Abstract
Angiotensin II is a key neuropeptide that acting within the brain hypothalamic paraventricular nucleus regulates neurohumoral outflow to the circulation. Moreover, an exacerbated angiotensin II action within the paraventricular nucleus contributes to neurohumoral activation in hypertension. While angiotensin II effects involve changes in paraventricular nucleus neuronal activity, the precise underlying mechanisms, cellular targets, and distribution of angiotensin II receptors within the paraventricular nucleus remain largely unknown. Thus, whether angiotensin II effects involve direct actions on paraventricular neurons, or whether it acts via intermediary cells, such as astrocytes, is still controversial. To address this important gap in our knowledge, we used a multidisciplinary approach combining patch-clamp electrophysiology in presympathetic paraventricular neurons and astrocytes, along with in vivo sympathetic nerve recordings and astrocyte-targeted gene manipulations. We present evidence for a novel mechanism underlying central angiotensin II actions, which involves astrocytes as major intermediary cellular targets. We found that angiotensin II AT1 receptor mRNA is expressed in paraventricular astrocytes. Moreover, we report that AngII inhibited glutamate transporter function, increasing in turn extracellular glutamate levels. This resulted in the activation of neuronal extrasynaptic NMDA-receptors, increased presympathetic neuronal activity, enhanced sympathoexcitatory outflow, and increased blood pressure. Together, our studies support astrocytes as critical intermediary cell types underlying brain angiotensin II regulation of the circulation, and indicate that angiotensin II-mediated neuronal and sympathoexcitatory effects are dependent on a unique neuro-glial signaling modality involving non-synaptic glutamate transmission.
Keywords: Angiotensin, astrocyte, hypothalamus, glutamate, transporter, sympathetic, autonomic
INTRODUCTION
Angiotensin II (AngII) is a critical neuropeptide involved in cardiovascular and fluid homeostasis. In addition to its peripheral actions, AngII acts upon central nervous system (CNS) type 1 receptors (AT1rR) to stimulate sympathetic and neuroendocrine outputs to influence cardiovascular function1. Importantly, AngII-mediated sympatho-humoral activation is now recognized as a critical mechanism in neurogenic hypertension and heart failure2–4.
Within the CNS circuitry involved in sympathetic regulation, the paraventricular nucleus of the hypothalamus (PVN) is a major center mediating central AngII action5, 6. AngII within the PVN stimulates sympathetic activity, whereas blockade of PVN AT1 receptors reduced sympathetic activity under different conditions, including challenges to fluid balance6, 7. Moreover, AngII actions within the PVN have been implicated in sympatho-humoral activation during hypertension and heart failure8, 9. Sympathetic outflow from the PVN is largely determined by the activity of presympathetic neurons that project to the rostral ventrolateral medulla (RVLM) and preganglionic sympathetic neurons in the spinal cord10, 11. Both in vivo5 and in vitro12, 13 studies compellingly showed that AngII stimulates PVN neuronal activity. However, reports on underlying mechanisms have been less consistent, and included activation of a postsynaptic mixcationic conductance12, oxidative stress14, and changes presynaptic input activity13. Moreover, recent studies showing that activation of both AngII and glutamate receptors within the PVN were shown to be necessary for maintaining blood pressure during water deprivation15, while blockade of glutamate receptors abrogated the antihypertensive effect of AT1 receptor blockade within the PVN16 support interactions between AngII and glutamate in the modulation of PVN activity. These studies altogether support the notion that AngII effects within the PVN are mediated by direct actions on PVN neurons. Still, a model that mechanistically explains these seemingly disparate mechanisms involving AngII actions in the PVN is missing.
We recently showed that in addition to transient synaptic glutamate actions, PVN neurons also display a persistent excitatory modality mediated by activation of extrasynaptic NMDARs by ambient glutamate levels17–19. Importantly, we showed that the magnitude of this tonic modality is dictated by the activity of astrocyte glutamate transporters (GLT1) that actively buffer extracellular glutamate17. Based on recent studies supporting the expression of AngII AT1 receptors in glial cells20, 21, and a contribution of astrocytes to AngII-driven sympathetic activity during heart failure22, we tested the hypothesis that astrocytes are key intermediaries for AngII actions in the PVN. Using a multidisciplinary approach combining patch-clamp recordings from presympathetic PVN neurons and astrocytes, along with whole-animal sympathetic nerve recordings and astrocyte-targeted gene manipulations, we show that AngII inhibits astrocyte GLT1 activity, enhancing extrasynaptic glutamate excitatory tone, thus indirectly stimulating neuronal activity and sympathoexcitatory outflow from the PVN, and that this effects involve astrocyte-derive oxidative stress.
METHODS
Animals
All the procedures were approved by Georgia Regents University and the University of Nebraska Institutional Animal Care and Use Committee guidelines. Details about strains are provided in the Supplemental Information.
Retrograde tracing
Presympathetic RVLM-projecting PVN neurons (PVN-RVLM) were identified by injecting rhodamine beads unilaterally into the brainstem region containing the RVLM. The location of the tracer was verified histologically. A sample of an RVLM injection is shown in Fig. S1. Animals were used for 3–4 days after surgery.
