Abstract
The renin-angiotensin system (RAS) in the brain is a critical determinant of blood pressure but the mechanisms regulating RAS activity in the brain remain unclear. Expression of brain renin (renin-b) occurs from an alternative promoter-first exon. The predicted translation product is a non-secreted enzymatically active renin whose function is unknown. We generated a unique mouse model by selectively ablating the brain-specific isoform of renin (renin-b) while preserving expression and function of the classical isoform expressed in the kidney (renin-a). Preservation of renal renin was confirmed by measurements of renin gene expression and immunohistochemistry. Surprisingly, renin-b-deficient mice exhibited hypertension, increased sympathetic nerve activity to the kidney and heart, and impaired baroreflex sensitivity. Whereas these mice displayed decreased circulating RAS activity, there was a paradoxical increase in brain RAS activity. Physiologically, renin-b-deficient mice exhibited an exaggerated depressor response to intracerebroventricular administration of losartan, captopril or aliskiren. At the molecular level, renin-b-deficient mice exhibited increased expression of AT1 receptor in the paraventricular nucleus, which correlated with an increased renal sympathetic nerve response to leptin which was dependent upon AT1 receptor activity. Interestingly, despite an ablation of renin-b expression, expression of renin-a was significantly increased in rostral ventral lateral medulla. These data support a new paradigm for the genetic control of RAS activity in the brain by a coordinated regulation of the renin isoforms, with expression of renin-b tonically inhibiting expression of renin-a under baseline conditions. Impairment of this control mechanism causes neurogenic hypertension.
Keywords: Renin, angiotensin II, brain, sympathetic nervous system, hypertension
Introduction
It is well known that the renin-angiotensin system (RAS) plays a crucial role in regulating blood pressure (BP) and fluid homeostasis. Many tissues express all components of the RAS and have the capacity for the synthesis and action of angiotensin-II (ANG). The importance of the tissue RAS as an independently regulated system distinct from the circulating or endocrine RAS has become an accepted paradigm.1 Whereas all components of the RAS are present in the brain, and accumulating evidence indicates that the brain RAS regulates BP, the mechanisms regulating RAS activity in the brain remain unclear.2,3
Renin expression in the kidney and most other tissues (except brain) is initiated from a strong promoter upstream of the classical first exon (exon 1a), leading to production of preprorenin, the precursor for secreted active renin (termed renin-a) (Figure 1A). In the brain, renin is transcribed from an alternative promoter-first exon (termed exon 1b) which does not encode the initiation codon present in exon 1a.4,5 It is predicted that translation initiates at the next ATG in exon 2, a codon which is both in frame and evolutionarily conserved.6 This translation product (termed renin-b) is virtually brain-specific and lacks both the signal peptide and the first third of the prosegment. Consequently, renin-b cannot enter the secretory pathway and should remain intracellular. Renin-b has been reported to be enzymatically active.4 Although renin-b is the dominant isoform of renin in the brain under normal conditions, the function of renin-b in the brain and whether it encodes a functional intracellular renin is unknown.5,7
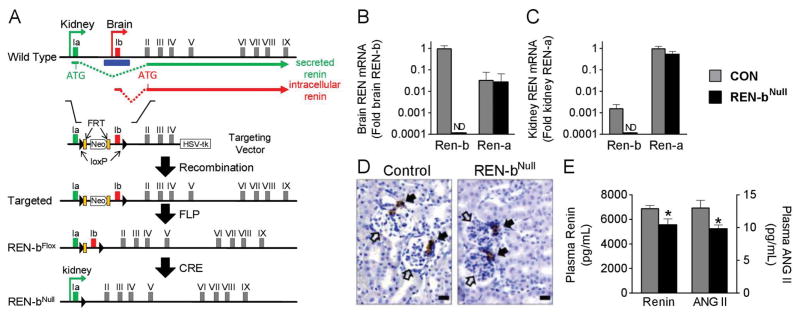
Figure 1. Generation of Renin-bNull Mice.
(A) Strategy for generating renin-bNull mice. The locations of exon 1a (green) and exon 1b (red) along with FRT (orange block) and loxP (black triangle) sites are indicated. The location of isoform-specific translation start sites and splice sites to exon II are indicated. Homologous recombinant founder mice containing the targeted allele were bred with FLPase mice to generate the floxed allele. The null allele was then generated by breeding with EIIA-Cre transgenic mice. Exon 1a and the common portions (exon 2 to 9) of the renin gene were retained in the Ren-bNull allele to preserve renin-a expression. (B–C) Renin-b and renin-a mRNA expression were measured in brain (B) and kidney (C) by real-time quantitative RT-PCR analysis (n=5 per group). ND, not detected. Graphs are mean±SEM. (D) Immunohistochemistry for renin in the kidney. Scale bars: 10 μm. Open arrow head, glomerulus; closed arrowhead, juxtaglomerular apparatus. (E) Plasma renin and angiotensin peptide levels were measure by ELISA (n=5 per group). *, P<0.05 vs control.
