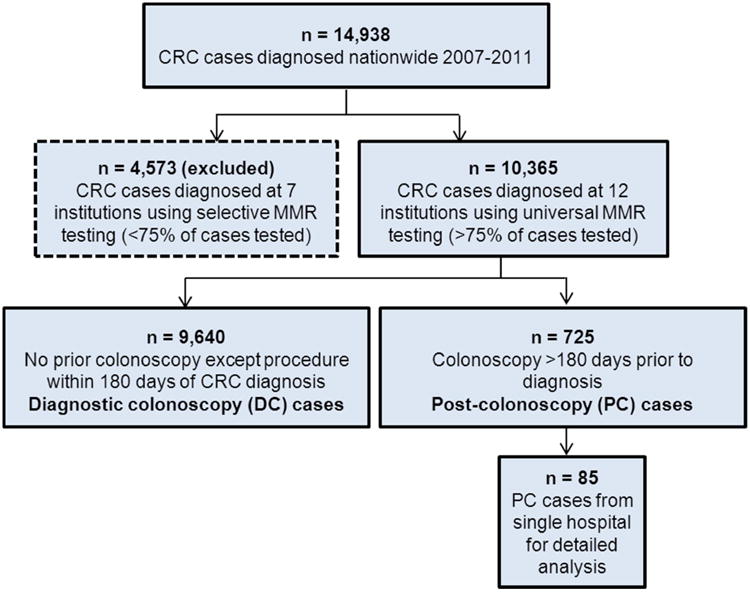
Flow chart of colorectal cancer (CRC) cases diagnosed nationwide in Denmark during 2007-2011, showing post-colonoscopy (PC) vs. diagnostic colonoscopy (DC) cases, and a hospital-based subsample of PC cases.
Hospital-based subset of Post-colonoscopy cases
Using the DCR, we ascertained CRC cases diagnosed at Aalborg University Hospital, serving a population of approximately 640,000. We identified 101 candidate post-colonoscopy cases identified >90 days after colonoscopy, with available endoscopic records and formalin-fixed paraffin-embedded tumor blocks. Medical records were reviewed to confirm post-colonoscopy status. The latest colonoscopy performed >90 days prior to CRC diagnosis was defined as the “index” examination. After record reviews, 16 (16%) cases were excluded: the index exam was flexible sigmoidoscopy, not colonoscopy (n=5); the CRC diagnosis was known at the time (or within 90 days) of the index colonoscopy (n=7); or pathology review did not confirm colorectal adenocarcinoma (n=4). Eighty-five post-colonoscopy CRC tumors remained for molecular analysis. Colonoscopy reports were abstracted to determine indication, bowel preparation quality, completeness of examination, findings including polyps (histology, number, and location), and polypectomy technique. Advanced adenomas were defined as those ≥10mm in size, and/or with high-grade dysplasia or villous histology.
An expert gastrointestinal pathologist (SRH) reviewed slides recut from the CRC tumor blocks without access to clinical information. For each tumor, nuclear expression of four DNA MMR proteins (MLH1, MSH2, MSH6, and PMS2) was evaluated by IHC.23,24 DNA extracted from microdissected cancer tissue was evaluated for somatic mutations in BRAF (V600E), KRAS (codons 12,13, 61, and 146), NRAS (codons 12,13), and PIK3CA (exons 9 and 20) using Sequenom MassARRAY methodology.25,26
CRCs demonstrating absent nuclear expression of one or more MMR proteins were characterized as dMMR. Cases with equivocal IHC status were investigated using PCR amplification of a DNA microsatellite marker panel (BAT-25, BAT-26, BAT-40, TGFbRII, D2S123, D5S345, and D17S250).27 dMMR tumors were further characterized as “sporadic” or “Lynch syndrome” using current diagnostic algorithms.28 Tumors with loss of MLH1 protein on IHC with BRAF gene mutation were classified as sporadic. Tumors with loss of MLH1 protein without BRAF mutation were studied further for MLH1 promoter hypermethylation using pyrosequencing.29 Tumors with loss of MLH1 protein without BRAF mutation or MLH1 promoter hypermethylation, and those showing loss of both MSH2 and MSH6 proteins, or MSH6 or PMS2 proteins only, were classified as consistent with Lynch syndrome.