Patch clamp electrophysiology
Conventional whole-cell patch-clamp recordings from PVN-RVLM neurons were obtained from acute hypothalamic slices (250 μm thick). The internal solution in patch pipettes (3–7 MΩ) contained in mM: 140 potassium gluconate, 5 EGTA, 10 HEPES, 10 KCl, 0.9 MgCl2, 0.5 CaCl2, 4 MgATP, 0.3 NaGTP, and 20 phosphocreatine (Na+). Recordings were obtained with an Axopatch 700A amplifier (Axon Instruments, Foster City, CA). For current-clamp recordings, the mean firing rate was analysed in segments of 2 min before, during and after bath-applied drugs.
Microinjections into the PVN
Rats were anesthetized with α-chloralose (70-140 mg/kg, i.p) and urethane (0.75-1.5 g/kg, i.p).. The coordinates of the right PVN with reference to bregma were calculated as being, 1.5 mm posterior, 0.4 mm lateral, and 7.8 mm ventral to the dura. A needle (0.2 mm OD) connected to a microsyringe (0.5 μl) was lowered into the PVN and various drugs (50 – 100 nl) injected. Two days after injection, the rats were used for functional and immunohistological experiments. Representative examples and summary of injection locations into the PVN are shown in Figs. S2,S3.
Renal sympathetic nerve activity and arterial pressure measurements
Rats were anesthetized with α-chloralose (70-140 mg/kg, i.p) and urethane (0.75-1.5 g/kg, i.p). The left renal nerve was isolated and the electrical signal was recorded with the PowerLab (AD Instruments, CO) to monitor RSNA. The changes in integration and frequency of the nerve discharge were expressed as a percentage from basal value. Responses of MAP and HR were expressed as the absolute difference between the basal value and the value after each dose of a drug.
Western blotting
PVN punches were lysed in 50 μl lysis buffer (10 mM Tris, 1 mM EDTA, 1% SDS, 0.1% Triton X-100 containing complete protease inhibitor cocktail from BaculoGold) for 20 min at 4° C and samples subjected to electrophoresis. Protein content of lysates was estimated using the bicinchoninic acid method with bovine serum albumin as a standard (Pierce, Rockford, IL). An enhanced chemiluminescence substrate (Pierce Chemical, Rockford, IL) was used to visualize the signals, which were detected by Worklab digital image system. Image J (NIH) was used to quantify the signal. GAPDH was used as the housekeeping gene.
Hypothalamic astrocyte Isolation
Enriched astrocytes were isolated from the hypothalamus using Percoll density gradient. The resulting homogenate was centrifuged and the cell pellet was re-suspended in 70% isotonic Percoll. Cell counting and morphological analysis was performed using a handheld automatic cell counter (Scepter instrument, EMD Millipore, USA). Standard immunofluorescence was performed in order to characterize the population collected. To evaluate whether AT1a receptor mRNA was expressed in the collected astrocytes, we performed real time PCR experiments. β-actin was used as housekeeping gene. Formation of PCR product was monitored in real time using the 7500 Fast Real time PCR System (Applied Biosystems, USA). DNA bands were visualized through an ethidium bromide- treated 2% agarose gel electrophoresis. A PCR product band of 285bp for GLT1; 193bp for AT1a and 61bp for β-actin were expected.
Statistical Analysis
All values are expressed as mean± standard error mean (SEM), and passed a test for normality (D'agostino-Pearson test). Differences between groups were determined using paired or unpaired t tests, and were considered significant at p<0.05. All statistical analyses were conducted using GraphPad Prism 5.00 (GraphPad Software).
RESULTS
AngII increases PVN presympathetic neuronal firing activity and sympathetic outflow in a glutamate-dependent manner
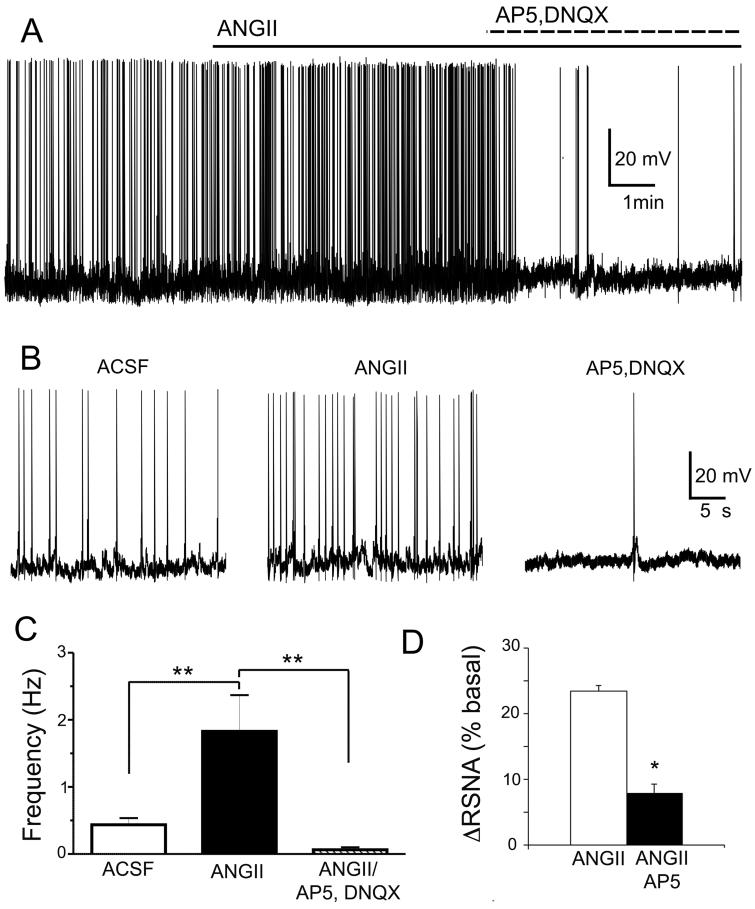
Bath-applied AngII (0.5μM) increased PVN-RVLM firing activity (~430%, P< 0.01, n=13), an effect that was largely blocked following glutamate receptor blockade (100 μM AP5 + 10 μM DNQX, P< 0.01 vs AngII alone, Fig.1A–C). Moreover, AngII directly microinjected into the PVN (0.2 nmol) induced a significant increase in RSNA, an effect blunted by a prior microinjection of the NMDA receptor blocker AP5 (2 nmol) (P< 0.05, n=5, Fig.1D,). As previously reported6 microinjection of AngII injection by itself increased RSNA, AP and HR (not shown).