There is a long held hypothesis that ANG acts as a neurotransmitter.8 Although this is supported by functional evidence, the criteria for de novo intracellular synthesis of ANG has yet to be satisfied.9–11 Renin is expressed in neurons along with its substrate angiotensinogen (AGT) in regions of the brain controlling cardiovascular function.12,13 Therefore, the hypothesis for an intracellular renin in neurons is particularly compelling as it may offer the missing mechanistic link defining ANG as a neurotransmitter. There is a second hypothesis that expression of renin-a and renin-b are differentially but coordinately regulated in the brain.14 At baseline, renin-a expression in brain is undetectable, and the predominant isoform is renin-b (albeit expressed at a level orders of magnitude lower than in the kidney). However, in response to deoxycorticosterone (DOCA)-salt, there is an induction of renin-a mRNA expression concomitant with a suppression of renin-b mRNA expression. This induction of renin-a occurs concordantly with a state of brain RAS activation. Thus the balance between these two isoforms may dictate brain RAS activity which could have profound effects on BP. This is further complicated by the fact that the protein products of renin-a and renin-b mRNAs likely differ. In brain, expression of renin-a should produce and release prorenin, whereas expression of renin-b should support production of a non-secreted intracellularly-retained form of active renin. Herein, we used an unconventional genetic approach to generate mice that selectively lack renin-b in the brain to investigate the importance of renin-b and differentiate between these two hypotheses. A notable feature of the model is the preservation of renin-a expression by the kidney and secretion of renin. This is critical because renin-a-deficient mice exhibit post-natal lethality.15
Methods
Generation of renin-bNull mice
A targeting vector was designed to delete 500 bp upstream and downstream of exon 1b using a bacterial artificial chromosome clone carrying the mouse Ren-1c gene as a template. Gene targeting was performed in C57BL/6 inbred ES cells (IC1) by the inGenious Targeting Laboratory (Ronkonkoma, NY). Neomycin resistance and HSV thymidine kinase gene were used for positive and negative selection, respectively. Recombinant clone ITL4D5 successfully passed all quality control tests for the presence of loxP and FRT sites and was used for blastocyst injection. Chimeras were bred with C57BL/6J mice. Positive offspring were bred to C57BL/6J congenic B6.129S4-Gt(ROSA)26Sortm1(FLP1)Dym/RainJ (a FLP deleter strain, Jax 009086) to eliminate the neomycin gene, and then to C57BL/6J to ensure removal of FLP. Offspring were bred to C57BL/6J congenic B6.FVB-Tg(EIIa-cre)C5379Lmgd/J (Jax 003724) to generate the REN-bNull allele. Mice carrying the REN-bNull allele were maintained by backcross breeding to C57BL/6J, and heterozygotes were intercrossed to generate the renin-bNull mice.
In this first study, aged matched male REN-bNull mice were examined. Control littermates carrying both wildtype alleles were used as controls in all experiments. Studies are currently in progress to assess sex differences in both cardiovascular and metabolic parameters. All mice were fed standard laboratory chow (NIH-31 modified 6% mouse diet, Harlan Teklad) and tap water ad libitum. All studies were approved by the University of Iowa Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Design
Measures of cardiovascular parameters, spectral analysis of heart rate, sympathetic nerve activity (performed in chloralose-anesthetized mice), plasma renin and angiotensin II levels, isolation of specific brain regions and gene expression, and renin immunohistochemistry as detailed in the Online-only Supplement. The doses of drugs used in this study were propranolol (5 mg/kg, i.p.), methyl-atropine (2 mg/kg, i.p.), hexamethonium bromide (1 mg/kg, i.p.), losartan (5 μg/h, i.c.v.), captopril (5 μg/h, i.c.v.).
Statistics
Data were analyzed using t-tests or ANOVA with repeated measures as appropriate, followed by Tukey multiple-comparisons procedures. Differences were considered significant if p<0.05. All data are presented as mean ± SEM.
Results
We employed a gene-targeting strategy to selectively delete renin-b while preserving renin-a, by ablating exon 1b, its promoter and surrounding sequences in the mouse renin gene, while exon 1a and the common portions (exon 2 to 9) of the renin gene were retained (Figure 1A). The selective deletion of exon 1b in renin-bNull mice was confirmed by Southern blot (Figure S1). Importantly, there was no decrease in survival of renin-bNull mice to adulthood as has been reported for renin-aNull mice.15
Renin-b mRNA has a very limited tissue distribution and is the predominant isoform of renin mRNA in the brain.5,15 Thus, renin-bNull mice are essentially brain-specific knockouts of renin-b. Real-time quantitative RT-PCR established a loss of renin-b mRNA in the brain (Figure 1B), but a preservation of renin-a mRNA in the kidney (Figure 1C). Immunostaining studies revealed normal expression and distribution of renin protein in juxtaglomerular areas in the kidneys of both control and renin-bNull mice (Figure 1D). For reasons explained below, plasma levels of renin and ANG were modestly but significantly decreased in renin-bNull mice (Figure 1E). We conclude that renin-b was selectively eliminated from the brain without altering expression of renin-a in the kidney.
