Abstract
Purpose
Cognitive behavioral stress management (CBSM) is an empirically-validated group-based psychosocial intervention. CBSM is related to decreased self-reported indicators of psychological adversity during breast cancer treatment and greater disease-free survival (DFS) vs. a control condition. This study examined relationships between CBSM, DFS, and a potential biobehavioral pathway linking these variables in breast cancer patients through a gene expression composite representing the leukocyte conserved transcriptional response to adversity (CTRA).
Design
Women with stage 0-IIIb breast cancer completed questionnaires and provided blood samples post-surgery. Participants were randomized to 10-week group-based CBSM or a psychoeducation control group and followed at 6 months, 12 months, and median 11 years. In total, 51 participants provided blood data for longitudinal analyses (CBSM n = 28; Control n = 23). Mixed model analyses examined CBSM effects on 6–12 month changes in CTRA expression (53 indicator genes representing pro-inflammatory, anti-viral and antibody production signaling). Cox regression models assessed the relationship between 6–12 month changes in CTRA expression and 11-year DFS.
Results
Patients randomized to CBSM showed attenuated 6–12 month change in CTRA gene expression, whereas patients randomized to control showed increased CTRA expression (p = 0.010). Average DFS was 5.92 years (SD = 3.90). Greater 6–12 month CTRA increases predicted shorter 11-year DFS controlling for covariates (p = 0.023).
Conclusions
CBSM attenuated CTRA gene expression during the initial year of breast cancer treatment. In turn, greater increases in CTRA gene expression predicted shorter long-term DFS. These findings identify a biobehavioral oncology pathway to examine in future work.
Keywords: Conserved Transcriptional Response to Adversity, cognitive behavioral stress management, leukocyte gene expression, breast cancer recurrence, disease-free survival
1. Introduction
1.1. Cognitive behavioral interventions and breast cancer outcomes
Cognitive behavioral interventions focused on stress management can improve the quality of life of breast cancer patients undergoing primary treatment (e.g., Andersen et al., 2004;,Antoni et al., 2006a). Evidence suggests that such interventions may affect long-term clinical outcomes in women with non-metastatic breast cancer (Andersen et al., 2008; Stagl et al., 2015) although these effects have not been consistently observed in the field (Antoni et al., 2006b; Coyne et al., 2007; Daniels and Kissane, 2008; Newell et al., 2002). Andersen et al. (2004, 2008) tested a 4-month weekly cognitive-behavioral therapy intervention (with 8 additional monthly maintenance sessions) among women with stage II-III breast cancer, and reported improved psychological symptoms and immune functioning compared to a treatment as usual control group at 4-month follow-up. At 7–13 year follow-up (11-year median) women in the intervention group had longer disease free survival (DFS; i.e., time to breast cancer recurrence), time to breast cancer specific mortality, and time to all-cause mortality relative to women in the control condition (Andersen et al., 2008). This suggests that offering psychosocial intervention during primary treatment may potentially influence long-term health outcomes for patients with breast cancer.
In our work, a 10-week cognitive behavioral stress management (CBSM) intervention designed to improve coping and psychological adaptation was shown to improve self-reported indicators of psychological adversity (cancer-associated intrusive thoughts, interviewer rated anxiety symptoms and negative affect), immune system functioning, and inflammatory signaling over the initial year of follow-up compared to an active psychoeducational control group in women with stage 0-IIIb breast cancer (Antoni et al., 2006a, 2006b, 2009, 2012). At 8–15 year follow-up (11-year median), women in CBSM had more favorable overall and disease free survival (DFS) compared to women in the control group (Stagl et al., 2015). While studies such as those by Andersen (2008) and Stagl (2015) reveal a link between psychosocial interventions and breast cancer recurrence, more research is needed to identify possible mechanistic pathways. The present study focuses on identifying mechanistic pathways underlying such intervention effects on breast cancer recurrence in a secondary analysis of a previously published trial (Stagl et al., 2015).
1.2. Biological mechanisms of breast cancer recurrence
Breast cancer recurrence is linked to pro-metastatic molecular processes such as inflammation, angiogenesis, proliferation, invasion, and evasion of immune system surveillance (Fidler, 2003). Stress-related neuroendocrine signaling stimulates many of these molecular processes (Antoni et al., 2006c, Cole et al., 2015). In experimental mouse models of breast cancer, stress-induced activation of the sympathetic nervous system (SNS) can promote a macrophage-mediated metastatic switch within a growing primary tumor and thereby increase the burden of distant metastasis (Sloan et al., 2010). In other solid epithelial tumors (e.g., ovarian carcinoma), adversity as indicated by low social support is associated with multiple metastasis-related processes including greater concentrations of the SNS neurotransmitter norepinephrine within tumor tissue (Armaiz-Pena et al., 2009; Lutgendorf et al., 2011), increased angiogenesis due to upregulation of vascular endothelial growth factor (VEGF) (Lutgendorf et al., 2002, 2008), and increased activity of pro-inflammatory transcription control pathways (Lutgendorf et al., 2009).
