Abstract
The unusual subset of patients with severe hepatitis, hypergammaglobulinemia, arthritis, and LE cells in the blood reported by Henry Kunkel and others suggested to these investigators that “lupoid” hepatitis might share pathogenic mechanisms with SLE. More than half a century later, the etiology of autoimmune hepatitis remains unclear. The occurrence of autoimmune hepatitis in a small fraction (about 3%) of SLE patients in our lupus cohort and in two mouse models of SLE supports their conclusion that lupoid hepatitis may be share pathogenic mechanisms with SLE. The development of autoimmune hepatitis in mice with pristane-induced lupus provides an opportunity to further explore the potential link between these two autoimmune disorders.
INTRODUCTION
Autoimmune “lupoid” hepatitis was described independently in the 1950’s by Henry Kunkel and others (1). For an extensive review of the history of lupoid hepatitis, see (2). The initial patients in Kunkel’s case series, predominantly young women, had a history suggestive of acute hepatitis and developed cirrhosis (1). Other manifestations included arthritis, amenorrhea, fatigue, unexplained fever, extreme hypergammaglobulinemia, and the presence of LE cells in the blood. In most cases, the patients died within 10 years of diagnosis from liver failure or bleeding esophageal varices. Liver pathology showed nodular cirrhosis with small areas of necrosis, fibrosis, and intense lymphoplasmacytic infiltrates containing numerous plasma cells (1). The disease responded to cortisone treatment, which along with the systemic manifestations and the presence of LE cells led the authors to suggest a relationship to collagen vascular diseases, although it was noted that many of the signs and symptoms of classic systemic lupus erythematosus (SLE) were lacking and that cirrhosis is rare in SLE.
Although the term “lupoid” hepatitis has fallen out of favor today, a mechanistic link between autoimmune hepatitis and systemic lupus erythematosus (SLE) has not been excluded. Recent data suggest that this link may involve more than just the presence of LE cells and production of antinuclear antibodies (ANA). We review the clinical features of lupoid hepatitis and present evidence for an overlap syndrome of SLE and lupoid hepatitis in humans and mice.
Clinical features of lupoid hepatitis
Clinically, lupoid hepatitis is characterized by elevated transaminases (AST, ALT), specific autoantibodies, elevated γ-globulin levels (hypergammaglobulinemia) that is usually confined to the IgG fraction, and a liver biopsy showing interface hepatitis (3). Most commonly, the disease progresses slowly (if untreated) to cirrhosis and is associated with an increased risk of hepatocellular carcinoma. The disease was classified into Type 1 and Type 2 in 1987 based on autoantibody specificities (4, 5). Autoantibodies associated with Type 1 autoimmune hepatitis (“lupoid” hepatitis”) include anti-smooth muscle antibodies (SMA), anti-filamentous (F)-actin antibodies (AAA), atypical anti-neutrophil cytoplasmic antibodies (p-ANCA), and ANA. Autoantibodies associated with Type 2 autoimmune hepatitis, which occurs primarily in young women and girls, include anti-liver-kidney-microsomal 1 (LKM1, specific for the cytochrome P450 enzyme CYP2D6) and anti-liver cytosol 1 (LC1, specific for formiminotransferase cyclodeaminase) antibodies (3). Anti-soluble liver antigen (SLA) and liver-pancreas (LP) antibodies are identical (5), and may be seen in both Type 1 and Type 2 autoimmune hepatitis. Anti-SLA may be the only autoantibody detected in some patients. There is little evidence that these autoantibodies are directly involved in disease pathogenesis.
Type 1 disease has a 4:1 female to male ratio and Type 2 a 10:1 ratio. The incidence in white Europeans is 0.1–1.9 per 100,000 per year with a prevalence of 16.9 per 100,000. Some patients have overlapping features of autoimmune hepatitis along with either primary biliary cirrhosis (PBC), which is characterized by anti-mitochondrial antibodies, or primary sclerosing cholangitis. The existence of other autoimmune hepatitis overlap syndromes with cryptogenic hepatitis, sarcoidosis, hepatitis C, or non-alcoholic steatohepatitis (NASH) is more controversial (5). However, chronic portal inflammation is present in over half of adults and children with NASH (6). Autoantibodies may be seen in these patients, including ANA (15–30% positive) and SMA (~5% positive) (6–8), but they have no relationship to the presence/absence or severity of portal inflammation (6). However, in one study 4% of autoantibody positive patients had a liver biopsy showing overlapping features of NASH with autoimmune hepatitis and there was an association of high titer ANA (but not SMA) with insulin resistance (7).