Statistical Analysis
In the nationwide analysis, we used standard descriptive statistics, t-tests, and contingency table analyses to examine differences between clinical and molecular features of diagnostic colonoscopy and post-colonoscopy CRC cases diagnosed at various intervals after the index colonoscopy. For dichotomous risk factors, we used restricted cubic splines30 (knots at 1, 3, 6, 9, and 12 years) to assess variation in the post-colonoscopy:diagnostic prevalence ratio over time, adjusted for age at CRC diagnosis and gender (age only in analyses of gender). We visually estimated the duration of time that the post-colonoscopy:diagnostic differences persisted after the index colonoscopy, and the period during which they were greatest. Subsequently, we used logistic regression to compute odds ratios (ORs) and 95% confidence limits (CIs) to evaluate the associations between patient and tumor characteristics and post-colonoscopy vs. diagnostic status within the selected time periods after the index colonoscopy. As index colonoscopy exams were performed at >70 endoscopy centers, we grouped CRC cases into 12 study centers corresponding to the regional pathology institutes serving each center as a proxy to adjust for potential differences by hospital. Interactions were assessed using Wald tests. Models included age, sex, site of cancer (proximal vs. distal), MMR status (deficient vs. proficient), and stage (metastatic vs. not metastatic), with adjustment for hospital. Goodness of fit for the logistic regression models was assessed using the Hosmer Lemeshow test10. To assess the impact of our definition of diagnostic CRC, we performed a sensitivity analysis extending the definition of diagnostic CRC cases to include those diagnosed within 90 days following the index colonoscopy.
For post-colonoscopy cases from the hospital sample, we used descriptive statistics to characterize patient and index procedure factors and tumor molecular characteristics among CRC cases diagnosed <1 year, 1 - 10 years, and >10 years following their index colonoscopy. Proportions were compared with Fisher's exact tests.
Analyses were conducted using SAS statistical software (version 9.3 SAS Institute, Cary, North Carolina). This study was approved by the Danish Data Protection Agency (2011-41-5913), the Danish Ethics Board (M-20110163), and the Institutional Review Board of the University of Texas MD Anderson Cancer Center.
Results
Analysis of Nationwide Sample
In the national sample 10,365 incident CRC cases were identified during the study period at the pathology institutes included in the study: 725 (7%) post-colonoscopy cases and 9,640 (93%) diagnostic CRC cases (Table 1). Sixty eight (9%) post-colonoscopy cases were diagnosed within the first year after the index colonoscopy, 373 (51%) in the first 5 years, 566 (78%) in the first 10 years, and 159 (22%) more than 10 years later. Post-colonoscopy cases were older than diagnostic CRC cases and included a higher proportion of individuals with IBD (Table 1, p<0.001). However, there were no substantial differences by gender. Post-colonoscopy tumors were more often proximal and more often diagnosed at localized stages (p<0.001).
Table 1.
Characteristics of colorectal cancer cases diagnosed nationwide (2007-2011) according to time from index colonoscopy to diagnosis (N=10,365).a
| Tumor/patient characteristics | Diagnostic colonoscopy CRC | Post-colonoscopy CRC (N=725) by interval from index colonoscopy to CRC diagnosise | |||
|---|---|---|---|---|---|
|
| |||||
| <3y | <5y | <10y | ≥10y | ||
|
| |||||
| Number of cases | 9640 | 243 | 373 | 566 | 159 |
|
| |||||
| Age at CRC diagnosis (years) mean ± SD | 69.8 ± 11.1 | 73.5 ± 10.7 | 73.2 ± 10.4 | 73.8 ± 10.3 | 73.3 ± 11.5 |
|
| |||||
| Gender | |||||
| Male, N (%) | 5132 (53.2) | 131 (53.9) | 187 (50.1) | 261 (46.1) | 66 (41.5) |
| Female, N (%) | 4508 (46.8) | 112 (46.1) | 186 (49.9) | 305 (53.9) | 93 (58.5) |
|
| |||||
| Inflammatory Bowel Disease, N (%) | 56 (0.6) | 18 (7.4) | 31 (8.3) | 55 (9.7) | 16 (10.1) |
|
| |||||
| Tumor locationb | |||||
| Proximal, N (%) | 2948 (30.6) | 136 (56.0) | 200 (53.6) | 306 (54.1) | 80 (50.3) |
| Distal, N (%) | 6379 (66.2) | 89 (36.6) | 151 (40.5) | 233 (41.2) | 71 (44.7) |
|
| |||||
| Stagec | |||||
| Localized, N (%) | 3833 (39.8) | 104 (42.8) | 165 (44.2) | 253 (44.7) | 79 (49.7) |
| Regional, N (%) | 2995 (31.1) | 69 (28.4) | 106 (28.4) | 167 (29.5) | 45 (28.3) |
| Metastatic, N (%) | 1414 (14.7) | 29 (11.9) | 42 (11.3) | 62 (11.0) | 21 (13.2) |
|
| |||||
| MMR Statusd | |||||
| Proficient, N (%) | 6952 (72.1) | 150 (61.7) | 232 (62.2) | 341 (60.2) | 107 (67.3) |
| Deficient (Loss), N (%) | 1385 (14.4) | 59 (24.3) | 92 (24.7) | 150 (26.5) | 30 (18.9) |
| Absent MLH1 | 1190 (12.3) | 54 (22.2) | 82 (22.0) | 135 (23.9) | 25 (15.7) |
| Absent MSH2 | 127 (1.3) | 7 (2.9) | 9 (2.4) | 11 (1.9) | 3 (1.9) |
| Absent MSH6 only | 80 (0.8) | 0 | 2 (0.5) | 5 (0.9) | 3 (1.9) |
| Absent PMS2 only | 20 (0.2) | 0 | 1(0.3) | 1(0.2) | 0 |
Data restricted to 10,365 cases evaluated at 12 Danish institutes performing immunohistochemical testing for MMR proteins on >75% of CRC tumors, 2007-2011.