Given these relationships between stress biology and pro-metastatic molecular processes, clinical researchers are now considering whether psychosocial interventions designed to mitigate adversity in cancer patients affect disease recurrence in part by down-regulating similar pathways (for reviews see Antoni, 2013 and Lutgendorf et al., 2010). Preclinical research has implicated inflammation and monocyte/macrophage lineage cell recruitment in the effects of stress on breast cancer progression (Sloan et al., 2010), and translational research has begun to identify up-regulation of inflammation-related gene expression in circulating monocytes.
Circulating leukocytes have shown a conserved transcriptional response to adversity (CTRA) across a variety of adverse life circumstances (Chen et al., 2009, 2011; Cole et al., 2007, 2011). The CTRA gene response involves increased expression of pro-inflammatory genes (e.g., IL1B, IL6, IL8, and TNF) and decreased expression of genes associated with antibody synthesis and Type I interferon antiviral responses (e.g., IGJ, IFNB, IFIs, MX, OAS; Cole, 2009, 2010; Cole et al., 2012; Irwin and Cole, 2011; Miller et al., 2009). These effects are mediated primarily by monocytes (Miller et al., 2008, 2014; Powell et al., 2013). If stressful circumstances are associated with a CTRA response involving this pattern of leukocyte gene expression in patients with breast cancer, then it is plausible that CBSM may mitigate or reverse the CTRA response in these patients. Such effects could help explain the established relationship between CBSM and improved DFS in breast cancer patients.
1.3. Background to the present study
We previously found that CBSM was associated with favorable changes in leukocyte pro-inflammatory gene expression over 6–12 months in women undergoing treatment for breast cancer (Antoni et al., 2012). Subsequent analyses of long-term clinical outcomes in the same study found that CBSM is related to improved DFS (Stagl et al., 2015). In the present study, we sought to integrate these molecular and clinical findings with the existing CTRA literature by directly assessing 53 canonical CTRA indicator genes (Fredrickson et al., 2013, 2015) to (a) determine whether CBSM is associated with significant attenuation in CTRA gene expression relative to an active control condition during the first year of primary treatment, and (b) determine whether such attenuation in CTRA gene expression predicts longer DFS over 8–15 years of follow-up.
2. Materials and Methods
2.1. Study design and participants
Participants were women with stage 0-IIIb breast cancer recruited up to 12 weeks post-primary surgery and pre-adjuvant therapy between 1998–2005. The study was a single center, single blind, randomized, parallel assignment efficacy trial of CBSM that obtained approval by the University of Miami (UM) Institutional Review Board (IRB) in 1998 (National Institutes of Health Clinical Trial NCT01422551). The original study design is described in previous reports (e.g., Antoni et al., 2006a; Vargas et al., 2014). Briefly, women were recruited from surgical oncology practices in South Florida through advertising and private physician referrals, and at the UM/Sylvester Cancer Center. Exclusion criteria were age outside 21–75 years old, lack of fluency in English, diagnosis of stage IV breast cancer, prior cancer diagnosis (except minor skin cancers such as basal cell carcinoma), onset of adjuvant treatment, diagnosis of major medical conditions other than cancer, previous psychiatric hospitalization, and current endorsement of psychosis, suicidality, major depressive disorder, or panic disorder. After the initial assessment, participants were randomized on a 1:1 ratio to either a 10-week, group-based CBSM intervention focusing on anxiety reduction, cognitive restructuring, and coping skills, or an active contact psychoeducational control condition as previously described (Antoni, 2003; Antoni et al., 2006a, 2006b).
2.2. Procedures
Women signed informed consent and completed baseline assessments including blood samples, mood, demographic, and health information. Participants were randomized on a 1:1 basis to either the 10-week CBSM intervention or a 1-day psychoeducational control group. Follow-up assessments were conducted at 6-months and 12-months post-study enrollment. Due to the original study design and funding restrictions, only a relatively small proportion of participants from the larger study had available blood samples for the current analyses. The original study design did not include collection of blood samples, and this procedure became possible midway through the trial through supplemental funding and study amendments. Available blood sample data initially focused on serum cortisol (Phillips et al., 2008) and leukocyte challenge studies (e.g., Antoni et al., 2009), after which 80 baseline samples had remaining cryopreserved cells to complete gene expression assays. From the 80 baselines venous blood samples, 3–10 × 106 peripheral blood mononuclear cells (PBMC) were isolated as previously described (Antoni et al., 2009). Subsequently, 51 participants provided venous blood samples at 6-month and/or 12-month follow-up, allowing for analyses of change in gene expression over time. For participants with blood samples at 6-month and 12-month follow-up, change was assessed using the 12-month value.