Detection of autoantibodies in autoimmune hepatitis
Initially, the levels of various autoantibodies associated with AIH were determined by indirect immunofluorescence using rodent tissues. However, as more has become known about the antigenic specificities, labor-intensive immunofluorescence tests are being replaced by ELISAs, particularly in the case of anti-SMA (5, 9). The commercial anti-F-actin ELISA (Inova Diagnostics) has a high sensitivity (100% in one study) to detect anti-SMA positive autoimmune hepatitis sera and ELISA units correlate strongly with anti-SMA titers (9). Moreover, the sensitivity of the ELISA for detecting patients meeting the diagnostic criteria for autoimmune hepatitis is higher than that of the anti-SMA immunofluorescence assay (74% vs. 34%) and there is no added benefit of testing for both anti-SMA and AAA (9).
MATERIALS AND METHODS
Patients
Patients with SLE previously enrolled in the UF Lupus Registry were screened for the clinical diagnosis of autoimmune hepatitis, which was based on histological evaluation of liver biopsies, elevated serum IgG or globulin, and elevated serum transaminases (AST, ALT). Anti-SMA and anti-LKM-1 were determined by indirect immunofluorescence assay (IFA, Quest Diagnostics, Madison, NJ). ANA were detected on Hep-2 cells (Inova Diagnostics, San Diego, CA) using 1:100 diluted mouse serum and FITC-conjugated goat anti-human IgG antibodies following the manufacturer’s protocol. Anti-Sm, RNP, Ro/SS-A, and La/SS-B antibodies were detected by ELISA (Inova) at a 1:100 serum dilution. All patients had a positive ANA, as expected based on their primary diagnosis of lupus or lupus-related disease. The diagnosis of SLE was established by ACR criteria (10). Healthy controls without a known history of autoimmune disease were recruited from staff and other individuals without autoimmune disease who were seen in the Rheumatology Clinic. Type I interferon levels were evaluated by measuring CD64 expression on blood monocytes and patients were classified as “low”, “intermediate”, or “high” interferon producers as described (11, 12). All patients gave informed consent before they were included in the study.
Mice
Mice were maintained under specific pathogen-free conditions. C57BL/6 (B6) and BALB/c mice were from Jackson Laboratory (Bar Harbor, ME). To induce lupus, 0.5 mL of pristane (Sigma-Aldrich, St. Louis, MO) was administered i.p. Controls were left untreated. Serum was tested at 6-months for anti-U1A (anti-RNP) autoantibodies by ELISA (12). Mice were euthanized at 10–11 months and the liver was fixed in formalin and paraffin-embedded. These studies were approved by the Institutional Animal Care and Use Committee.
Pathological evaluation of liver biopsies
Formalin-fixed, paraffin-embedded human and mouse liver tissue was sectioned (4 μm) and stained with hematoxylin & eosin (H&E), periodic acid-Schiff reagent (PAS), or trichrome. After heat-induced epitope retrieval, double immunohistochemistry (IHC) of human liver sections was performed as previously described (#23966} using the following primary antibodies: anti-κ and anti-λ light-chain, anti-CD4, anti-CD8, anti-CD68 (prediluted, from Dako, Carpinteria, CA), and anti-cleaved caspase 3 (1:300, Cell Signaling Technology, Danvers, MA). Reaction product was visualized using an ultraView DAB detection kit (κ L-chain, CD4, CD68, brown) or an ultraView Universal Alkaline Phosphatase Red Detection Kit (λ L-chain, CD8, cleaved caspase-3, red) (Vertana).
Anti-F-actin ELISA
Anti-F-actin antibodies (AAA) were detected by commercial ELISA (Inova Diagnostics, San Diego, CA) following the manufacturer’s protocol. For the second antibody, we substituted 1:1000 alkaline phosphatase-conjugated goat anti-human IgG or goat anti-mouse IgG antibodies (Southern Biotechnology, Birmingham, AL). Serum samples were tested at a 1:100 dilution. Murine serum samples were tested 6 months after pristane treatment.
Statistical analysis
Data are presented as mean ± SD. For normally distributed data, comparisons between mean values were performed by the unpaired 2-tailed Student t test using GraphPad (San Diego, CA) Prism version-5 software. Comparisons for non-normally distributed data were made using the Mann-Whitney test. Correlation was analyzed using Spearman’s rank correlation coefficient (GraphPad). p < 0.05 was considered significant.
RESULTS
Although abnormal liver enzymes are common in SLE patients, they are most frequently explained by hepatotoxic drug exposure, viral hepatitis, or NASH (13). The liver lesions of “classic” SLE are relatively trivial compared with those of autoimmune “lupoid” hepatitis (2, 14). However, cases of lupoid hepatitis have been reported in SLE patients, suggesting that an overlap syndrome of SLE-AIH may exist (13). We examined the UF Lupus Registry to identify patients with autoimmune hepatitis.