Unknown,tumor location: DC cases: 313 (3.2%); PC cases: 35 (4.8%).
Unknown stage: DC cases: 1398 (14.5%); PC cases: 98 (13.5%).
Not tested: DC cases: 1303 (13.5%); PC cases: 97 (13.4%).
Only tumors diagnosed > 180 days after index colonoscopy were included as PC cases. See Methods for details
Tumor MMR testing was available for 8,965 (86%) CRC cases in the study population; 1,565 (17%) were dMMR; of these, 1,350 (86%) exhibited loss of MLH1 expression, and 844 (5.4%) loss of MSH2, MSH6, or PMS2 proteins. The prevalence of dMMR tumors was higher among post-colonoscopy than diagnostic cases in all the follow-up periods studied, across all clinical sites (Table 1, Supplementary Figure 1). Including CRCs diagnosed > 90 days (rather than >180 days) after the index colonoscopy in the post-colonoscopy case definition did not change these results (Supplementary Table 1).
Compared to diagnostic cases, post-colonoscopy cancers were more often proximal and dMMR for up to 10 years after the index examination. The prevalence of these subtypes was highest among post-colonoscopy patients diagnosed 3-6 years after their index colonoscopies (Supplementary Figure 1).
During the 3-6 years following the index colonoscopy, when post-colonoscopy and diagnostic cases differed most, post-colonoscopy tumors were more likely to be located in the proximal colon [OR: 1.92 (95% CI 1.36, 2.72)] and to have evidence of dMMR [OR: 1.53 (95% CI 1.06, 2.23)]. Older age and earlier stage were also independently associated with post-colonoscopy tumor status (Table 2). After adjustment for age and sex, dMMR was not independently associated with post-colonoscopy status in the proximal colon [OR: 1.40 (95% CI 0.91, 2.14)], but approached statistical significance among the small numbers of distal tumors [OR 2.08 (95% CI 1.01, 4.26); p for interaction = 0.41] (Table 2). There were broadly similar findings for post-colonoscopy cases during the entire 10 year period during which the post-colonoscopy cases differed from the diagnostic cases (Table 2, Figure 2). Results were similar when using a more sensitive definition of post-colonoscopy cases diagnosed >90 days to 10 years after the index examination (data not shown).
Table 2.
Multivariate analysis of factors independently associated with post-colonoscopy colorectal cancer between 180 days and 10 years after index colonoscopy (n = 566 PC cases).
| Patient or tumor characteristic | PC cases 3 - 6 years mean ± SD or n (%) | PC cases 180 days - 10 years mean ± SD or n (%) | 3 - 6 years OR (95% CI) | 180 days - 10 years OR (95% CI) |
|---|---|---|---|---|
| Age at CRC diagnosis (years) | 73.2 ± 9.9 | 73.8 ± 10.3 | 1.02 (1.01, 1.04) | 1.03 (1.02, 1.04) |
| Female sex | 116 (59.8) | 305 (53.9) | 0.74 (0.54, 1.02) | 0.91 (0.75, 1.10) |
| Proximal tumor location | 104 (53.6) | 306 (54.1) | 1.92 (1.36, 2.72) | 2.33 (1.89, 2.88) |
| Metastatic at presentation | 21 (10.8) | 62 (11.0) | 0.68 (0.41, 1.13) | 0.66 (0.48, 0.90) |
| Mismatch repair-deficient | ||||
| Overall | 55 (28.4) | 140 (24.7) | 1.53 (1.06, 2.23) | 1.28 (1.02, 1.62) |
| Proximal | 46 (23.7) | 120 (21.2) | 1.40 (0.91, 2.14) | 1.18 (0.91, 1.54) |
| Distal | 9 (4.6) | 20 (3.5) | 2.08 (1.01, 4.26) | 1.85 (1.14, 3.01) |
Logistic regression modeling used to estimate odds ratios and 95% CI for post-colonoscopy cancer over specified time intervals, adjusting for age, age, sex, site of cancer (proximal vs. distal), MMR status (deficient vs. proficient), stage (metastatic vs. not metastatic), and clinical center
Figure 2.