Women were re-contacted in 2013 at 8–15 years post-study enrollment (11-year median) and study personnel obtained self-reported information about participant disease status that was confirmed via medical chart reviews. Information regarding recurrence history was collected from the medical charts of participants who had expired by time of follow-up. Importantly all participants had consented at study entry to having their medical/health status followed over time. Information regarding self-reported demographic, medical, and treatment-related information was verified during medical chart reviews. DFS was computed as days elapsed from date of randomization (2–12 weeks post-surgery) to documented breast cancer recurrence or new cancer.
2.3. Gene expression profiling
Detailed methods for gene expression profiling have been reported previously (Antoni et al., 2012). Briefly, RNA was extracted from PBMC, quality assured for mass and integrity, and subject to genome-wide transcriptional profiling using Illumina Human HT-12 v3 Expression BeadChips (Illumina Inc., San Diego, California) in the UCLA Neuroscience Genomics Core Laboratory. Data are deposited as National Center for Biotechnology Information Gene Expression Omnibus series GSE24079.
2.4. Statistical analysis
Quantile-normalized gene expression values were log2-transformed and z-score standardized within gene. A CTRA summary score was formed by averaging standardized values of 53 CTRA indicator genes which were chosen a priori and which have been used in previous research (Fredrickson et al., 2013, 2015; Knight et al., 2015). The 53 CTRA indicator genes include 19 pro-inflammatory transcripts (IL1A, IL1B, IL6, IL8, TNF, PTGS1, PTGS2, FOS, FOSB, FOSL1, FOSL2, JUN, JUNB, JUND, NFKB1, NFKB2, REL, RELA, RELB) and 34 transcripts inversely related to the CTRA profile involving Type I interferon responses (GBP1, IFI16, IFI27, IFI27L1-2, IFI30, IFI35, IFI44, IFI44L, IFI6, IFIH1, IFIT1-3, IFIT5, IFIT1L, IFITM1-3, IFITM4P, IFITM5, IFNB1, IRF2, IRF7-8, MX1-2, OAS1-3, OASL) and antibody synthesis (IGJ, IGLL1, IGLL3), which were sign inverted prior to averaging. Thus, larger values represent a more adverse profile.
For analyses of differential change in CTRA gene expression from pre- to post-intervention, a change score was defined for each gene (follow-up – baseline) and tested for difference in average value in CBSM vs. control conditions using mixed effect linear models treating the 53 gene-specific change scores as repeated measures, as in previous research (Fredrickson et al., 2015). Mixed models were estimated by maximum likelihood using SAS PROC MIXED (SAS Institute, Cary, NC, USA) and included a fixed effect of gene and random individual-specific intercepts to control for correlation across genes. We conducted both simple effect analyses testing average difference across groups and covariate-adjusted analyses that additionally controlled for age at diagnosis (continuous), disease stage (4-level categorical variable; 0/I/II/III), a set of 3 dichotomous variables indicating treatment with chemotherapy, radiation therapy, and endocrine therapy, time of post-intervention follow-up gene expression sample (6-vs. 12-months), and baseline gene expression levels. Ancillary analyses also controlled for baseline gene expression values. To facilitate the use of CTRA change scores as predictors of survival, we also formed single composite average change score (i.e., averaged across genes). Ancillary analyses verified that composite change scores also differed by group using standard univariate linear statistical models (SAS PROC GLM).
Survival analyses tested whether composite CTRA change scores predicted DFS (days from study entry to disease recurrence or new cancer diagnosis) above and beyond the effects of intervention group. Ancillary analyses again controlled for age, disease stage, treatment exposures, and gene expression follow-up time. Data were censored on the date of last study contact for women who did not have a breast cancer recurrence or new cancer at time of follow up, were lost to follow-up, or had previously dropped out of the trial. To accommodate data from 7 patients with confirmed breast cancer recurrence but unknown recurrence dates (i.e., interval censored between randomization and end of study/death), our previous analysis of DFS in this trial utilized Weibull Accelerated Failure Time (AFT) models (Stagl et al., 2015). For consistency with those analyses, this study also utilized Weibull AFT models fit using SAS PROC LIFEREG. However, the 7 interval censored patients did not fall within the cohort for which RNA samples were available, and all findings reported here also emerged in Cox proportional hazards analysis of DFS interval using SAS PROC PHREG. All analyses were based on intention to treat and 2-tailed significance was set at p < 0.050.