Lupoid hepatitis in patients with systemic autoimmune disease
Of 174 patients with extensively characterized systemic autoimmune disease, we identified 5 with autoimmune hepatitis, 3 of 109 who met ACR criteria for SLE (2.8%) and 1 patient each with Raynaud’s phenomenon and Hashimoto thyroiditis, CREST syndrome (Table 1). All had elevated transaminases (AST/ALT) and histological evidence of autoimmune hepatitis on liver biopsy.
Table 1.
Lupoid hepatitis patients in a systemic autoimmune disease cohort
| Patient | Age, race, gender, Dx (# of SLE criteria) | RNP/Sm1 | Ro/La1 | SMA2 (AAA)3 | LKM2 | Globulin4 (total IgG)5 |
|---|---|---|---|---|---|---|
| 1839 | 62 y.o. WF, AIH, Raynaud’s, Hashimoto (1) | RNP | (−) | 1:80 (0.96) | n.d.* | (n.d.) (globulin 4.2 g/dL) |
| 4031 | 29 y.o. BF, SLE, Graves’, AIH (4) | RNP, Sm | Ro | Negative (negative) | (negative) | 1.8 g/dL |
| 4134 | 24 y.o. BF, SLE/PM, AIH (5) | RNP, Sm | Ro | 57 (1.82) | (negative) | 2.0 g/dL |
| 4111 | 21 y.o. LF, SLE, uveitis, AIH (6) | RNP, Sm | Ro, La | unable to interpret (negative) | (negative) | 4.9 g/dL |
| 4135 | 71 y.o. BF, CREST, PBC/AIH (1) | (negative) | (negative) | Negative (negative) | (negative) | 1.9 g/dL |
commercial ELISA;
commercial immunofluorescence assay;
ELISA (Fig. 2);
commercial chemistry assay;
commercial nephelometry (mg/dL);
n.d., not done
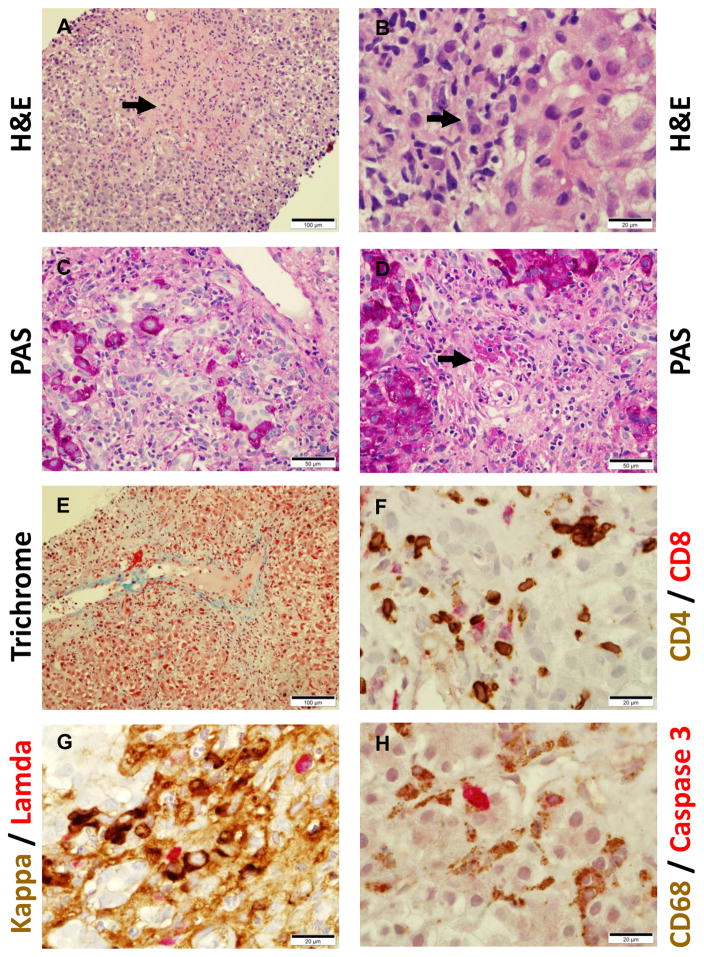
A representative liver biopsy from patient #4031 showed severe active hepatitis (mixed inflammation of portal tracts and lobules including plasma cells), confluent necrosis, architectural collapse, and regenerative changes (Fig. 1). This patient is a 26-year-old woman with a past history of Graves’ disease seen for evaluation of SLE. Clinical manifestations included arthritis, class V lupus nephritis with nephrotic syndrome, and elevated AST and ALT (3096 and 1344 units/L, respectively) with an alkaline phosphatase of 240 units/L. She had a strongly positive ANA (> 1:1280 speckled) and positive anti-Sm, -RNP, -ribosomal P, -Ro, and -dsDNA antibodies (1:10,240 by Crithidia luciliae kinetoplast staining assay), and hypocomplementemia (C3 52 mg/dL, C4 15 mg/dL). Serum protein and albumin were 4.1 g/dL and 1.7 g/dL (globulin 2.7 g/dL), respectively and serum IgG was 1829 mg/dL with a normal IgA (275 mg/dL) and IgM (97 mg/dL). Hepatitis B and C screening and both anti-SMA and LKM-1 antibodies were negative. She was treated initially with prednisone 60 mg/d, hydroxychloroquine 