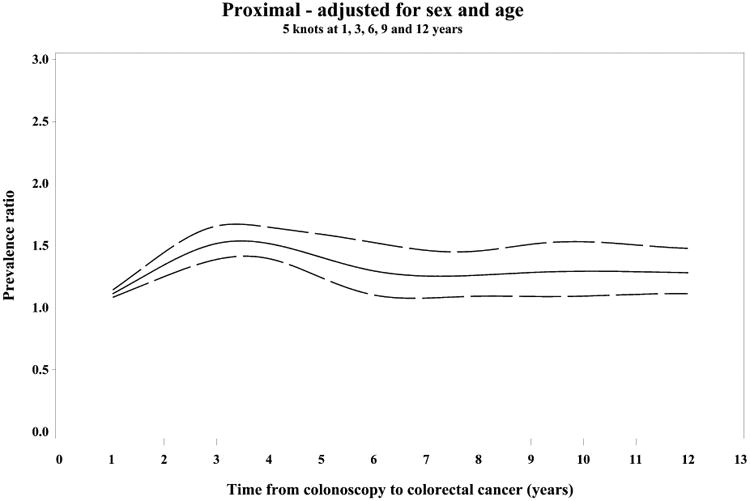
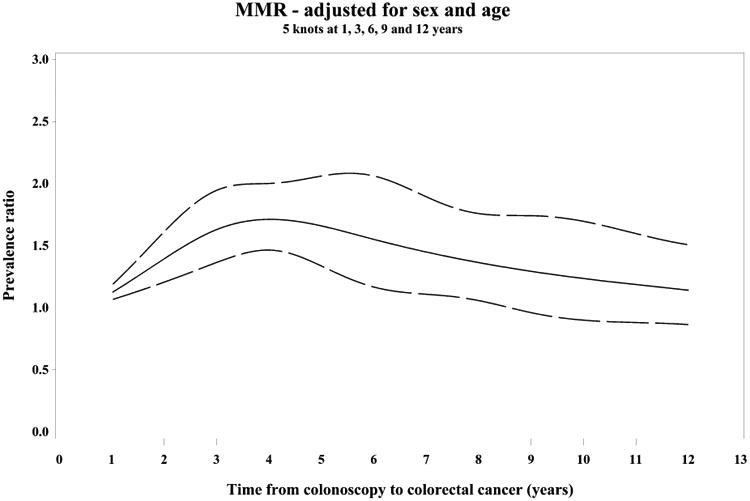
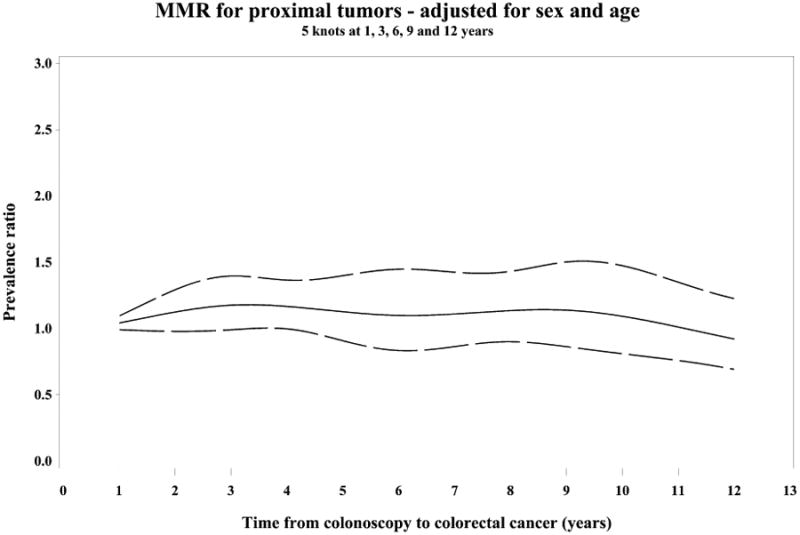
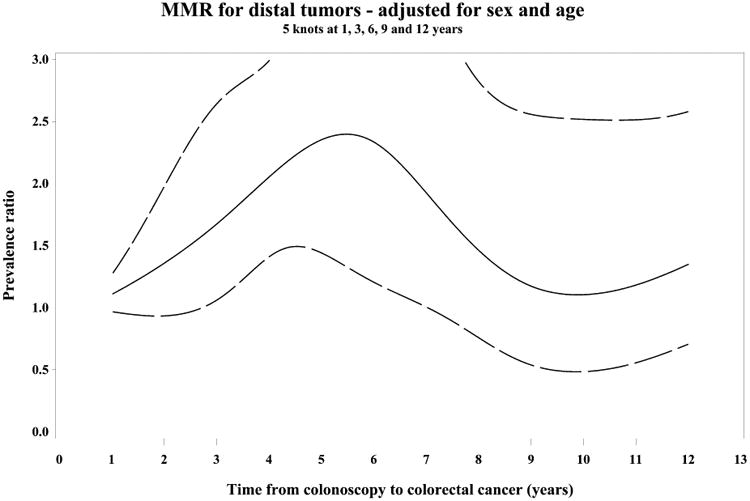
Restricted cubic spline analyses showing variation in the PC/DC prevalence ratio (PR) of patient and tumor characteristics over time since prior colonoscopy, adjusted for sex and age (PR= solid line, 95% CI=dashed lines)
(A) PR of proximal tumor location, (B) PR of mismatch repair deficiency (MMR)
PR of MMR stratified by tumor location proximal (C) and distal (D)
Splines have 5 knots at 1, 3, 6, 9 and 12 years.