Figure 1. AngII-driven presympathetic PVN neuronal activity and sympathoexcitatory output requires functional glutamate receptors.
A, Bath-applied AngII (0.5 μM) increases the firing activity of a presympathetic PVN neuron, an effect blocked by glutamate receptor antagonism. B, Representative segments of firing activity for each recording condition in A are shown at an expanded time scale. C, Summary data showing mean firing activity in each recording condition (n=13). D, Summary data showing mean percent changes in renal sympathetic nerve activity (RSNA) evoked by AngII (0.2 nmol) in control conditions, or in the presence of the NMDA receptor blocker AP5 (2 nmol, n=5). *P< 0.05
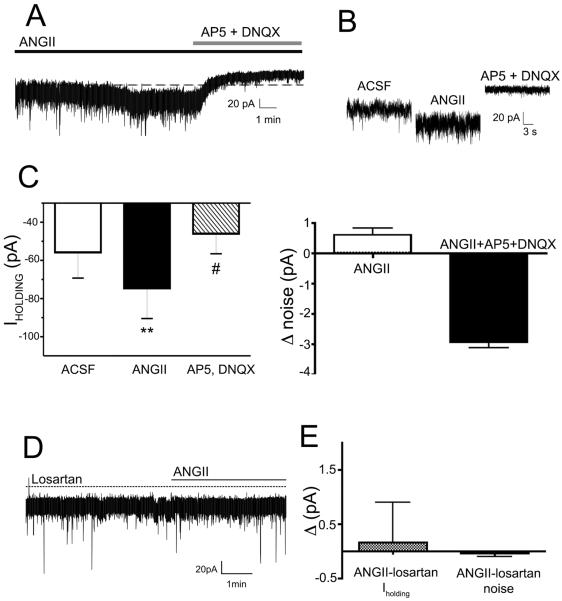
To further characterize the mechanism underlying the glutamate-dependent AngII effect within the PVN, we performed in vitro voltage-clamp experiments. Bath-applied AngII (0.5 μM) evoked a slow-developing inward current in PVN-RVLM neurons (ΔIholding −18.9 ± 4.8 pA, P< 0.01, n= 7), which also corresponded with an increase in RMS noise (Δ0.60 ± 0.2 pA, P< 0.05) (Fig.2A–C). The AngII-mediated inward current persisted in the presence of the Na+ channel blocker tetrodotoxin (TTX, 0.5 μM, ΔIηOλδινγ −17.6 ± 2.4 pA, P< 0.01, n= 5), but was largely blocked following glutamate receptor blockade, which shifted Iholding and reduced RMS noise beyond the baseline control levels (Fig.2A–C, P<0.05 for Iholding and RMS, n=7). Conversely, fast synaptic inward currents (representing a mix of glutamate and GABA PSCs), were not affected by AngII (PSC frequency: ACSF: 5.6 ± 1.6 Hz; AngII: 6.7 ± 1.8 Hz, n= 7, P> 0.2). In the presence of the AT1 receptor blocker losartan (10μM), AngII failed to induce a change in Iholding or RMS (P> 0.5, n=7, Fig.2D, E).
Figure 2. AngII evokes a sustained, glutamate-mediated inward current in presympathetic PVN neurons.
A, Bath-applied AngII (0.5 μM) induced an inward shift in Iholding that was blocked by glutamate receptor antagonism. Note the outward shift beyond baseline evoked by glutamate receptor blockade. B, Representative current segments for each recording condition in A are shown at an expanded time scale. C, Summary data showing mean Iholding values (left) and changes in mean RSM noise level (right) in each recording condition (n= 7). D, Preincubation of slices with the AT1a receptor blocker losartan (10 μM) occluded AngII effects on Iholding and RSM noise (n=7). **P< 0.01 vs. ACSF and AP5+DNQX; #P< 0.05 vs. ACSF.