400 mg/d, and mofetil mycophenolate (MMP) 1500 mg twice daily, but due to development of an MMP allergy, was subsequently treated for lupus nephritis with cyclophosphamide 500 mg i.v. every 2 weeks X 6 doses (Euro-Lupus protocol). Her renal disease responded dramatically to therapy, but after stopping cyclophosphamide, her AST and ALT levels rebounded. She was subsequently treated with azathioprine 150 mg/d with normalization of her transaminases. The patient’s liver biopsy showed bridging fibrosis (Fig. 1A, D, E), mixed lymphoplasmacytic infiltrates with polyclonal (both κ and λ L-chain+) plasma cells and CD4+ > CD8+ T cells involving the portal tracts and lobules with marked interface activity (Fig. 1B, D, F, and G), confluent liver cell necrosis (Fig. 1B and D), bile duct proliferation and disorganization (Fig. 1C), and fibrosis (Fig. 1D, E). Cell death could be demonstrated by IHC using an antibody to cleaved caspase-3, and uptake of the dead cells by CD68+ liver macrophages appeared to be impaired (Fig. 1H). There were no diagnostic inclusions on PAS/PAS-D stains and iron stain was negative (not shown).
Figure 1.
Liver pathology of formalin-fixed tissue from a 29-year-old Black woman with SLE and autoimmune hepatitis. A, hematoxylin & eosin (H&E) stain showing bridging fibrosis (arrow); B, H&E stain showing plasma cell (arrow) and mononuclear cell infiltrates; C, periodic acid-Schiff (PAS) stain showing bile duct proliferation and disorganization; D, PAS stain showing liver cell necrosis, inflammatory cell infiltrates, and fibrosis; E, trichrome stain showing portal tract collagen fibrosis. F–H show double immunohistochemistry (IHC, peroxidase staining is shown in brown and alkaline phosphatase staining in red) after antigen recovery. F, double IHC for CD4+ (brown) and CD8+ (red) T cells; G, double IHC of plasma cells for intracellular for κ (brown) and λ (red) L-chain; H, double IHC of CD68+ liver macrophages (brown) and cleaved caspase-3+ dead cells (red).
Of the five patients, four (3 SLE and 1 Raynaud’s) had only autoimmune hepatitis and one (with CREST syndrome) had evidence of overlapping autoimmune hepatitis and primary biliary cirrhosis (Table 1). All 5 patients’ sera contained ANA, and 4 of 5 (80%) were anti-Sm and/or RNP positive vs. 3 of 5 (60%) anti-Ro or La positive (Table 1). Two sera were anti-SMA positive. Anti-LKM antibodies were not detected. All of the patients had elevated IgG or γglobulin.