3. Results
3.1. Patient characteristics
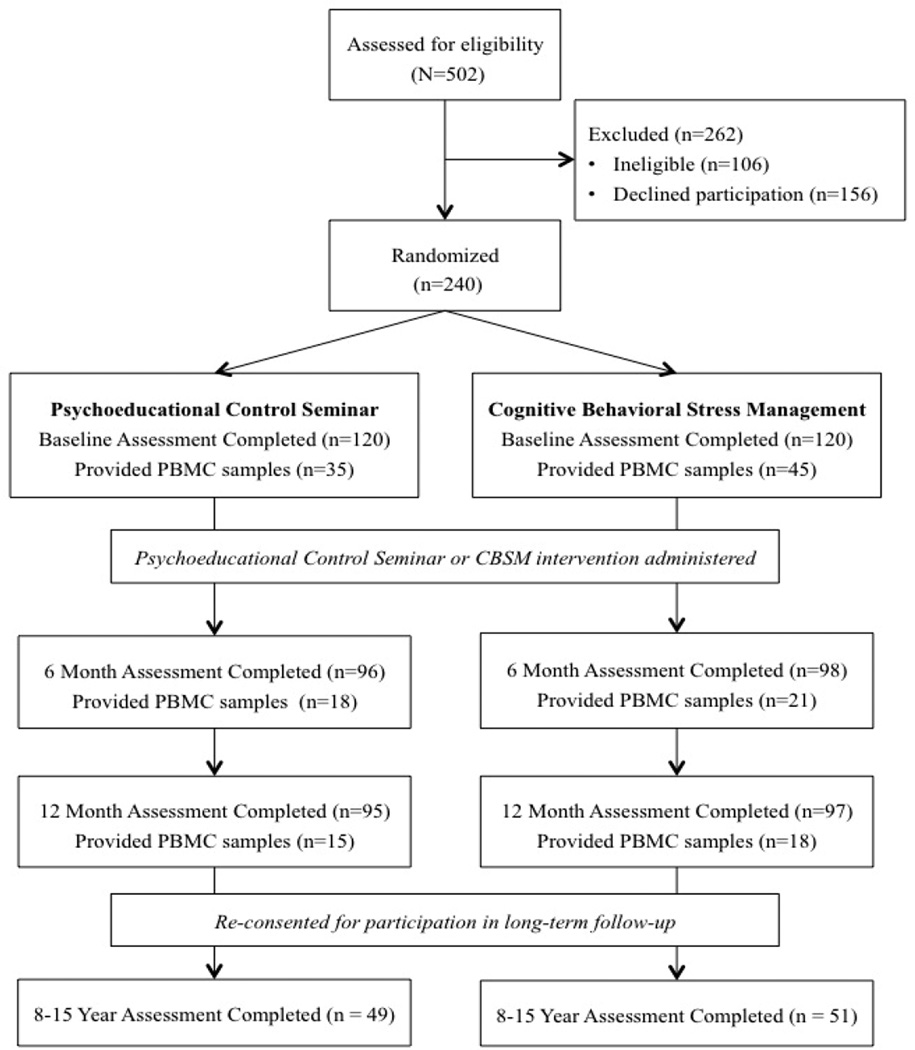
The Consolidated Standards of Reporting Trials (CONSORT) diagram of study enrollment and retention is provided in Figure 1. Women who provided blood samples for longitudinal analysis of change in CTRA gene expression were mostly similar to women in the larger study who did not. Women in these analyses did not differ from women excluded from these analyses with regard to age, education, annual household income, menopausal status, stage of disease, number of positive lymph nodes removed during surgery, size of tumor, PR status, HER2/neu status, surgical procedure, receipt of chemotherapy, receipt of hormonal therapy, receipt of radiation therapy, or study condition (all ps > 0.10).
Figure 1.
CONSORT Diagram; n = 10 participants in the psychoeducational control seminar provided PBMC samples at both T2 and T3; n = 11 participants in the cognitive behavioral stress management condition provided PMBC samples at both T2 and T3.
Women included in these analyses significantly differed from women excluded from these analyses by ethnicity (χ²[3] = 8.51, p = 0.037) and employment status (χ²[1] = 6.69, p = 0.010). The majority of women included in these analyses identified as White, Non-Hispanic (39 women, 76.5%) followed by Black or Hispanic (6 women each, 11.8% each). Women excluded from these analyses mostly identified as White, Non-Hispanic (113 women, 60.1%) followed by Hispanic (55 women, 29.3%), Black (15 women, 8.0%), and Asian (5 women, 2.7%). Women included in these analyses were significantly more likely to be employed (45 women, 88.2%) compared to women excluded from these analyses (113 women, 70.4%). Women included in these analyses were marginally more likely to be ER positive (38 women, 88.4%) compared to women excluded from these analyses (118 women, 76.1%; χ²[1] = 3.02, p = 0.082).