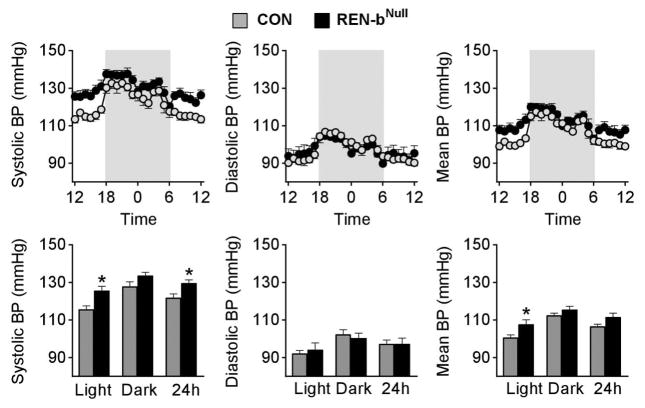
We predicted loss of renin-b to decrease BP. In contrast to our expectation, renin-bNull mice exhibited a significant increase in systolic BP compared to controls (Figure 2). There was no difference in diastolic BP during the day or night between groups. The differential effect on systolic and diastolic BP was reflected in a large increase in pulse pressure in renin-bNull mice (Figure S2). The increased BP likely caused feedback inhibition which decreased plasma renin and angiotensin peptides as noted above.
Figure 2. Arterial Pressure Phenotyping.
Systolic, diastolic, and mean BP are plotted hourly (top panel) and averaged across the light, dark, and 24-hour phases (bottom panel). Shaded areas indicate the dark phase (control n=7, renin-bNull mice n=8). *, P<0.05 vs control.
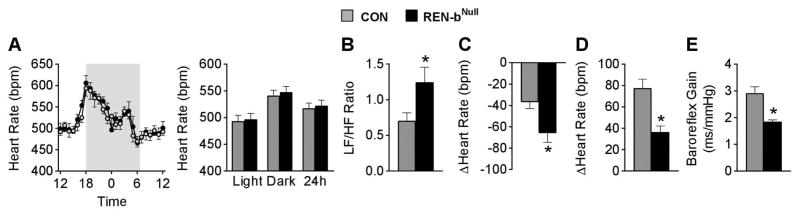
There was no difference in baseline heart rate between renin-bNull and control mice (Figure 3A). Power spectral analysis of heart rate variability showed increased indices of sympathetic outflow and decreased indices of parasympathetic tone (Figure 3B). To test this directly, we treated a separate cohort of mice with the β-adrenergic antagonist propranolol and the parasympathetic agonist atropine. We observed a larger bradycardic response after propranolol (Figure 3C), and a blunted tachycardic response induced by atropine in renin-bNull mice (Figure 3D). Baroreflex gain was decreased in renin-bNull mice, indicating impaired baroreflex sensitivity and autonomic dysfunction (Figure 3E).
Figure 3. Heart Rate Phenotyping.
(A) Heart rate is plotted hourly (left panel) and averaged across the light, dark, and 24-hour phases (right panel). Shaded areas indicate the dark phase (control n=7, renin-bNull mice n=8). (B) Relative low frequency, high frequency (LF/HF) ratio derived from power spectral analysis of heart rate variability (n=7 per group). (C–D) Heart rate responses to i.p. injection of propranolol (C) or atropine (D) (n=7 per group). (E) Baroreflex gain derived from Sequence method (n=7 per group). *, P<0.05 vs control.
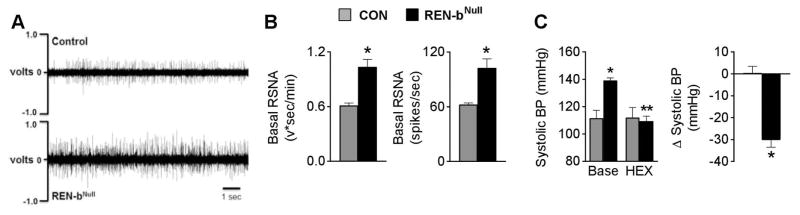
Direct measurement of sympathetic nerve activity (SNA) subserving the kidney, a key cardiovascular organ, revealed higher baseline renal sympathetic tone in renin-bNull mice (Figure 4A–B). A higher resolution recording is shown in Figure S3. The sensitivity of ganglionic blockade to the elevated BP in renin-bNull mice was investigated using a dose of hexamethonium that did not change BP in control mice. Renin-bNull mice exhibited increased sensitivity to hexamethonium as illustrated by a 30 mmHg decrease in BP (Figure 4C), and a similar decrease in HR (data not shown). A comparison of the effect of hexamethonium on systolic, diastolic and mean BP is shown in Figure S4. Collectively, these data suggest that mechanistically, hypertension in renin-bNull mice is sympathetically mediated.