AngII-driven PVN presympathetic neuronal activity and sympathoexcitatory outflow is prevented by blockade of astrocyte glutamate transporters
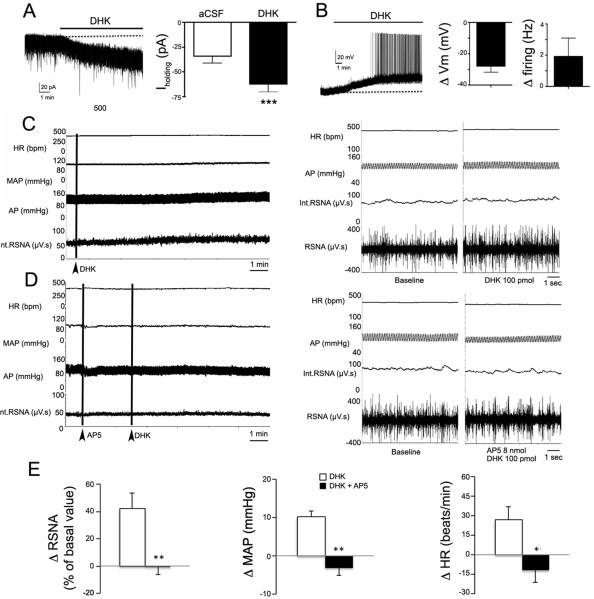
The glutamate-dependent, AngII-mediated slow inward current in PVN-RVLM neurons resembled the inward current that we recently reported in SON and PVN neurosecretory neurons following blockade of astrocyte glutamate transporters, which lead to a build-up of extracellular glutamate, subsequently activating an NMDAR-mediated inward shift in Iholding and increased RMS noise17, 19. Similar to neurosecretory neurons, selective GLT1 blockade (DHK, 300 μM) induced in PVN-RVLM neurons an inward shift in Iholding (ΔIholding −28.4 ± 5.7 pA, P< 0.01, n= 6, Fig.3A), membrane depolarization (ΔVm= 27.9 ± 3.8 mV, P< 0.01) and firing activity (Δfiring= 1.9 ± 1.1 Hz P< 0.05) (n= 4, Fig.3B), effects that as we previously described17 were almost completely blocked by glutamate receptor blockade (not shown).
Figure 3. Blockade of astrocyte GLT1 transporters increases firing activity of presympathetic neurons and sympathoexcitatory outflow from the PVN.
A, Bath-applied DHK (300 μM) induced a slowly developing inward shift in Iholding (left) in a presympathetic PVN neuron. The mean Iholding before and after DHK is shown in the right panel (n=6). B, Bath-applied DHK (300 μM) induced a slowly developing membrane depolarization and evoked firing discharge in a presympathetic PVN neuron. The mean change (delta) in Vm and firing rate evoked by DHK is shown in the right panels (n= 4). C, Representative sample showing that DHK microinjections directly into the PVN of an anesthetized rat (100 pmol) increased heart rate (HR), arterial pressure (AP), mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA; the integrated (int) and raw traces are shown). The left panel shows continuous traces, whereas the right panel shows segments of traces at expanded time scale. D, Representative traces showing that the DHK effect was prevented by the simultaneous microinjection of AP5 (2 nmol) into the PVN. The left panel shows continuous traces, whereas the right panel shows segments of traces at expanded time scale. E, Summary data showing changes in mean RSNA (% of basal value), MAP (Δ mmHg) and HR (Δ beats/min) evoked by DHK or DHK+AP5 (n=6). *P< 0.05; ** P < 0.01 and *** P < 0.001.
Microinjections of DHK (100 pmol) into the PVN increased RSNA, MAP and HR (n=6). These effects were completely blocked by simultaneous injections with the NMDA receptor blocker AP5 (2 nmol; P< 0.01 for RSNA and MAP, and P< 0.05 for HR, Fig.3C–E). These results indicate that astrocyte GLT1 transporters tonically restrain ambient glutamate levels, limiting in turn glutamate excitatory actions on PVN-RVLM neurons, as well as glutamate-mediated sympathetic outflow from the PVN.
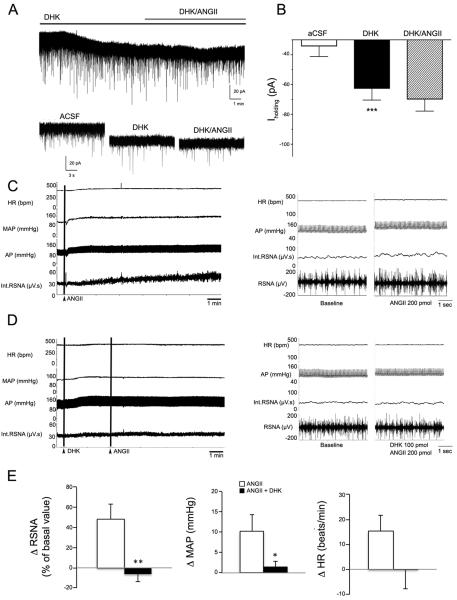
We found that in the presence of DHK, AngII failed to induce an inward shift in Iholding (P> 0.1, n=6, Fig.4A,B) as well as increases in RSNA and MAP (P<0.01 for RSNA and P< 0.05 MAP, n=9, Fig. 4C–E). Taken together, these studies suggest that AngII excitatory effects on PVN-RVLM neurons and sympathetic responses were mediated indirectly, via inhibition of glial GLT1 transporter activity.
Figure 4. Blockade of astrocyte GLT1 transporters occludes AngII-mediated stimulation of presympathetic neuronal activity and sympathoexcitatory outflow from the PVN.