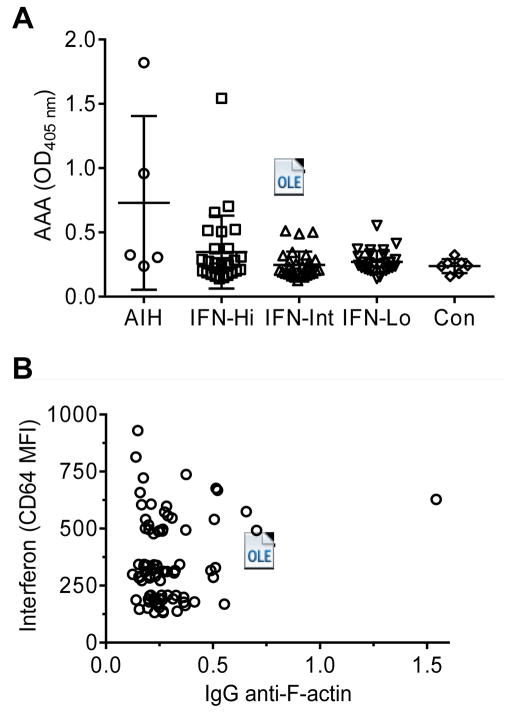
When sera from the five patients were screened by ELISA, both of the anti-SMA+ (immunofluorescence assay) sera were positive and all of the anti-SMA− sera were negative by ELISA (Fig. 2A). Cases of autoimmune hepatitis have been reported in multiple sclerosis patients treated with IFNβ1a (15, 16) and in a hepatitis C patient treated with IFNα2b (17). Since SLE is associated with elevated levels of type I IFN (IFNα, IFNβ), we examined whether the lupoid hepatitis was associated with high IFN-I levels in SLE patients. We previously defined groups of SLE patients who are IFN signature low (monocyte CD64 MFI < 270, mean 206), intermediate (MFI 271–392, mean 337), and high (MFI > 392, mean 517) (12). Range of the CD64 MFI in healthy controls was 96–384 (mean 249). Interferon levels (CD64 MFI) in the SLE patients with autoimmune hepatitis were 202 and 231 (not determined in one patient). We also tested sera from SLE patients in the IFN low, intermediate, and high groups for AAA and found one high-positive and 10 low-positive samples (Fig. 2A). The patient with high-positive AAA did not clinically exhibit evidence of liver disease. IgG AAA frequencies were not statistically different in the IFNhigh subset vs. the IFNintermediate and IFNlow subsets (Student t-test), although there was a trend toward more positive sera in the IFNhigh subset. The level of AAA was not significantly correlated with monocyte CD64 levels (Fig. 2B).
Figure 2.
Serum autoantibodies in SLE patients. A, levels of IgG anti-F-actin antibodies (AAA) (ELISA) in sera from patients with autoimmune hepatitis (AIH), in SLE patients with high, intermediate, or low levels of type I interferon (IFN), or in healthy controls (Con). High positive control O.D.405 nm = 2.24; low positive O.D.405 nm = 0.32; background O.D.405 nm = 0.08; B, correlation of IgG anti-F-actin autoantibody levels (ELISA) with interferon levels (monocyte CD64 expression, mean fluorescence intensity by flow cytometry).
Autoimmune hepatitis in murine lupus
Intraperitoneal injection of pristane (2,6,10,14 tetramethylpentadecane) induces a TLR7- and IFN-I-mediated autoimmune disease closely resembling human SLE in BALB/c, C57BL/6 (B6) and other non-autoimmune strains of mice (18). The disease is characterized by the production of lupus autoantibodies (anti-Sm, -RNP, -dsDNA, -Su, -ribosomal P and others), polyclonal hypergammaglobulinemia, over-production of IFN-I (IFNα and IFNβ), lupus nephritis, anemia, diffuse alveolar hemorrhage, and arthritis (18). The presence of pristane in the peritoneum results in the formation of chronic inflammatory tissue (“lipogranulomas”), some of which were attached to the surface of the liver (Fig. 3A, “Lipo”). At 10–12 months after pristane treatment of B6 mice, but not at 6-months, the liver underlying these lipogranulomas often exhibited coagulative necrosis and cell death (Fig. 3A, red box and yellow arrowhead; Fig. 3B, yellow arrowhead). Bile stasis (Fig. 3C, yellow arrowhead) and bile duct proliferation (Fig. 3D, yellow arrowhead) as well as inflammation (Fig. 3D, white arrowhead) were seen. Pristane-treated BALB/c mice also developed liver inflammation at 10-months (Fig. 3E–F). These changes were absent in liver from untreated mice (Fig. 3G, H). The cellular infiltrate consisted primarily of lymphocytes and plasma cells (Fig. 3F, *), primarily in a peri-portal distribution (Fig. 3E, F, yellow arrowheads). However, fibrosis was not seen, consistent with relatively mild liver inflammation.
Figure 3.
Liver pathology in pristane-treated mice. B6 (A–D) and BALB/c (E–F) mice were treated with pristane and 10 months later, liver was formalin-fixed and stained with hematoxylin & eosin. Controls were untreated B6 mice (G–H). A, liver underlying a pristane-induced lipogranuloma (Lipo) showing coagulative necrosis (yellow arrowhead). Examination of the area within the red box at high power (B) showing coagulative necrosis and cell death (yellow arrowhead). C, bile stasis (yellow arrowhead). D, extensive bile duct proliferation (yellow arrowhead) and lymphoplasmacytic inflammatory infiltrates (white arrow). E (low power) and F (high power), lymphoplasmacytic infiltrates in a peri-portal distribution (yellow arrowheads) in liver tissue from a 10-month pristane-treated BALB/c mouse. Plasma cells are indicated by *. G (low power) and H (high power), absence of liver inflammation in tissue from an untreated B6 mouse.