Analysis of hospital-based subset of post-colonoscopy cases
The 85 post-colonoscopy cases were similar to the nationwide post-colonoscopy cases in age, gender, cancer stage, tumor location, and time between index colonoscopy and CRC diagnosis (Supplementary Table 2). The most frequent indications for the index examinations were symptoms (49%), polyp follow up (26%), and history of colitis (9%). Adenomas had been found in 27 (31%) subjects including 26 with advanced adenomas. Overall, quality of bowel preparation was not reliably recorded; however 18 (21%) post-colonoscopy cases had endoscopic reports from the index colonoscopy which noted that the exam was incomplete (specifically that the cecum was not reached), and this was more common in cases diagnosed at shorter intervals: 38% among those diagnosed in the first year following the index colonoscopy and 16% among those diagnosed 1 - 10 years after (p=0.07).
Overall, 20 (24%) post-colonoscopy tumors exhibited dMMR. In tumors diagnosed within 10 years of the index examination, BRAF mutations were identified in 16 (19%) tumors (12 were dMMR), KRAS/NRAS mutations in 23 (27%), and PIK3CA mutations in 16 (19%). A total of 6 (7%) tumors had molecular characteristics of Lynch syndrome (Figure 3). All Lynch syndrome cancers occurred within the first 10 years after index colonoscopy. Four post-colonoscopy cases (5%) occurring less than 10 years after the index colonoscopy had synchronous primary CRCs, three of which exhibited dMMR with somatic BRAF mutations, which are features of the serrated pathway.31
Figure 3.
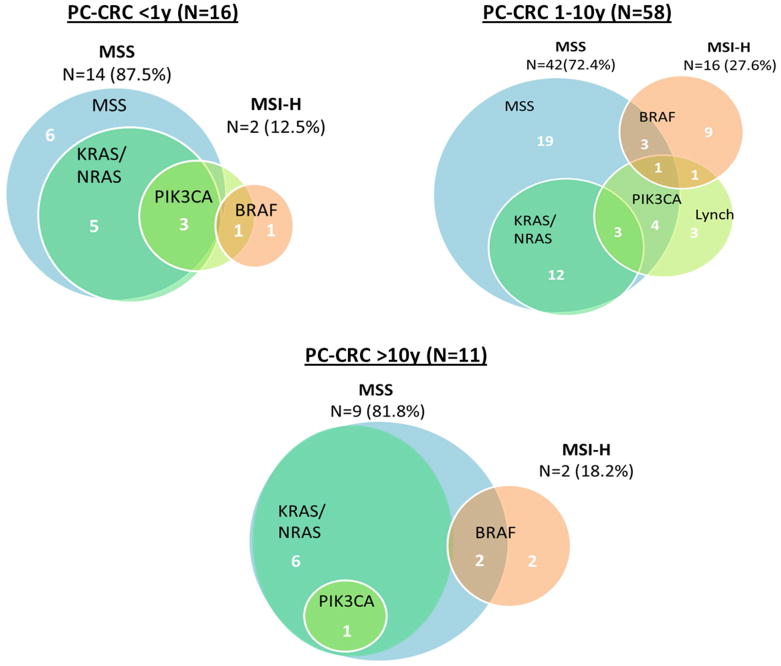
Distribution of molecular characteristics among hospital-based PC cases, by interval between colonoscopy and CRC diagnosis (n=85).
Note: Figures are not proportional between time intervals.
Area exterior to MSS circle represents MSI-high tumors.
Discussion
In this large population-based study of more than 700 CRC cases diagnosed 180 days to 20+ years after colonoscopy, we found that proximal location and dMMR were overrepresented among post-colonoscopy cancers diagnosed up to 10 years later. The characteristics of post-colonoscopy cases varied over time, with the highest prevalence of proximal location and dMMR 3 to 6 years after the index colonoscopy. The relative excess of dMMR tumors was more pronounced among cancers located in the distal colon. The nationwide findings were confirmed in analyses of post-colonoscopy cancers diagnosed up to 10 years after colonoscopy at a single center. Of these, 24% were dMMR, with 7% exhibiting molecular features of Lynch syndrome.
Our findings agree with previous research showing that CRCs diagnosed within 3 or 5 years after colonoscopy are more likely to be located in the proximal colon and exhibit dMMR than those diagnosed without a previous colonoscopy.5,14,15 Although some studies have suggested that the risk of interval cancer may be higher for women than men,32 we found no gender differences during the overall 10 year follow-up period.