A, Sample of DHK-induced inward shift in Iholding. Note that in the presence of DHK, AngII (0.5 μM) failed to induce any further shift in Iholding. Lower traces show expanded segments of the trace above. B, Summary data showing mean Iholding values in each recording condition (n=6). C, Representative sample showing that ANGII microinjections directly into the PVN of an anesthetized rat (200 pmol) increased heart rate (HR), arterial pressure (AP), mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA; the integrated (int) and raw traces are shown). The left panel shows continuous traces, whereas the right panel shows segments of traces at expanded time scale. D, Representative traces showing that the ANGII effects were occluded by the simultaneous microinjection of DHK (100 pmol) into the PVN. The left panel shows continuous traces, whereas the right panel shows segments of traces at expanded time scale. E, Summary data showing changes in mean RSNA (% of basal value), MAP (Δ mmHg) and HR (Δ beats/min) evoked by DHK or DHK+AP5 (n=6). *P< 0.05; ** P < 0.01 and *** P < 0.001.
AngII inhibits astrocyte glutamate transporter activity
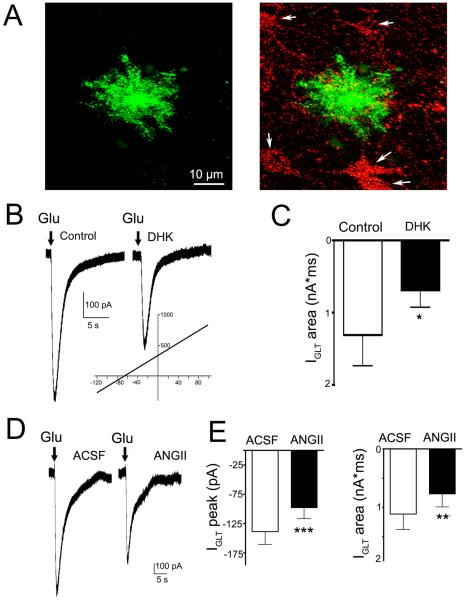
To directly test whether AngII modulated astrocyte glutamate transporter function, we obtained patch-clamp recordings from PVN astrocytes (n=16). Astrocytes were identified by their small rounded appearance, absence of action potentials, and by a characteristic linear current-voltage relationship (range: −120 mV – +100 mV, reversal potential −87.3 ± 2.5 mV) (Fig.5B, inset). Their identity was confirmed by intracellular staining, unveiling a typical astrocytic morphology, including thin and extended processes (Fig.5A). Astrocyte glutamate transporter currents (IGLT) were activated by puffing glutamate (5 mM, 250 ms duration, 3~5 PSI) onto the patched astrocytes in the presence of ionotropic and metabotropic glutamate receptor blockers. Glutamate evoked an inward current whose magnitude was partially but significantly reduced by the GLT1 blocker DHK (300 μM) (~40% inhibition; P< 0.05, n=7, Fig.5B,C), as previously reported in astrocytes elsewhere23, 24. Importantly, AngII (0.5μM) significantly diminished both the peak and the area of the evoked IGLT (~35% inhibition; P< 0.001 and P< 0.02 for peak and area, n=10, Fig.5D–E). A similar AngII-mediated inhibition of IGLT was observed in a small subset of astrocytes recorded from the SON (n=4, not shown).
Figure 5. AngII inhibits astrocyte glutamate transporter currents.
A, Representative example of a patched and intracellularly labeled PVN astrocyte (green, left) in very close proximity to retrogradely-labeled, RVLM-projecting PVN neurons (red, arrows). B, A brief puff of glutamate (5 mM, 250 ms, in the presence of ionotropic glutamate receptor blockers, Methods) onto the patched astrocyte evoked and inward current (left) that was partially blocked by glutamate transporter blockade (right, 300 μM DHK). Inset: A voltage ramp (−120 mV − +100 mV) evoked a characteristic linear I/V plot with a reversal potential of ~−80 mV). C, Summary of the mean astrocyte glutamate transporter current (IGLT) area in control and in the presence of DHK (n=7). D, Representative sample obtained from another patched astrocyte showing that in the presence of AngII (0.5 μM), the glutamate-mediated IGLT was diminished. E, Summary of the Mean astrocyte IGLT peak and area in control and in the presence of AngII (n= 10).
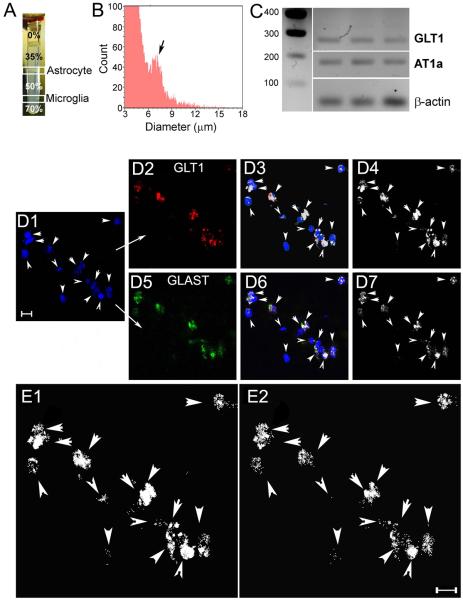
AT1 receptor mRNA is expressed in SON and PVN astrocytes
We performed real time PCR for AT1 receptor mRNA in isolated hypothalamic astrocytes (Fig.6A,B). The enriched astrocyte cell population had a mean cell body diameter of 6.96 ± 0.05 μm, and showed positive immunostaining for the astrocyte specific markers GLT1 and GFAP (Fig.6D–E). PCR performed on the isolated hypothalamic astrocytes showed amplification product for both GLT1 and for the AT1R mRNAs (Fig.6C). Conversely, no amplification was detected for IBA1 mRNA (a marker for microglia cells) or for VP mRNA, an abundant neuronal neuropeptide widely expressed in the hypothalamus. Similar results were observed in 6 independently performed studies.