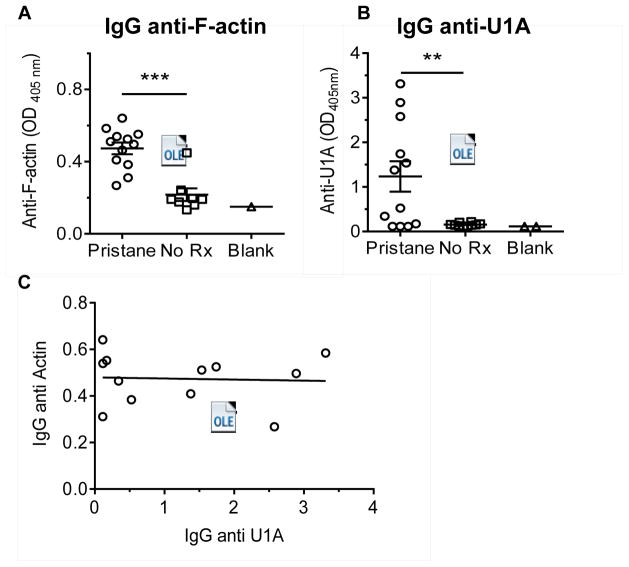
Sera from pristane-treated B6 mice (6-months after injection) contained increased levels of IgG AAA antibodies by ELISA compared with untreated age-, sex-, and strain-matched controls (Fig. 4A). Consistent with our previous observations, anti-U1A (RNP) autoantibodies also were increased in the pristane-treated mice (Fig. 4B). Since anti-U1A autoantibodies in both mice (18, 19) and humans (20) are associated with over-production of IFN-I, we examined the levels of IgG AAA vs. anti-U1A antibodies. However, levels of the two autoantibodies were not correlated (Fig. 4C).
Figure 4.
Autoantibodies in 6-month pristane-treated B6 mouse sera. A, levels of IgG anti-F-actin autoantibodies (AAA) in sera from pristane-treated mice and untreated controls were determined by ELISA at a serum dilution of 1:100. B, levels of IgG anti-U1A (RNP) autoantibodies were determined by ELISA using recombinant human U1-A protein (1:100 serum dilution). C, correlation of the levels of IgG AAA and IgG anti-U1A autoantibodies in the sera.
DISCUSSION
Type 1 autoimmune (“lupoid”) hepatitis occurs preferentially in women and is associated with joint symptoms, polyclonal hypergammaglobulinemia, LE cells, and ANA. These features led Kunkel, et al. in an early report to suggest a link with SLE. More recently, the disease has been considered to be unrelated to SLE. Here we describe a subset of patients who meet established clinical criteria for SLE (~3% of our lupus cohort) plus three additional patients with other systemic autoimmune diseases (Sjogren’s syndrome, CREST syndrome, Raynaud’s phenomenon) who have an overlap syndrome with biopsy-proven lupoid hepatitis. Four of the five patients (and all three of the SLE patients) had anti-Sm and/or RNP autoantibodies, a specificity associated with the overproduction of IFN-I in mice and humans (18, 20). There are reports of autoimmune hepatitis developing in the setting of IFNα or IFNβ therapy (15–17). However, the SLE-lupoid hepatitis patients did not have extraordinarily high levels of IFN-I and there was not a significant correlation between IgG AAA and CD64 MFI on monocytes (Fig. 2). There is substantial evidence that some drugs, mainly minocycline and nitrofurantoin, can induce autoimmune hepatitis (21, 22). Interestingly, these drugs also are associated with ANA and drug-induced lupus (23, 24). To begin to address the pathogenic mechanisms, we turned to pristane-induced lupus, a mouse model in which disease and autoantibody production are dependent on TLR7-stimulated IFN-I production (18).
Lupoid hepatitis in mice
Although an animal model of type 2 autoimmune hepatitis has been developed by immunization of mice with human liver autoantigens (FTCA and CYP450 2D6) (25), it has proven difficult until recently to develop an animal model of type 1 autoimmune hepatitis (2). It has been found that mice deficient in the phosphatidylserine receptors tyro3, axl, and mertk (“TAM” receptors) develop a spontaneous liver disease closely resembling autoimmune hepatitis (26). TAM receptors play a key role in the non-inflammatory clearance of apoptotic cells and mice deficient in all three receptors or in mertk alone develop manifestations of lupus, including nephritis, arthritis, and production of anti-dsDNA autoantibodies, but not anti-Sm/RNP (27, 28). Engagement of apoptotic cells by the TAM receptors negatively regulates TLR signaling (29). In addition to histological lesions consistent with autoimmune hepatitis, triple TAM-deficient mice develop elevated transaminases (AST and ALT) at 6 months, high levels of ANA at age 12 months, and low levels of anti-SMA (26). Moreover, IFN-I, IL-6, and TNFα levels are upregulated.