Molecular characteristics of post-colonoscopy CRC have not been as well investigated as clinical features. Ours is the first population-based study to incorporate molecular phenotypes. Previous investigations of post-colonoscopy cases that reported molecular features included a total of 167 tumors diagnosed within 5 years of a colonoscopy (with a maximum cohort size of 63),5,13-16,17 in contrast to 725 included in our analysis (566 diagnosed within 10 years). Our findings demonstrate once again that proximal tumors and dMMR tumors are overrepresented among post-colonoscopy CRC cases.13,32,5,14 Our large sample size allowed use of multivariable analyses to estimate the independent effects of tumor location and molecular characteristics. Proximal location and dMMR commonly occur together in sporadic colorectal cancer, and we were able to demonstrate that while the association of proximal location with post-colonoscopy CRC is clear, the independent effect of dMMR is mostly exhibited in the distal colorectum.
Our analysis of the 85 hospital-based post-colonoscopy cases provided an opportunity to examine colonoscopy quality and molecular characteristics of tumors in more detail. Our finding of incomplete index examinations in 38% of post-colonoscopy cases diagnosed within the first year following a colonoscopy suggests that exam quality is important, especially in diagnoses made soon after colonoscopy. While the majority of sporadic as well as post-colonoscopy tumors develop through the chromosomal instability pathway, our molecular analyses confirmed that dMMR tumors are overrepresented among post-colonoscopy CRCs. Lynch syndrome-associated neoplasms arise through this mutator pathway and are known to progress rapidly to cancer (often within 3 years after colonoscopy).33,34 Interestingly, we did find that the prevalence of Lynch syndrome among post-colonoscopy cases (7%) was more than double the reported population prevalence of 2-3%35 and these tumors represented 1/3rd of dMMR cases within 10 years of a prior colonoscopy. We also found that 1 in 5 post-colonoscopy cases were BRAF-mutated, a feature of serrated pathway carcinogenesis.31 However, the lack of controls in this analysis makes these findings difficult to interpret.
A novel feature of our analysis is that we included post-colonoscopy CRCs diagnosed during a wide range of time intervals after index colonoscopy and found considerable heterogeneity in the proportions of post-colonoscopy cancers that were proximal and dMMR, depending on time since the index examination. Other strengths of this study include the large population-based sample with availability of data regarding MMR status. Our detailed review of medical records and tumors of a representative subset of post-colonoscopy cancers corroborates the nationwide findings.
However, we acknowledge that our study has certain limitations. Quality of colonoscopy exams is a determinant of effectiveness in preventing CRC, and is impacted by the success of the patient's bowel cleanout as well as the expertise of the endoscopist. While 80% of colonoscopies in Denmark are performed by gastrointestinal specialists36, detailed data regarding endoscopist and procedure characteristics (eg. adenoma detection rate, withdrawal time, cleanliness) were not available for the nationwide cases and thus the quality of individual index colonoscopy exams could not be assessed. A number of reviews of outcomes of CRC screening exams performed in Denmark have reported 89-93% of colonoscopies are “complete” 36,37,38 which is slightly below the quality benchmark of 95% cecal intubation rate, and lower than that reported in a European randomized clinical trial39. It is also worth noting that since colonoscopic screening for average risk individuals was not current practice in Denmark during the study period, most individuals who underwent colonoscopies had symptoms or previous polyps that prompted colonoscopic evaluation. Consequently, it is possible our findings may not be generalizable to populations in which high quality colonoscopy screening of asymptomatic average-risk individuals is more widely employed. We recognize that our analysis depends on the accuracy of our data. While the DCR offers near-complete ascertainment of cancer cases, it is possible that post-colonoscopy and diagnostic CRC cases may have been misclassified, as occurred in 15% of post-colonoscopy cases reviewed in our hospital sample (10% in the national sample with the 180 day time window).
Our finding that 7% of CRC cases in Denmark occurred in individuals who had undergone a prior colonoscopy is a clinical and medicolegal concern. Our data imply that the problem of post-colonoscopy cancers, though greatest about 3-6 years following colonoscopy, persists to some extent far longer than the 5-year time frame commonly used to define so-called “interval” cancers. The high proportion of proximal tumors, in conjunction with the frequency of incomplete colonoscopy among post-colonoscopy cases diagnosed within the first year following colonoscopy, supports the popular assumption that many cancers diagnosed soon after colonoscopy result from missed lesions. 40,36 However, the heterogeneity in clinical and molecular features of cancers diagnosed at different time intervals suggests post-colonoscopy CRCs are likely multifactorial in their etiology and clinical behavior. Studies consistently show that colonoscopy affords less protection against proximal cancers3,7,41,42 and dMMR tumors are more frequent among proximal cancers. The proximal and distal colon differ with respect to embryologic origin and gene expression profiles, prompting some to suggest that these might be considered as two distinct organs.43 Whether the precursors of post-colonoscopy CRCs are simply harder to detect and/or resect endoscopically, or whether their behavior differs on the basis of anatomic location and/or molecular subtype remains unclear and warrants further investigation. Our study adds to the literature supporting that a paramount concern is effective visualization of the proximal large bowel to maximize the effectiveness of colonoscopy.