Figure 4. Sympathetic Nervous System Activation.
(A) Representative raw tracing of renal sympathetic nerve activity in chloralose-anesthetized control and renin-bNull mice. (B) Basal RSNA (control n=5, renin-bNull mice n=7). (C) BP responses to ganglionic blockade (control n=5, renin-bNull mice n=8). Base, baseline; HEX, hexamethonium. *P<0.05 vs Control, **P<0.05 vs Baseline.
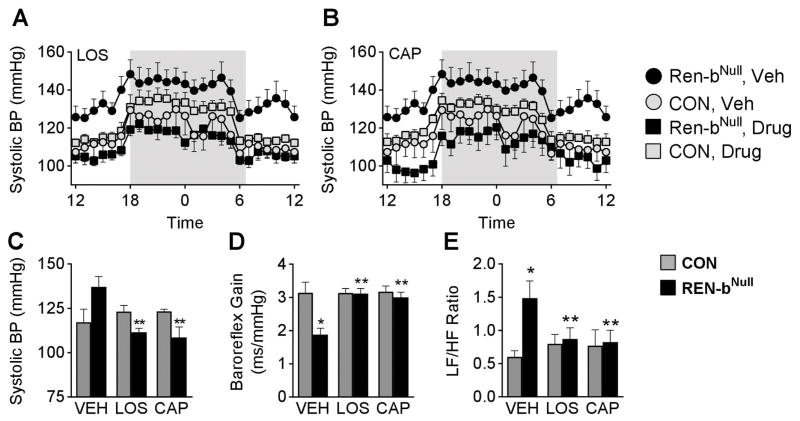
The increased BP and SNA are phenotypes consistent with increased brain RAS activity. Although antithetical to our initial hypothesis, we next considered the possibility that deletion of renin-bNull in the brain was causing a paradoxical increase in brain RAS activity. To test this, we measured BP in mice chronically treated with the AT1 receptor antagonist losartan or the angiotensin converting enzyme (ACE) inhibitor captopril. Chronic i.c.v. losartan not only abolished the elevated BP in renin-bNull mice, but reduced it below the baseline in untreated or losartan-treated control mice (Figure 5A). Similarly, chronic i.c.v. infusion of captopril caused an exaggerated BP reduction in renin-bNull mice (Figure 5B). Summary data for both treatments is shown in Figure 5C, and a comparison of systolic, diastolic and mean BP is shown in Figure S5. Interestingly, inhibition of the brain RAS normalized the impairment of baroreflex sensitivity (Figure 5D) and autonomic nervous function (Figure 5E) in renin-bNull mice. We conclude that the hypertension is due to increased activity of the brain RAS in renin-bNull mice, likely reflecting increased local synthesis of ANG and action at the AT1 receptor within the brain.
Figure 5. Activation of the Brain RAS.
(A–B) Effect of chronic RAS inhibition in response to i.c.v. losartan (LOS, A) and i.c.v. captopril (CAP, B) on BP. BP was measured by radiotelemetry and is plotted hourly (A–B). Shaded area reflects the dark cycle. Sample size was 5 control vehicle, 6 control LOS, 5 control CAP, 7 renin-bNull vehicle, 6 renin-bNull LOS, 6 renin-bNull CAP. Note that the vehicle groups in A and B are the same and were repeated for clarity and easy comparison. (C–E) Summary data of 24 hour systolic BP (C), baroreflex gain (D) and spectral analysis (E). *P < 0.05 vs. control. **P < 0.05 vs. vehicle.
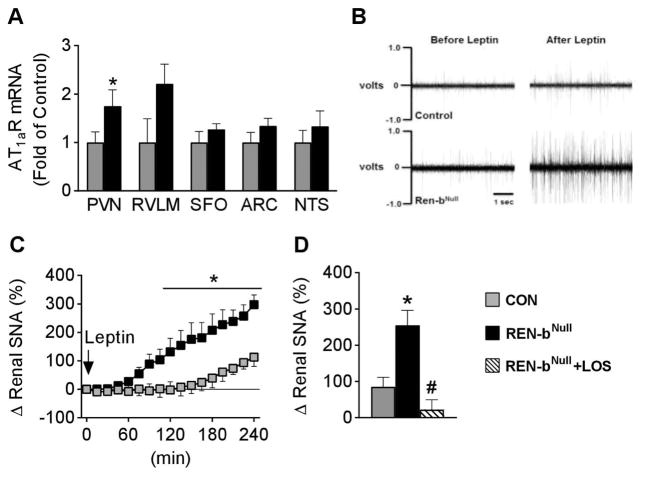
To identify the potential nuclei underlying the effects evoked by brain RAS activation in renin-bNull mice, we measured expression of RAS genes in several cardiovascular control regions of the brain. There was no significant difference in expression of angiotensinogen, prorenin receptor, or Mas receptor mRNA (Table S1). Expression of ACE was increased in the subfornical organ (SFO) but not in other nuclei. Expression of AT1a receptor mRNA was significantly increased in the paraventricular nucleus (PVN) and there was a trend for an increase in the rostral ventral lateral medulla (RVLM, Figure 6A). There was no change in expression of AT1a receptor mRNA in the SFO, arcuate nucleus (ARC) and nucleus tractus solitarius (NTS).