The studies presented here suggest that pristane-induced lupus is a new animal model of type 1 autoimmune hepatitis. The key criteria for diagnosis include elevated transaminases, anti-SMA or AAA, ANA, γ-globulins or IgG ≥ 1.5 times the upper limit or normal, and characteristic liver biopsy changes (interface hepatitis with lymphoplasmacytic infiltrates in the portal triads extending beyond the limiting plate). Most B6 and BALB/c mice develop high levels of IgG ANA at 3-months (30) and moderate levels of AAA by 6 months after pristane treatment (Fig. 4A). IgG3 hypergammaglobulinemia develops as early as 2-weeks after pristane treatment and increased levels of IgG1, IgG2a, and IgG2b at 3-months (30). The liver pathology in pristane-treated mice was consistent with autoimmune hepatitis, but fibrosis was absent at 10-months after pristane treatment, suggesting that the peri-portal inflammatory process is relatively mild. As these studies were carried out with previously stored tissue and serum samples, we did not analyze serum AST or ALT, which requires fresh serum or plasma. We also have not carried out longitudinal studies to precisely time the onset of AAA production and to determine whether autoantibody levels increase over time. A limitation of this model is the relatively long period between pristane injection and the onset of liver pathology. We did not see liver inflammation at 6-months after pristane injection, but it was present in all mice (with varying severity) at 10–11 months. Interestingly, the development of autoimmune hepatitis also is a late manifestation of lupus-like disease in mertk−/− and triple TAM receptor deficient mice (26). Further studies will be necessary to determine if BALB/c mice develop more severe disease than B6, as suggested by examination of a limited number of liver specimens (Fig. 3). The pristane-lupus model is highly amenable to the evaluation of disease mechanisms. In view of suggestions that IFN-I is involved in the pathogenesis of human autoimmune hepatitis, it will be of interest to assess whether autoimmune hepatitis is induced by pristane in mice lacking TLR7 or the type I interferon receptor.
HIGHLIGHTS.
Autoimmune “lupoid” hepatitis shares certain clinical and serological features with SLE
Although the diseases are clinically distinct, about 3% of SLE patients develop typical manifestations of autoimmune hepatitis
Mice with pristane-induced also develop features of autoimmune hepatitis as a late manifestation of their systemic autoimmune disease
These observations suggest that there may be common mechanisms in the immunopathogenesis of autoimmune hepatitis and SLE
Acknowledgments
Supported by research grants R01-AR44731 from NIH/NIAMS (WR), the Lupus Research Institute (LY). We thank Annie Chan, R.N. for assistance with the collection of clinical data and samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bearn AG, Kunkel HG, Slater RJ. The problem of chronic liver disease in young women. AmJMed. 1956;21:3–15. doi: 10.1016/0002-9343(56)90003-1. [DOI] [PubMed] [Google Scholar]
- 2.Mackay IR. A 50-year experience with autoimmune hepatitis: and where are we now? JGastroenterol. 2011;46(Suppl 1):17–28. doi: 10.1007/s00535-010-0325-2. [DOI] [PubMed] [Google Scholar]
- 3.Sahebjam F, Vierling JM. Autoimmune hepatitis. FrontMed. 2015;9(2):187–219. doi: 10.1007/s11684-015-0386-y. [DOI] [PubMed] [Google Scholar]
- 4.Homberg JC, Abuaf N, Bernard O, Islam S, Alvarez F, Khalil SH, et al. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of “autoimmune” hepatitis. Hepatology. 1987;7(6):1333–9. doi: 10.1002/hep.1840070626. [DOI] [PubMed] [Google Scholar]
- 5.Krawitt EL. Discrimination of autoimmune hepatitis: autoantibody typing and beyond. JGastroenterol. 2011;46(Suppl 1):39–41. doi: 10.1007/s00535-010-0324-3. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(3):809–20. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loria P, Lonardo A, Leonardi F, Fontana C, Carulli L, Verrone AM, et al. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. DigDisSci. 2003;48(11):2173–81. doi: 10.1023/b:ddas.0000004522.36120.08. [DOI] [PubMed] [Google Scholar]
- 8.Cotler SJ, Kanji K, Keshavarzian A, Jensen DM, Jakate S. Prevalence and significance of autoantibodies in patients with non-alcoholic steatohepatitis. JClinGastroenterol. 2004;38(9):801–4. doi: 10.1097/01.mcg.0000139072.38580.a0. [DOI] [PubMed] [Google Scholar]