Table 1 reports characteristics of the subsample of 51 patients with data available for longitudinal analysis of change in CTRA gene expression. Within the cohort, patients randomized to CBSM (n = 28) did not differ from controls (n = 23) on any baseline characteristics (all ps > 0.05; Table 1). Gene expression data were available at 6-month follow-up for 18 women (35.3%), and at 12-month follow-up or both 6 and 12-month follow up for the remaining 33 women (64.7%) with similar follow-up distributions in controls and CBSM (p > 0.10).
Table 1.
Means, standard deviations, and frequencies of demographic, medical, and treatment variables by study group; ER=estrogen receptor; PR=progesterone receptor; HER2/neu=human epidermal growth factor.
| Variable | n | Total | Control | Intervention | p |
|---|---|---|---|---|---|
| Age at diagnosis (years) | 51 | 49.67 (7.15) | 50.83 (6.84) | 48.71 (7.37) | 0.298 |
| Race/ethnicity | 51 | 0.645 | |||
| White non-Hispanic | 39 (76.5%) | 19 (82.6%) | 20 (71.4%) | ||
| Hispanic | 6 (11.8%) | 2 (8.7%) | 4 (14.3%) | ||
| African American | 6 (11.8%) | 2 (8.7%) | 4 (14.3%) | ||
| Employment Status | 51 | 0.258 | |||
| Not employed | 6 (11.8%) | 4 (17.4%) | 2 (7.1%) | ||
| Employed | 45 (88.2%) | 19 (82.6%) | 26 (92.9%) | ||
| Education (years) | 51 | 15.61 (2.50) | 15.22 (2.59) | 15.93 (2.42) | 0.317 |
| Income (thousands of dollars) | 78.16 (53.56) | 80.48 (71.23) | 76.04 (31.35) | 0.788 | |
| Menopausal status | 51 | 0.683 | |||
| Premenopausal | 26 (51.0%) | 11 (47.8%) | 15 (52.6%) | ||
| Postmenopausal | 25 (49.0%) | 12 (52.2%) | 13 (46.4%) | ||
| Stage | 51 | 0.538 | |||
| 0 | 10 (19.6%) | 4 (17.4%) | 6 (21.4%) | ||
| I | 22 (43.1%) | 10 (43.5%) | 12 (42.9%) | ||
| II | 17 (33.3%) | 9 (39.1%) | 8 (28.6%) | ||
| III | 2 (3.9%) | 0 (0.0%) | 2 (7.1%) | ||
| Positive lymph nodes | 51 | 0.90 (3.02) | 0.17 (4.91) | 1.5 (3.99) | 0.120 |
| Size of tumor (cm) | 23 | 2.07 (1.29) | 2.12 (1.09) | 1.02 (1.47) | 0.864 |
| ER status | 43 | 0.841 | |||
| Positive | 38 (88.4%) | 17 (89.5%) | 21 (87.5%) | ||
| Negative | 5 (11.6%) | 2 (10.5%) | 3 (12.5%) | ||
| PR status | 36 | 0.498 | |||
| Positive | 26 (72.2%) | 11 (78.6%) | 15 (68.2%) | ||
| Negative | 10 (27.8%) | 3 (21.4%) | 7 (31.8%) | ||
| HER2/neu status | 24 | 0.324 | |||
| Positive | 7 (29.2%) | 4 (40.0%) | 3 (21.4%) | ||
| Negative | 17 (70.8%) | 6 (60.0%) | 11 (78.6%) | ||
| Procedure type | 51 | 0.357 | |||
| Lumpectomy | 23 (45.1%) | 12 (52.2%) | 11 (39.3%) | ||
| Mastectomy | 28 (54.9%) | 11 (47.8%) | 17 (60.7%) | ||
| Received chemotherapy | 50 | 0.854 | |||
| Yes | 28 (56.0%) | 12 (54.6%) | 16 (57.1%) | ||
| No | 22 (44.0%) | 10 (45.5%) | 12 (42.9%) | ||
| Received radiation therapy | 48 | 0.096 | |||
| Yes | 26 (54.2%) | 8 (40.0%) | 18 (64.3%) | ||
| No | 22 (45.8%) | 12 (60.0%) | 10 (35.7%) | ||
| Received endocrine therapy | 50 | 0.852 | |||
| Yes | 38 (76.0%) | 17 (77.3%) | 21 (75.0%) | ||
| No | 12 (24.0%) | 5 (22.7%) | 7 (25.0%) | ||
Note. Means, standard deviations, and frequencies of demographic, medical, and treatment variables by study group; ER=estrogen receptor; PR=progesterone receptor; HER2/neu=human epidermal growth factor.