Figure 6. Role of AT1 Receptors.
(A) AT1aR mRNA expression in PVN (n=10 per group), RVLM (n=10 control, n=9 renin-bNull), SFO (n=10 per group), ARC (n=9 per group) and NTS (n=9 control, n=10 renin-bNull). *, P< 0.05 vs control. (B) Representative tracings of RSNA in chloralose-anesthetized mice before and during the 4th hour following i.c.v. administration of leptin (5 μg). (C–D) Renal sympathetic nerve activity (SNA) response to i.c.v. injection of leptin. Percent changes in renal SNA for 4 hours after i.c.v. leptin are plotted and the last 60 minutes are summarized (control n=10, renin-bNull n=5, renin-bNull + i.c.v. losartan n=5). *, P<0.05 vs control; #, P<0.05 vs renin-bNull without losartan.
To address the functional importance of increased AT1a receptor, we took advantage of our previous observation that increased renal SNA in response to leptin is dependent upon AT1a receptors in the brain.16 Consistent with this, the renal SNA response to i.c.v. leptin was markedly augmented in renin-bNull mice compared with controls (Figure 6B–C). Importantly, this augmented response was ablated by pretreatment with losartan (Figure 6D). These data suggest that increased sympathoexcitation in renin-bNull mice is due to increased activity of AT1a receptors.
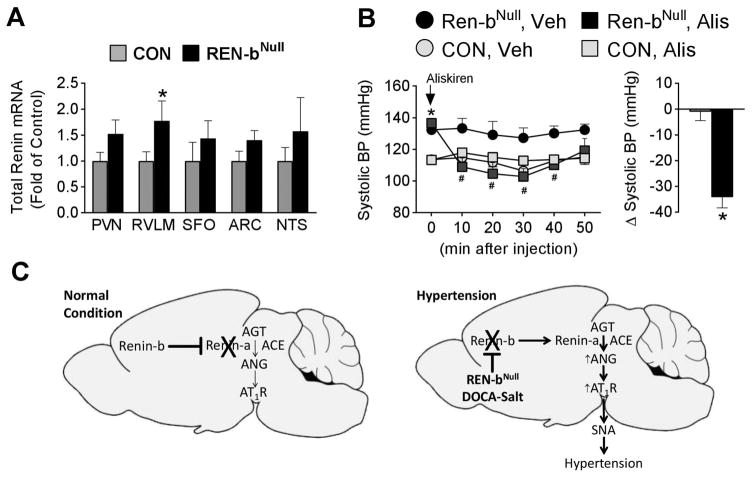
Finally, given the coordinated regulation of renin-a and renin-b mRNA in the brain of DOCA-salt mice, we considered the possibility that the loss of renin-b mRNA might trigger an increase in renin-a mRNA.14 We employed a sensitive TaqMan assay designed to detect total renin mRNA instead of an isoform-specific assay because: a) the level of renin-a mRNA within individual regions of the brain is extremely low and below the level of detection, b) the assays measuring each renin isoform selectively are inefficient because the respective exons are very small and primers cannot be optimized, and c) renin-b mRNA is the dominant form under baseline conditions. Thus, any renin mRNA detected in renin-bNull mice must be, by definition, renin-a. Like AT1a receptor, expression of renin-a mRNA was significantly increased in the RVLM, and there was a trend for an increase in the PVN in renin-bNull mice (Figure 7A). There was no increase in renin mRNA in the SFO, ARC or NTS. Given the increase in renin mRNA, which in renin-bNull mice must be renin-a mRNA, we tested if the hypertensive response is renin-dependent. Acute i.c.v. injection of aliskiren, a renin inhibitor, significantly reduced BP in renin-bNull but not control mice (Figure 7B).
Figure 7. Role of Brain Renin.
(A) Total renin mRNA expression in PVN (n=9 per group), RVLM (n=9 per group), SFO (n=8 control, n=9 renin-bNull), ARC (n=9 per group) and NTS (n=9 per group). *, P<0.05 vs control. (B) Acute BP effect of i.c.v. infusion of aliskiren over 30 min (control n=5, renin-bNull n=7). *, P<0.05 vs control; #, P<0.05 vs. i.c.v. vehicle. (C) Model illustrating coordinate regulation of renin-a expression by renin-b in the brain under normal (left) and hypertension-inducing conditions (right).