Figure 6. AT1a receptor mRNA expression in isolated hypothalamic astrocytes.
A, astrocytes were isolated from rat brain hypothalami using a Percoll density gradient. Note the enriched astrocyte cell layer at the 35/50 interphase, which was clearly differentiated from the enriched microglia cell layer at the 50/75 interphase. B, A distribution histogram of extracted cell diameter showing the astrocyte cell population (arrow), with a peak diameter at ~ 7 μm is shown to the right. C, Ethidium bromide-stained gel showing real time PCR product amplification of GLT1 (285 bp); AT1a (193 bp) and b-actin (61 bp) in the extracted astrocyte cell population (n=3 rats). D1, Sample of an isolated astrocyte cell population (TOTO, blue, arrowheads) that displayed GLT1 positive staining (D2, red). D3 shows the overlap of TOTO and GLT1. The white color displays pixels containing both signals (colocalization, ImageJ plugin). In D4, only those pixels showing colocalization of TOTO and GLT1 are displayed. D5, GFAP staining (green) in the same isolated astrocyte population. D6 shows the overlap of TOTO and GFAP. The white color displays pixels containing both signals (colocalization, ImageJ plugin). In D7, only those pixels showing colocalization of TOTO and GFAP are displayed. E1 and E2, Images in D4 and D7 are shown at an expanded scale. Scale bars: 10 μm.
DISCUSSION
We present evidence for a novel mechanism underlying central AngII modulatory actions on neuronal activity and sympathetic outflow from the PVN, which involves astrocytes as major intermediary cell types. Specifically, we show that AngII inhibits astrocyte glutamate transporter function, leading to increased ambient glutamate levels, and subsequent activation of a sustained, extrasynaptic excitatory modality in presympathetic neurons.
AngII enhances tonic extrasynaptic glutamate function by inhibiting the activity of astrocyte glutamate transporters
A growing body of evidence supports that astrocytes regulate neuronal function and neurohumoral outflows from the SON and PVN25, 26. Astrocytes affect neuronal function partly via the release of gliotransmitters, including ATP, D-serine and nitric oxide27–29. Moreover, via the expression of selective neurotransmitter uptake transporters, including the GLT1 glutamate transporter, astrocytes influence the time course and concentration of neurotransmitters in the extracellular space. We recently showed that glutamate in the extracellular space in the PVN and SON evokes a persistent form of excitation that involves activation of extrasynaptic NMDA receptors, whose magnitude is determined by the activity of GLT1 expressed in astrocyte processes that enwrap SON/PVN neurons17–19. Thus, we found that either pharmacological blockade of GLT1 or activity-dependent removal of astrocyte processes from the vicinity of neurons induced a sustained NMDA-mediated inward current and increased firing activity17. These studies indicate that the basal activity of astrocyte GLT1 tonically influence neuronal excitability in the PVN. However, to what extent astrocyte GLT1 and the associated glutamate extrasynaptic modality are amenable to direct modulation by critical cardiovascular-related signals, remains unknown.
We report here that AngII evoked a slowly developing inward current in presympathetic neurons that was sensitive to both AT1 receptor and glutamate receptor blockade, supporting a contribution of glutamate to AngII excitatory actions. The interaction between AngII and glutamate could occur at different loci, including presynaptically13. However, the fact that blockade of GLT1 abrogated AngII effects suggests that AngII inhibited active astrocyte glutamate uptake, leading to a progressive increase in ambient glutamate levels and activation of extrasynaptic glutamate receptors17.
To more conclusively demonstrate a functional interaction between AngII and astrocyte GLT1 activity, we directly assessed GLT1 function in patched PVN astrocytes. Glutamate uptake is electrogenic, and can be quantitatively assessed as an inward current23. We found that the glutamate transporter-mediated inward current (IGLT) in PVN astrocytes was inhibited by AngII, blunting thus the glutamate buffering capacity of PVN astrocytes. These results are thus consistent with, and provide more mechanistic evidence for the AngII-evoked, glutamate-mediated increased firing activity in presympathetic neurons. The functional significance of the AngII effect on PVN astrocytes is further highlighted by our in vivo studies showing a contribution of PVN astrocytes to the AngII-evoked increase in sympathetic nerve activity and blood pressure. Our results are also consistent with a recent study showing that targeted-deletion of astrocyte AT1 receptors diminished sympathoexcitation in mice with heart failure22.
While previous studies demonstrated AngII effects on PVN neuronal activity and sympathetic outflow, whether these involved direct actions on presympathetic neurons, or alternatively targeted intermediary cells, was not systematically assessed. Taken together, our in vitro and in vivo studies combined support an indirect, astrocyte-dependent effect of AngII on PVN presympathetic neurons and sympathoexcitatory outflow, results that are consistent with earlier work demonstrating a lack of AT1 receptor expression in spinal cord- and medulla-projecting PVN neurons30. A caveat to take into account is that the RVLM is a cellularly heterogeneous nucleus 31, in which PVN projections likely innervate RVLM neurons other than those involved in regulating sympathetic outflow to the circulation. Thus, it is possible that our in vitro recordings included functionally diverse PVN-RVLM neuronal populations. Nevertheless, our in vivo studies do support that the basic mechanisms described in vitro play an important role in regulating cardiovascular-related sympathoexcitatory outflow.