3.2. Group differences in CTRA change
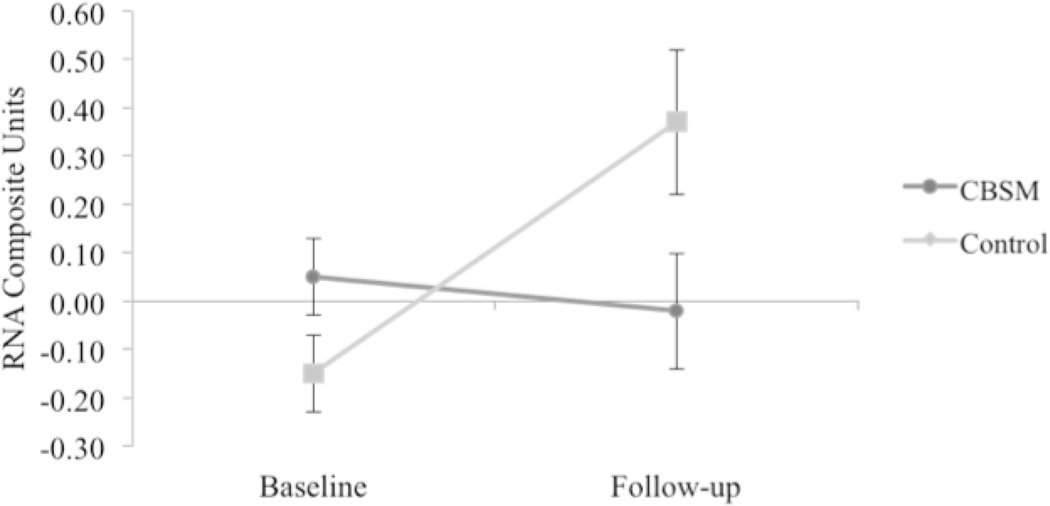
In mixed effect linear model analyses of change in expression of 53 CTRA indicator genes from pre-intervention baseline to post-intervention follow-up, patients in the control group showed a general increase (mean = +0.33 ± SE 0.13 standardized RNA change values) whereas those randomized to CBSM showed a slight reduction (−0.02 ± 0.08; difference from controls: t(49) = 2.57, p = .010). Similar effects emerged in analyses that controlled for age, disease stage, and treatment with chemotherapy, radiation, and endocrine therapy as well as gene expression follow-up time (6- vs. 12-months) (control: +0.34 ± 0.13 vs. CBSM: −0.02 ± 0.10; difference, t(38) = 2.77, p = .006). At baseline, patients randomized to CBSM showed a non-significant trend toward elevated CTRA gene expression values relative to controls (CBSM: +0.05 ± SE 0.08 RNA composite units; control: −0.15 ± 0.08; difference, p = 0.057). To ensure this trend did not account for the observed group differences in CTRA change over time, additional covariate-adjusted analyses were conducted which also controlled for baseline gene expression values in addition to other covariates. Results again indicated a reduction in the magnitude of CTRA increase over time in the CBSM-treated group (control: +0.25 ± 0.09 vs. CBSM: +0.04 ± 0.07; difference, t(37) = 2.39, p = .017). Thus, treatment effects emerged regardless of whether baseline variations in CTRA gene expression were controlled for or not.
To facilitate prediction of survival times by CTRA gene expression, we constructed a 1-number summary score averaging over the 53 gene-specific change scores. Composite change scores also differed across groups in both simple (unadjusted) analyses (control: +0.32 ± 0.11 vs. CBSM: −0.03 ± 0.10; difference, t(49) = 2.39, p = 0.021) and covariate-adjusted analyses (control: +0.37 ± 0.15 vs. CBSM: −0.02 ± 0.12; difference, t(38) = 2.57, p = 0.014; see Table 2 and Figure 2).
Table 2.
Mixed effect linear model of group differences in pre- to post-intervention change of CTRA gene expression (N = 51); SE=standard error.
| Effect | Estimate | SE | t-value | p |
|---|---|---|---|---|
| Condition (CBSM vs. control) | 0.39 | 0.15 | 2.57 | 0.014 |
| Age | −0.02 | 0.01 | −1.84 | 0.073 |
| Stage | ||||
| 0 (vs. III) | −0.28 | 0.42 | −0.67 | 0.507 |
| I (vs. III) | −0.18 | 0.38 | −0.46 | 0.650 |
| II (vs. III) | −0.11 | 0.38 | −0.28 | 0.780 |
| Chemotherapy received | −0.34 | 0.18 | −0.18 | 0.075 |
| Radiation therapy received | −0.01 | 0.15 | −0.08 | 0.935 |
| Endocrine therapy received | 0.30 | 0.17 | 1.79 | 0.081 |
| Time of follow-up (6 vs. 12 months) | 0.13 | 0.16 | 0.80 | 0.431 |
Note. Mixed effect linear model of group differences in pre- to post-intervention change of CTRA gene expression (N = 51); SE=standard error.