Discussion
Physiological phenotypes in renin-bNull mice, such as hypertension, elevated renal SNA, and the enhanced renal SNA response to leptin, are consistent with increased brain RAS activity.2 Deletion of renin-b paradoxically results in increased brain RAS activity strongly suggesting that expression of renin-b acts as an endogenous inhibitor of the brain RAS. Mechanistically, our data suggests that in the brain, expression of renin-b tonically inhibits expression of renin-a which encodes secreted renin. The processes controlling renin-a in the kidney have been extensively studied, but there are no data on the regulation or mechanisms of renin-b expression.17 That this mechanism occurs naturally is supported by our previous result showing the concomitant increase in renin-a and decrease in renin-b in the brain in DOCA-salt hypertension.14 A mechanism controlling the regulation of secreted renin in the brain is conceptually interesting, because the ANG precursor and renin substrate, AGT, is widely expressed in, and constitutively released from astrocytes and glia.18,19 Thus, the extracellular space is essentially bathed with the precursor to ANG. Renin and AGT are synthesized in neighboring cells in the RVLM.13 Deletion of AGT specifically in the brain of mice and rats decreases BP and SNA.20–23 Thus, without a control mechanism, the generation of extracellular ANG may directly activate AT1 receptors in this region of the brain leading to increased sympathetic outflow.24 Consequently, expression of renin-b might provide a counter regulatory control mechanism preventing widespread conversion of extracellular AGT to ANG peptides.
Our original provocative hypothesis was that renin-b encodes an intracellular isoform of renin which functions as part of the cellular machinery synthesizing ANG from AGT within neurons.7 Evidence supporting intracellular ANG synthesis could support the hypothesis that ANG functions as a neurotransmitter.8 The main weakness of the intracellular ANG hypothesis is in modeling how intracellular renin could interact with AGT within the cell. AGT is a secretory protein whereas renin-b lacks the signal peptide needed for incorporation of the protein into the secretory apparatus. Although we predicted that deletion of an intracellular mediator for ANG synthesis would be associated with decreased BP, it was in fact associated with increased BP. However, an argument can be made that intracellular renin might control an inhibitory neural circuit, and indeed ANG-dependent inhibitory circuits have been reported.25 Nevertheless, the strongest data implicating ANG as a neurotransmitter is in the SFO-PVN axis where ANG is strongly stimulatory.10 Moreover, disinhibition of an inhibitory neural circuit would not be expected to be RAS-dependent. Consequently, our data does not provide supporting evidence for a role for intracellular renin. Rather, our data supports a model in which the synthesis of ANG in the brain is controlled at the level of renin transcription (Figure 7C). We hypothesize that under normal conditions, expression of renin-b inhibits expression of renin-a thus limiting the synthesis and action of ANG (left panel). In the lifelong absence of renin-b (as in the renin-bNull mice), or in normal mice under conditions where renin-b is suppressed (e.g. DOCA-salt hypertension), the renin-a transcript is induced, renin (most likely prorenin) is synthesized and released, which converts AGT (in the presence of ACE) to ANG with consequent activation of neuronal AT1 receptors. Activation of AT1 receptors in the PVN and RVLM increase activity of the sympathetic nervous system. The mechanism converting prorenin to renin in the brain, and the requirement, if any, of (pro)renin receptor remains undefined. This model is supported by data showing that AT1 receptor blockade in the brain does not affect BP in normotensive models or under baseline conditions, wherein renin-b inhibits brain RAS activity.26 Similarly, knockout of renin-a specifically in the brain does not affect BP in the absence of a hypertension-inducing stress because renin-a is already silent.27 Both data are consistent with a state of renin-b synthesis and tonic inhibition of brain RAS activity under normal conditions. Hypertension-inducing signals, like DOCA-salt, cause a reprogramming of renin synthesis from renin-b to renin-a leading to local secretion of renin and local ANG synthesis and action.14
Perspectives
Perhaps the most important question to ask is whether our results have implications for blood pressure control in humans and if this pathway is involved in human hypertension. Two experimental findings suggest that our results may have applicability to humans. First, the initial identification of renin-b expression in the brain was in a transgenic animal model in which expression of the human renin gene was exquisitely regulated and responsive to physiological cues.5,28,29 This suggests that encoded in the human renin gene is the capacity for expression of both renin-a and renin-b. Our second experimental finding is that renin-b (not renin-a) is the primary isoform of renin expressed in human brain.5 Thus humans, like mice, express renin-b as the predominant isoform in the brain. Therefore, the main question that remains unresolved is whether the “molecular switch” we propose herein is active in humans. Future mechanistic studies in cultured neurons or neuronal cell lines may help to address this issue.