An important question however that remains to be more conclusively determined is whether AngII exerted a direct effect on PVN astrocytes to inhibit GLT1 function, or whether it involved other intermediary cells. Our studies showing the expression of AT1 receptor mRNA in an isolated hypothalamic astrocyte cell population would support the possibility of a direct AngII action. Moreover, the fact that AngII effects on the astrocyte-dependent extrasynaptic tonic glutamate current in PVN-RVLM neurons persisted in the presence of TTX would argue against AngII evoking an activity-dependent release of another intermediary signal from a different neuronal source. Admittedly however, the cellular distribution of AT1 receptors in the brain is still controversial, with studies supporting20, 21, or arguing against32, 33 the presence of AT1 receptors in non-neuronal cells. Moreover, the specificity of commercially available antibodies raised against the AT1 receptor is questionable34. Yet, numerous studies, including our own, have shown AT1 receptor-mediated actions on astrocytes and microglial cells20, 35. Conversely, there is more consensus supporting increased AT1 receptor expression in glial cells in pathological conditions, including inflammation, hypertension and heart failure 22, 36.
The specific source of the AngII that could activate the astrocyte-mediated stimulation of presympathetic neuronal activity within the PVN is at present unknown, but could include subfornical organ (SFO)-PVN projections that utilize AngII as a neurotransmitter37, 38, or locally produce AngII, given that all the RAS components have been shown to be present within the PVN1. Moreover, we recently showed that circulating AngII could leak into the PVN due to breakdown of the blood brain barrier integrity during hypertension39.
An important question that remains open is the precise signaling pathway by which AngII influences astrocyte GLT1 function. Superoxide is a key mediator of central AngII actions, contributing to AngII pressor and sympathoexcitatory effects, as well as to exacerbated sympathoactivation in hypertension and heart failure40, 41. Notably, a previous study showed that ROS inhibited glutamate transport activity in cortical astrocytes, leading to excitotoxicity42. Thus, future studies will be needed to determine whether AngII-induced ROS production in PVN astrocytes contributed to GLT1 inhibition.
PERSPECTIVES
It is long established that AngII is a critical neuropeptide that acting within the CNS, participates in the central regulation of cardiovascular function, and contributes to exacerbated sympathetic activation in hypertension. However, the precise cellular targets and underlying mechanisms mediating central AngII actions remain largely unknown. We provide here evidence for a novel mechanism by which AngII modulates neuronal activity and sympathetic outflow. Contrary to the prevailing notion that AngII directly targets CNS neurons, our study supports astrocytes and extrasynaptic glutamate signaling as key intermediaries and novel signaling mechanisms by which AngII stimulates presympathetic neuronal activity and sympathetic outflow from the PVN. We believe this is novel and fundamental information that will contribute to obtain a more comprehensive understanding of pathophysiological mechanisms contributing to AngII-dependent hypertension.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
We demonstrate for the first time that ANGII effects on presympathetic neuronal activity in the PVN and sympathetic regulation by these neurons, is largely mediated by AngII actions on local astrocytes. We also demonstrate for the first time that ANGII can modulate glutamate excitatory signaling by directly modulating astrocyte glutamate transporters.
What Is Relevant?
The present study unveils a novel cellular target and mechanism of action for ANGII in the brain that involves a unique neuro-glial signaling modality. This provides a mechanistic foundation for the development of novel glial-targeted therapies for the treatment of ANGII-dependent hypertension.
Summary
The present studies support astrocytes as novel cellular targets underlying brain AngII regulation of the circulation, and indicate that AngII-mediated neuronal and sympathoexcitatory effects are dependent on a unique neuro-glial signaling modality involving non-synaptic glutamate transmission
Acknowledgments
SOURCE OF FUNDING This work was supported by NIH grants R01 HL112225 and R01 HL090948 to JES and NIH grants R56 HL124104 and P01 HL62222 to KPP.