Figure 2.
Group differences in pre- and post-intervention mean CTRA gene expression (N = 51); Adjusted mean CTRA gene expression is depicted controlling for age, disease stage, treatment with chemotherapy, radiation therapy, endocrine therapy, and follow-up time (6 or 12-months). Error bars reflect standard error.
3.3. CTRA change and DFS
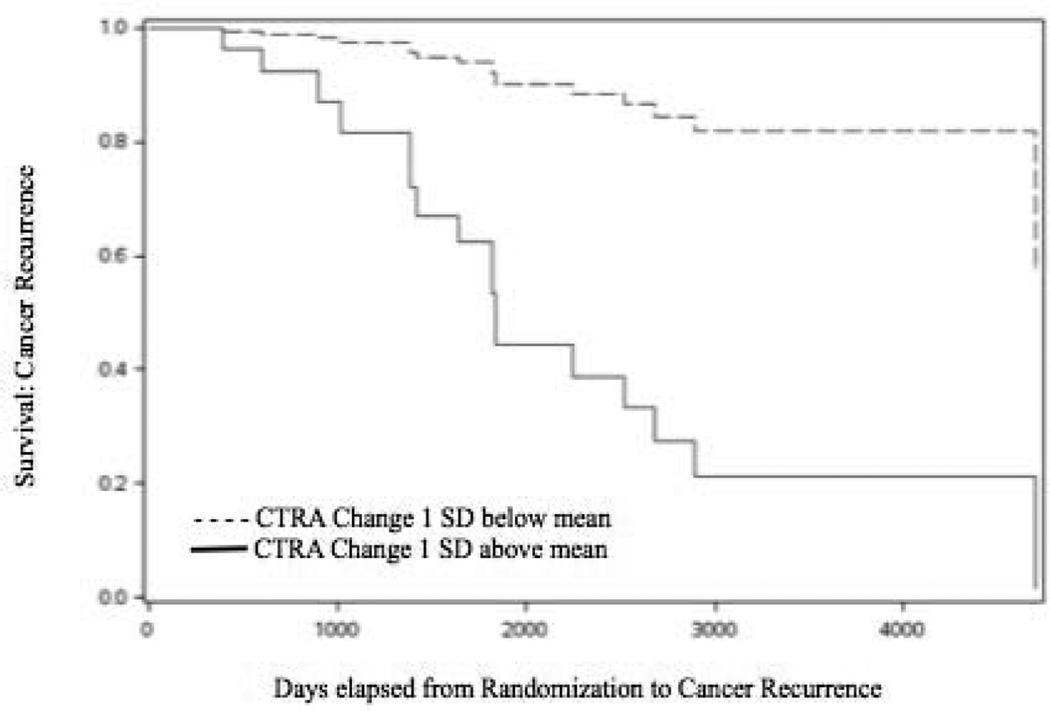
Among the cohort of 51 patients for whom survival and gene expression data were available, 17 (33.3%) reached a DFS endpoint (either recurrence or 2nd cancer diagnosis) over a median 11-years of follow-up (consistent with our previous DFS findings for the overall study; Stagl et al., 2015). In accelerated failure time survival analyses of DFS interval controlling for treatment condition, pre- to post-intervention change in CTRA gene expression was associated with a significant acceleration in time to recurrence in these 51 cases (log parameter: −0.83 ± 0.37, X2(1) = 5.00, p = .025). Similar effects were observed in analyses that additionally controlled for age, disease stage, and treatment with chemotherapy, radiation, and endocrine therapy as well as gene expression follow-up time (−1.09 ± 0.38, X2(1) = 4.92, p = 0.007). Similar effects were observed when DFS was analyzed using Cox proportional hazards regression (relative hazard of recurrence per standardized RNA composite unit = 4.02 [95% CI: 1.17–13.83], p = 0.028, and 6.32 [1.41–28.33], p = 0.016 additionally adjusted for age, stage, treatment, and follow-up time) (see Table 3). Results are represented in Figure 3, which shows the model-predicted DFS functions for hypothetical individuals with CTRA change scores falling 1 SD below the mean value on the continuous distribution of CTRA change scores vs 1 SD above the mean value (with otherwise identical average or modal values on all other covariates). Baseline levels of CTRA gene expression were not independently associated with DFS in this sample (i.e., above and beyond their contribution to change scores; p > 0.10 in both unadjusted and covariate-adjusted accelerated failure time analyses, and p > 0.20 in both unadjusted and covariate-adjusted proportional hazards analyses).
Table 3.