Are our findings applicable to human hypertension? It is interesting to note that increased sympathetic outflow has been attributed to be the basis of the failure of antihypertensive therapy in refractory hypertension.30 Refractory hypertension differs from resistant hypertension. Resistant hypertension is defined as uncontrolled high blood pressure despite the use of effective doses of 3 or more different classes of antihypertensive agents, including a diuretic.31 Refractory hypertension defines an extreme phenotype of antihypertensive treatment failure most recently classified by uncontrolled blood pressure with optimal dose of 5 or more different classes of antihypertensive agents, including chlorthalidone and a mineralocorticoid receptor antagonist.30,32 Whereas persistent intravascular fluid retention is a characteristic commonly associated with resistant hypertension, this is not the case in refractory hypertension. Instead, increased heart rate has been observed in refractory hypertension suggesting a neurogenic etiology. Although RAS blockers effectively lowered blood pressure in renin-bNull mice, it is important to recall that the drugs were administered intracerebroventricularly and therefore had direct access to the “compartment” where ANG was being generated and where it acted. We did not determine if systemic administration of RAS blockers lowered blood pressure. In retrospect, it would have been interesting to make this assessment. Antihypertensive agents differ in their ability to access to the central nervous system through the blood-brain barrier, which may differ if the blood-brain barrier becomes compromised in hypertension.33 Future studies will clearly be needed to determine if the renin-b/renin-a regulatory pathway is active in the central nervous system humans and to determine if it plays a role in some patients such as those with refractory hypertension. Unfortunately, we expect that assessing the importance of this pathway in humans may be a very challenging undertaking.
Supplementary Material
Novelty and Significance.
What Is New?
There are two forms of renin, renin-a, which is expressed in the kidney and encodes preprorenin, and renin-b, which is expressed in the brain and has been proposed to encode a non-secreted form of active prorenin.
We generated a unique mouse model by selectively ablating the brain-specific isoform of renin-b while preserving expression and function of renin-a in kidney.
Selective deletion of renin-b results in hypertension and increased sympathetic nerve activity due to activation of the brain RAS in response to an isoform switch which induces renin-a in the absence of renin-b in the brain.
What Is Relevant?
Expression of renin-b provides a sensitive control mechanism which limits activity of the brain renin-angiotensin system under normal conditions.
Summary
These data support a new paradigm for the genetic control of RAS activity in the brain by coordinated regulation of the renin isoforms with expression of renin-b tonically inhibiting expression of renin-a under baseline conditions.
This control mechanism becomes impaired under hypertension-inducing conditions.
Impairment of this control mechanism causes neurogenic hypertension.
Acknowledgments
Transgenic mice were maintained and genotyped at the University of Iowa Gene Editing Facility supported by grants from the NIH and from the Carver College of Medicine. We thank Bill Paradee, Norma Sinclair, JoAnne Schwarting, and Patricia Yarolem for genotyping mice.
Sources of Funding: This work was supported through research grants from the National Institutes of Health (NIH) to CDS (HL084207, HL048058, HL062984, HL125603), KR (HL084207), JLG (HL098276), MLSSL (DK091330, DK096373), and grants from the American Heart Association to CDS (15SFRN23480000) and KR (14EIA18860041), and the University of Iowa Fraternal Order of Eagles Diabetes Research Center to KR and JLG. The authors gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Footnotes
Conflict of Interest/Disclosure: None
References
- 1.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 2.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedans DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The Brain Renin-Angiotensin System Controls Divergent Efferent Mechanisms to Regulate Fluid and Energy Balance. Cell Metabolism. 2010;12:431–442. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension. 2011;57:600–607. doi: 10.1161/HYPERTENSIONAHA.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee-Kirsch MA, Gaudet F, Cardoso MC, Lindpaintner K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ Res. 1999;84:240–246. doi: 10.1161/01.res.84.2.240. [DOI] [PubMed] [Google Scholar]
- 5.Sinn PL, Sigmund CD. Identification of Three Human Renin mRNA Isoforms Resulting from Alternative Tissue-Specific Transcriptional Initiation. Physiol Genomics. 2000;3:25–31. doi: 10.1152/physiolgenomics.2000.3.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension. 2006;47:461–466. doi: 10.1161/01.HYP.0000203308.52919.dc. [DOI] [PubMed] [Google Scholar]
- 7.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology. 2008;23:187–193. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 2001;226:85–96. doi: 10.1177/153537020122600205. [DOI] [PubMed] [Google Scholar]
- 9.Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. American Journal of Physiology. 1993;265:R302–309. doi: 10.1152/ajpregu.1993.265.2.R302. [DOI] [PubMed] [Google Scholar]