Footnotes
DISCLOSURES None
REFERENCES
- 1.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: Location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 2.Fink GD. Long-term sympatho-excitatory effect of angiotensin ii: A mechanism of spontaneous and renovascular hypertension. Clin Exp Pharmacol Physiol. 1997;24:91–95. doi: 10.1111/j.1440-1681.1997.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 3.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin ii and dietary salt: Converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 4.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 5.Bains JS, Potyok A, Ferguson AV. Angiotensin ii actions in paraventricular nucleus: Functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- 6.Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the pvn: Role of nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1035–1043. doi: 10.1152/ajpregu.00338.2004. [DOI] [PubMed] [Google Scholar]
- 7.Chen QH, Toney GM. At(1)-receptor blockade in the hypothalamic pvn reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1844–1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- 8.Glass MJ, Wang G, Coleman CG, Chan J, Ogorodnik E, Van Kempen TA, Milner TA, Butler SD, Young CN, Davisson RL, Iadecola C, Pickel VM. Nmda receptor plasticity in the hypothalamic paraventricular nucleus contributes to the elevated blood pressure produced by angiotensin ii. J Neurosci. 2015;35:9558–9567. doi: 10.1523/JNEUROSCI.2301-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma NM, Llewellyn TL, Zheng H, Patel KP. Angiotensin ii-mediated posttranslational modification of nnos in the pvn of rats with chf: Role for pin. Am J Physiol Heart Circ Physiol. 2013;305:H843–855. doi: 10.1152/ajpheart.00170.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- 11.Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience. 1999;88:949–957. doi: 10.1016/s0306-4522(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 12.Cato MJ, Toney GM. Angiotensin ii excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: An in vitro patch-clamp study in brain slices. J Neurophysiol. 2005;93:403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li DP, Chen SR, Pan HL. Angiotensin ii stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G, Coleman CG, Chan J, Faraco G, Marques-Lopes J, Milner TA, Guruju MR, Anrather J, Davisson RL, Iadecola C, Pickel VM. Angiotensin ii slow-pressor hypertension enhances nmda currents and nox2-dependent superoxide production in hypothalamic paraventricular neurons. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1096–1106. doi: 10.1152/ajpregu.00367.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman KL, Brooks VL. At(1) and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1675–1682. doi: 10.1152/ajpregu.00623.2006. [DOI] [PubMed] [Google Scholar]
- 16.Gabor A, Leenen FH. Central neuromodulatory pathways regulating sympathetic activity in hypertension. Journal of applied physiology. 2012;113:1294–1303. doi: 10.1152/japplphysiol.00553.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming TM, Scott V, Joe N, Naskar K, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic nmda receptor signaling in neurosecretory neurons. J Physiol. 2011;589:3929–3941. doi: 10.1113/jphysiol.2011.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naskar K, Stern JE. A functional coupling between extrasynaptic nmda receptors and a-type k+ channels under astrocyte control regulates hypothalamic neurosecretory neuronal activity. J Physiol. 2014;592:2813–2827. doi: 10.1113/jphysiol.2014.270793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Astrocytes modulate a postsynaptic nmda-gabaa-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci. 2012;33:631–640. doi: 10.1523/JNEUROSCI.3936-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandalam U, Clark MA. Angiotensin ii activates jak2/stat3 pathway and induces interleukin-6 production in cultured rat brainstem astrocytes. Regul Pept. 2010;159:110–116. doi: 10.1016/j.regpep.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Sumners C, Tang W, Paulding W, Raizada MK. Peptide receptors in astroglia: Focus on angiotensin ii and atrial natriuretic peptide. Glia. 1994;11:110–116. doi: 10.1002/glia.440110206. [DOI] [PubMed] [Google Scholar]
- 22.Isegawa K, Hirooka Y, Katsuki M, Kishi T, Sunagawa K. Angiotensin ii type 1 receptor expression in astrocytes is upregulated leading to increased mortality in mice with myocardial infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2014;307:H1448–1455. doi: 10.1152/ajpheart.00462.2014. [DOI] [PubMed] [Google Scholar]
- 23.Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 24.Luscher C, Malenka RC, Nicoll RA. Monitoring glutamate release during ltp with glial transporter currents. Neuron. 1998;21:435–441. doi: 10.1016/s0896-6273(00)80552-8. [DOI] [PubMed] [Google Scholar]
- 25.Stern JE, Filosa JA. Bidirectional neuro-glial signaling modalities in the hypothalamus: Role in neurohumoral regulation. Auton Neurosci. 2013;35:8245–57. doi: 10.1016/j.autneu.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: From synapse to systems physiology. J Neuroendocrinol. 2012;24:566–576. doi: 10.1111/j.1365-2826.2011.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biancardi VC, Son SJ, Sonner PM, Zheng H, Patel KP, Stern JE. Contribution of central nervous system endothelial nitric oxide synthase to neurohumoral activation in heart failure rats. Hypertension. 2011;58:454–463. doi: 10.1161/HYPERTENSIONAHA.111.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial atp to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 29.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived d-serine controls nmda receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 30.Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (at1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol. 2001;13:139–146. doi: 10.1046/j.1365-2826.2001.00597.x. [DOI] [PubMed] [Google Scholar]
- 31.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, Marques-Lopes J, Young CN, Butler SD, Davisson RL, Iadecola C, Pickel VM, Pierce JP, Milner TA. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience. 2012;226:489–509. doi: 10.1016/j.neuroscience.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Callaghan EL, Bassi JK, Porrello ER, Delbridge LM, Thomas WG, Allen AM. Regulation of angiotensinogen by angiotensin ii in mouse primary astrocyte cultures. J Neurochem. 2011;119:18–26. doi: 10.1111/j.1471-4159.2011.07406.x. [DOI] [PubMed] [Google Scholar]
- 34.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension. 2013;61:253–258. doi: 10.1161/HYPERTENSIONAHA.112.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchtbauer L, Groth-Rasmussen M, Holm TH, Lobner M, Toft-Hansen H, Khorooshi R, Owens T. Angiotensin ii type 1 receptor (at1) signaling in astrocytes regulates synaptic degeneration-induced leukocyte entry to the central nervous system. Brain Behav Immun. 2011;25:897–904. doi: 10.1016/j.bbi.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: Critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–376. doi: 10.1159/000211202. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol. 1993;265:R302–309. doi: 10.1152/ajpregu.1993.265.2.R302. [DOI] [PubMed] [Google Scholar]
- 39.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin ii gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63:572–579. doi: 10.1161/HYPERTENSIONAHA.113.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res. 2004;94:402–409. doi: 10.1161/01.RES.0000112964.40701.93. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin ii infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 42.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.