Supplementary Material
Supplementary Figure 1. Prevalence of proximal tumor location (A) and mismatch repair deficiency (B) among post-colonoscopy colorectal cancer cases (N=628 cases with IHC results), stratified by sex. Female=blue, male=red. Dashed lines indicate prevalence of either factor among diagnostic colonoscopy cases for 3,926 females and 4,340 males; error bars indicate 95%
Supplementary Table 1. Sensitivity Analysis: Characteristics of CRC cases diagnosed nationwide (2007-2011) according to time from index colonoscopy to CRC diagnosis, defining PC as >90 days post-index colonoscopy (N=10,365)a
Supplementary Table 2. Endoscopic and tumor characteristics of post-colonoscopy CRC cases, by time from index colonoscopy to CRC diagnosis, Aalborg Hospital, 2000-2009 (N=85).
Acknowledgments
We appreciate the technical assistance provided by Dr. Raja Luthra and Trupti Methta for the laboratory work performed at MD Anderson.
Funding support: The study was supported by grants from the Danish Cancer Society (R73-A4284-13-S17), the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation, NIH/NCI Grant K07CA120448-5 (E. Stoffel), Cancer Center Support Grant CA16672 (MD Anderson Cancer Center), and The University of Texas Frederick Becker Distinguished University Chair in Cancer Research (S. Hamilton).
Footnotes
Conflicts of interest: No authors have conflicts to declare.
Author Contributions: Dr. Sørensen and Ms. Frøslev had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and Design: Baron, Erichsen, Stoffel, Sørensen
Acquisition, Analysis or Interpretation of Data: Baron, Crockett, Erichsen, Froslev, Koeppe, Hamilton, Pedersen, Stoffel, Sørensen, Vyberg
Drafting of the Manuscript: Stoffel, Baron, Sørensen, Erichsen, Crockett, Frøslev, Hamilton, Pedersen, Koeppe
Critical Revision of the Manuscript for Important Intellectual Content: Baron, Crockett, Erichsen, Frøslev, Hamilton, Koeppe, Pedersen, Stoffel, Sørensen, Vyberg
Statistical Analysis: Frøslev, Koeppe, Pedersen
Funding: Hamilton, Stoffel, Sørensen
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brenner H, Haug U, Arndt V, Stegmaier C, Altenhofen L, Hoffmeister M. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology. 2010 Mar;138(3):870–876. doi: 10.1053/j.gastro.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993 Dec 30;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011 Jan 4;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009 Mar;136(3):832–841. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006 Dec;131(6):1700–1705. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010 May 13;362(19):1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 7.Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012 Jun 15;118(12):3044–3052. doi: 10.1002/cncr.26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010 Dec;105(12):2588–2596. doi: 10.1038/ajg.2010.390. [DOI] [PubMed] [Google Scholar]
- 9.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014 Apr 3;370(14):1298–1306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosmer D, Lemeshow Stanley, editors. Applied Logistic Regression. 2nd. New York: Wiley and Sons; 2000. [Google Scholar]
- 11.Brenner H, Chang-Claude J, Jansen L, Seiler CM, Hoffmeister M. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: a population-based case-control study. Ann Intern Med. 2012 Aug 21;157(4):225–232. doi: 10.7326/0003-4819-157-4-201208210-00002. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014 Sep;109(9):1375–1389. doi: 10.1038/ajg.2014.171. [DOI] [PubMed] [Google Scholar]
- 13.Samadder NJ, Curtin K, Tuohy TM, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014 Apr;146(4):950–960. doi: 10.1053/j.gastro.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013 Sep 19;369(12):1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter JM, Pino MS, Austin TR, et al. Genetic mechanisms in interval colon cancers. Dig Dis Sci. 2014 Sep;59(9):2255–2263. doi: 10.1007/s10620-014-3134-2. [DOI] [PubMed] [Google Scholar]
- 16.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010 May;105(5):1189–1195. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 17.Shaukat A, Arain M, Thaygarajan B, Bond JH, Sawhney M. Is BRAF mutation associated with interval colorectal cancers? Dig Dis Sci. 2010 Aug;55(8):2352–2356. doi: 10.1007/s10620-010-1182-9. [DOI] [PubMed] [Google Scholar]
- 18.Frank L. Epidemiology. The epidemiologist's dream: Denmark. Science. 2003 Jul 11;301(5630):163. doi: 10.1126/science.301.5630.163. [DOI] [PubMed] [Google Scholar]
- 19.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scandinavian journal of public health. 2011 Jul;39(7 Suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 20.Erichsen R, Baron JA, Stoffel EM, Laurberg S, Sandler RS, Sorensen HT. Characteristics and survival of interval and sporadic colorectal cancer patients: a nationwide population-based cohort study. Am J Gastroenterol. 2013 Aug;108(8):1332–1340. doi: 10.1038/ajg.2013.175. [DOI] [PubMed] [Google Scholar]