- 11.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- 12.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of Renin Expressing Cells in the Brain Using a REN-eGFP Transgenic Model. Physiol Genomics. 2004;16:240–246. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- 13.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension. 2004;43:1116–1119. doi: 10.1161/01.HYP.0000125143.73301.94. [DOI] [PubMed] [Google Scholar]
- 14.Grobe JL, Rahmouni K, Liu X, Sigmund CD. Metabolic rate regulation by the renin-angiotensin system: brain vs. body. Pflugers Arch. 2012:167–175. doi: 10.1007/s00424-012-1096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu D, Borges GR, Grobe JL, Pelham CJ, Yang B, Sigmund CD. Preservation of intracellular renin expression is insufficient to compensate for genetic loss of secreted renin. Hypertension. 2009;54:1240–1247. doi: 10.1161/HYPERTENSIONAHA.109.138677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197–206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenn ST, Jones CA, Gross KW, Pan L. Control of renin gene expression. Pflugers Arch. 2013;465:13–21. doi: 10.1007/s00424-012-1110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stornetta RL, Hawelu Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- 19.Yang G, Gray TS, Sigmund CD, Cassell MD. The angiotensinogen gene is expressed in both astrocytes and neurons in murine central nervous system. Brain Res. 1999;817:123–131. doi: 10.1016/s0006-8993(98)01236-0. [DOI] [PubMed] [Google Scholar]
- 20.Gyurko R, Wielbo D, Phillips MI. Antisense inhibition of AT1 receptor mRNA and angiotensinogen mRNA in the brain of spontaneously hypertensive rats reduces hypertension of neurogenic origin. Regul Pept. 1993;49:167–174. doi: 10.1016/0167-0115(93)90438-e. [DOI] [PubMed] [Google Scholar]
- 21.Schinke M, Baltatu O, Bohm M, Peters J, Rascher W, Bricca G, Lippoldt A, Ganten D, Bader M. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci USA. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherrod M, Davis DR, Zhou X, Cassell MD, Sigmund CD. Glial-specific ablation of angiotensinogen lowers arterial pressure in renin and angiotensinogen transgenic mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1763–R1769. doi: 10.1152/ajpregu.00435.2005. [DOI] [PubMed] [Google Scholar]
- 23.Gomes da Silva AQ, Xavier CH, Campagnole-Santos MJ, Caligiorne SM, Baltatu OC, Bader M, Santos RA, Fontes MA. Cardiovascular responses evoked by activation or blockade of GABA(A) receptors in the hypothalamic PVN are attenuated in transgenic rats with low brain angiotensinogen. Brain Res. 2012;1448:101–110. doi: 10.1016/j.brainres.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 25.Tan PS, Potas JR, Killinger S, Horiuchi J, Goodchild AK, Pilowsky PM, Dampney RA. Angiotensin II evokes hypotension and renal sympathoinhibition from a highly restricted region in the nucleus tractus solitarii. Brain Res. 2005;1036:70–76. doi: 10.1016/j.brainres.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Davisson RL, Yang G, Beltz TG, Cassell MD, Johnson AK, Sigmund CD. The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res. 1998;83:1047–1058. doi: 10.1161/01.res.83.10.1047. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Borges GR, Davis DR, Agassandian K, Sequeira Lopez ML, Gomez RA, Cassell MD, Grobe JL, Sigmund CD. Neuron- or glial-specific ablation of secreted renin does not affect renal renin, baseline arterial pressure, or metabolism. Physiol Genomics. 2011;43:286–294. doi: 10.1152/physiolgenomics.00208.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinn PL, Davis DR, Sigmund CD. Highly Regulated Cell-Type Restricted Expression of Human Renin in Mice Containing 140 Kb or 160 Kb P1 Phage Artificial Chromosome Transgenes. J Biol Chem. 1999;274:35785–35793. doi: 10.1074/jbc.274.50.35785. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Davis DR, Sigmund CD. The human renin kidney enhancer is required to maintain baseline renin expression but is dispensable for tissue-specific, cell-specific and regulated expression. J Biol Chem. 2006;281:35296–35304. doi: 10.1074/jbc.M608055200. [DOI] [PubMed] [Google Scholar]
- 30.Acelajado MC, Pisoni R, Dudenbostel T, Dell’Italia LJ, Cartmill F, Zhang B, Cofield SS, Oparil S, Calhoun DA. Refractory hypertension: definition, prevalence, and patient characteristics. J Clin Hypertens (Greenwich) 2012;14:7–12. doi: 10.1111/j.1751-7176.2011.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqui M, Dudenbostel T, Calhoun DA. Resistant and Refractory Hypertension: Antihypertensive Treatment Resistance vs Treatment Failure. Can J Cardiol. 2016;32:603–606. doi: 10.1016/j.cjca.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calhoun DA, Booth JN, 3rd, Oparil S, Irvin MR, Shimbo D, Lackland DT, Howard G, Safford MM, Muntner P. Refractory hypertension: determination of prevalence, risk factors, and comorbidities in a large, population-based cohort. Hypertension. 2014;63:451–458. doi: 10.1161/HYPERTENSIONAHA.113.02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biancardi VC, Stern JE. Compromised blood-brain barrier permeability: novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. J Physiol. 2016;594:1591–1600. doi: 10.1113/JP271584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.