- 9.Frenzel C, Herkel J, Luth S, Galle PR, Schramm C, Lohse AW. Evaluation of F-actin ELISA for the diagnosis of autoimmune hepatitis. AmJGastroenterol. 2006;101(12):2731–6. doi: 10.1111/j.1572-0241.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 10.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Lee PY, Kellner E, Paulus M, Switanek J, Xu Y, et al. Monocyte surface expression of Fcgamma receptor RI (CD64), a biomarker reflecting Type-I interferon levels in systemic lupus erythematosus. Arthritis ResTher. 2010;12(3):R90. doi: 10.1186/ar3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang H, Han S, Li Y, Kienhöfer D, Lee P, Shumyak S, et al. A novel mechanism for generating the interferon signature in lupus: opsonization of dead cells by complement and IgM. Arthritis Rheumatol. 2016 doi: 10.1002/art.39781. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tojo J, Ohira H, Abe K, Yokokawa J, Takiguchi J, Rai T, et al. Autoimmune hepatitis accompanied by systemic lupus erythematosus. Internal medicine. 2004;43(3):258–62. doi: 10.2169/internalmedicine.43.258. [DOI] [PubMed] [Google Scholar]
- 14.Mackay IR, Taft LI, Cowling DC. Lupoid hepatitis and the hepatic lesions of systemic lupus erythematosus. Lancet. 1959;1(7063):65–9. doi: 10.1016/s0140-6736(59)91136-5. [DOI] [PubMed] [Google Scholar]
- 15.Mishra A, Guindi M, Kandel G, Streutker CJ. Autoimmune hepatitis-like reaction developing in a patient treated with interferon-beta1a. Histopathology. 2015;66(4):605–7. doi: 10.1111/his.12449. [DOI] [PubMed] [Google Scholar]
- 16.Villamil A, Mullen E, Casciato P, Gadano A. Interferon beta 1a-induced severe autoimmune hepatitis in patients with multiple sclerosis: report of two cases and review of the literature. AnnHepatol. 2015;14(2):273–80. [PubMed] [Google Scholar]
- 17.Aguilera I, Sousa JM, Gomez-Bravo MA, Nunez-Roldan A. De novo autoimmune hepatitis after interferon treatment in a liver transplant recipient with common variable immunodeficiency. DigLiver Dis. 2014;46(7):663–4. doi: 10.1016/j.dld.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30(9):455–64. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Sobel E, Satoh M, et al. Deficiency of the Type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–83. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang H, Narain S, Sobel E, Lee PY, Nacionales DC, Kelly KM, et al. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus. ClinImmunol. 2005;117(3):238–50. doi: 10.1016/j.clim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Schattner A, Von der WJ, Kozak N, Sokolovskaya N, Knobler H. Nitrofurantoin-induced immune-mediated lung and liver disease. AmJMedSci. 1999;317(5):336–40. doi: 10.1097/00000441-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Bjornsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51(6):2040–8. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 23.Fritzler MJ, Tan EM. Antibodies to histone in drug-induced and idiopathic lupus erythematosus. JClinInvest. 1978;62:560–7. doi: 10.1172/JCI109161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson TM, Amos N, Bulgen D, Williams BD. Minocycline-induced lupus: clinical features and response to rechallenge. Rheumatology (Oxford) 2001;40(3):329–35. doi: 10.1093/rheumatology/40.3.329. [DOI] [PubMed] [Google Scholar]
- 25.Lapierre P, Djilali-Saiah I, Vitozzi S, Alvarez F. A murine model of type 2 autoimmune hepatitis: Xenoimmunization with human antigens. Hepatology. 2004;39(4):1066–74. doi: 10.1002/hep.20109. [DOI] [PubMed] [Google Scholar]
- 26.Qi N, Liu P, Zhang Y, Wu H, Chen Y, Han D. Development of a spontaneous liver disease resembling autoimmune hepatitis in mice lacking tyro3, axl and mer receptor tyrosine kinases. PloS one. 2013;8(6):e66604. doi: 10.1371/journal.pone.0066604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. NatRevImmunol. 2008;8(5):327–36. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 29.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–36. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda Y, Akaogi J, Nacionales DC, Szabo NJ, Reeves WH, Satoh M. Distinctive patterns of autoimmune response induced by different types of mineral oil. ToxicolSci. 2004;78:222–8. doi: 10.1093/toxsci/kfh063. [DOI] [PubMed] [Google Scholar]