- 21.Gjerstorff ML. The Danish Cancer Registry. Scandinavian journal of public health. 2011 Jul;39(7 Suppl):42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 22.Erichsen R, Lash TL, Hamilton-Dutoit SJ, Bjerregaard B, Vyberg M, Pedersen L. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and Data Bank. Clin Epidemiol. 2010;2:51–56. doi: 10.2147/clep.s9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Jesus-Monge WE, Gonzalez-Keelan C, Zhao R, Hamilton SR, Rodriguez-Bigas M, Cruz-Correa M. Mismatch repair protein expression and colorectal cancer in Hispanics from Puerto Rico. Fam Cancer. 2010 Jun;9(2):155–166. doi: 10.1007/s10689-009-9310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer prevention research. 2012 Feb;5(2):320–327. doi: 10.1158/1940-6207.CAPR-11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greaves WO, Verma S, Patel KP, et al. Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. J Mol Diagn. 2013 Mar;15(2):220–226. doi: 10.1016/j.jmoldx.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portier BP, Kanagal-Shamanna R, Luthra R, et al. Quantitative assessment of mutant allele burden in solid tumors by semiconductor-based next-generation sequencing. American journal of clinical pathology. 2014 Apr;141(4):559–572. doi: 10.1309/AJCP1JUGQMW7ZNTL. [DOI] [PubMed] [Google Scholar]
- 27.Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012 Jul 19;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellizzi AM, Frankel WL. Colorectal cancer due to deficiency in DNA mismatch repair function: a review. Advances in anatomic pathology. 2009 Nov;16(6):405–417. doi: 10.1097/PAP.0b013e3181bb6bdc. [DOI] [PubMed] [Google Scholar]
- 29.Djordjevic B, Barkoh BA, Luthra R, Broaddus RR. Relationship between PTEN, DNA mismatch repair, and tumor histotype in endometrial carcinoma: retained positive expression of PTEN preferentially identifies sporadic non-endometrioid carcinomas. Mod Pathol. 2013 Oct;26(10):1401–1412. doi: 10.1038/modpathol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. Journal of clinical epidemiology. 2009 May;62(5):511–517 e511. doi: 10.1016/j.jclinepi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012 Sep;107(9):1315–1329. doi: 10.1038/ajg.2012.161. quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based case-control study. Gut. 2012 Nov;61(11):1576–1582. doi: 10.1136/gutjnl-2011-301531. [DOI] [PubMed] [Google Scholar]
- 33.Engel C, Rahner N, Schulmann K, et al. Efficacy of Annual Colonoscopic Surveillance in Individuals With Hereditary Nonpolyposis Colorectal Cancer. Clin Gastroenterol Hepatol. 2009 Oct 14; doi: 10.1016/j.cgh.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Jarvinen HJ, Renkonen-Sinisalo L, Aktan-Collan K, Peltomaki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009 Oct 1;27(28):4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 35.Ladabaum U, Ford JM, Martel M, Barkun AN. American Gastroenterological Association Technical Review on the Diagnosis and Management of Lynch Syndrome. Gastroenterology. 2015 Sep;149(3):783–813 e720. doi: 10.1053/j.gastro.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 36.Andersen FH. Ugeskr Laeger (Danish) 2007 Feb 5;169(6):514–517. [PubMed] [Google Scholar]
- 37.Rolighed LALM. Ugeskr Laeger (Danish) 2008 Jun 16;170(25):2232–2234. [PubMed] [Google Scholar]
- 38.Dansk tarmkræftscreeningsdatabase Årsrapport 2014 Første 10 måneder 1. nationale screeningsrunde. https://www.sundhed.dk/content/cms/45/61245_dts%C3%A5rsrapport-2014_8-1-16_final_inklbilag.pdf2016.
- 39.Bretthauer M, Kaminski MF, Loberg M, et al. Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial. JAMA Intern Med. 2016 May 23; doi: 10.1001/jamainternmed.2016.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rex DK. Avoiding and defending malpractice suits for postcolonoscopy cancer: advice from an expert witness. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768–773. doi: 10.1016/j.cgh.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009 Jan 6;150(1):1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 42.le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2013 Jun 6; doi: 10.1136/gutjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 43.Carethers JM. One colon lumen but two organs. Gastroenterology. 2011 Aug;141(2):411–412. doi: 10.1053/j.gastro.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Prevalence of proximal tumor location (A) and mismatch repair deficiency (B) among post-colonoscopy colorectal cancer cases (N=628 cases with IHC results), stratified by sex. Female=blue, male=red. Dashed lines indicate prevalence of either factor among diagnostic colonoscopy cases for 3,926 females and 4,340 males; error bars indicate 95%
Supplementary Table 1. Sensitivity Analysis: Characteristics of CRC cases diagnosed nationwide (2007-2011) according to time from index colonoscopy to CRC diagnosis, defining PC as >90 days post-index colonoscopy (N=10,365)a
Supplementary Table 2. Endoscopic and tumor characteristics of post-colonoscopy CRC cases, by time from index colonoscopy to CRC diagnosis, Aalborg Hospital, 2000-2009 (N=85).