Adjusted models with change in CTRA gene expression and disease free survival at 11-year follow-up (N = 51); CI=confidence interval; HR=hazard ratio.
| Variable | Weibull Accelerated Failure Time Model | Cox Proportional Hazards Model | |||||
|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Chi-square | p | HR | 95% CI | p | |
| Change in CTRA gene expression | −1.09 | −1.87 – −0.30 | 7.33 | 0.007 | 6.32 | 1.41 – 28.34 | 0.016 |
| Age | 0.02 | −0.04 – 0.07 | 0.43 | 0.511 | 0.96 | 0.86 – 1.07 | 0.461 |
| Stage | |||||||
| 0 (vs. III) | −0.76 | −2.07 – 0.54 | 1.31 | 0.253 | 4.35 | 0.36 – 53.13 | 0.249 |
| I (vs. III) | −0.02 | −1.22 – 1.18 | 0.00 | 0.972 | 1.15 | 0.12 – 11.37 | 0.903 |
| II (vs. III) | 0.37 | −0.94 – 1.69 | 0.31 | 0.578 | 0.72 | 0.06 – 8.39 | 0.792 |
| Chemotherapy received | −0.59 | −1.48 – 0.29 | 1.73 | 0.189 | 2.90 | 0.58 – 14.64 | 0.197 |
| Radiation therapy received | 0.17 | −0.55 – 0.89 | 0.22 | 0.642 | 0.63 | 0.17 – 2.30 | 0.479 |
| Endocrine therapy received | 0.64 | −0.03 – 1.31 | 3.48 | 0.062 | 0.39 | 0.10 – 1.50 | 0.172 |
| Time of follow-up (6 vs. 12 months) | 0.12 | −0.53 – 0.77 | 0.14 | 0.708 | 0.78 | 0.22 – 2.68 | 0.688 |
| Condition | −0.42 | −1.06 – 0.23 | 1.61 | 0.205 | 0.27 | 0.57 – 7.51 | 0.265 |
Note. Adjusted models with change in CTRA gene expression and disease free survival at 11-year follow-up (N = 51); CI=confidence interval; HR=hazard ratio.
Figure 3.
Cox Proportional Hazard Model-predicted survival functions for hypothetical individuals with CTRA change scores falling 1 SD below the mean value on the continuous distribution of CTRA change scores vs. 1 SD above the mean value (and otherwise having identical average or modal values on age, disease stage, treatment with chemotherapy, radiation therapy, and hormone therapy).
4. Discussion
The study reported here found a favorable CBSM intervention effect on change in expression of a composite of adversity-related gene transcripts in circulating leukocytes representing a conserved transcriptional response to adversity (CTRA; Irwin and Cole, 2011; Fredrickson et al., 2013). Greater CTRA gene expression is indicative of greater expression of pro-inflammatory genes and reduced expression of genes associated with antibody synthesis and Type I interferon antiviral responses. Compared to those in an active psychoeducation control group, who showed up-regulated CTRA gene expression at 6–12-month follow-up, those in CBSM showed no such changes. This suggests that CBSM may have blunted such transcriptional changes during active primary treatment for non-metastatic breast cancer. Separately, greater magnitude of relative increase in CTRA gene expression over this 6–12 month period was associated with shorter DFS at median 11-year follow-up. Findings were consistent when controlling for age, disease stage, exposure to adjuvant treatments (chemotherapy, radiation therapy, and endocrine therapy) and gene expression follow-up time (6 vs. 12-months). Taken together these findings provide preliminary evidence that CBSM delivered during primary cancer treatment may reduce adversity-associated transcriptional changes that themselves predict disease progression up to 15 years later.
This study builds upon previous research finding that CBSM is empirically associated with down-regulation of genes linked to pro-inflammatory transcription factors and up-regulation of genes linked to type I interferon response factors (Antoni et al., 2012). The present analyses used a standard externally defined panel of canonical CTRA indicator transcripts that were derived from the broader social genomics literature analyzing the molecular impact of adverse life circumstances ranging from social isolation to low socioeconomic status and Post-Traumatic Stress Disorder (Cole, 2013, 2014; Irwin and Cole, 2011; Fredrickson et al., 2013). This canonical CTRA indicator set was analyzed here to assess whether it too could detect CBSM effects (after all, it is tautological that the other specific gene expression indicators identified in Antoni et al. 2012 would differ across groups), to maintain consistency with other recent studies of CTRA correlates of cancer survival (Knight et al., 2015), and to provide a common molecular metric for contextualizing the impact of CBSM within the broader literature on social genomics.
The inflammation and interferon components of the CTRA profile also parallel the profile of molecular alterations observed in preclinical models of stress effects on cancer metastasis (Sloan et al., 2010). It is possible that CBSM effects gene expression of the CTRA indicator genes (and others) via effects on autonomic nervous system and HPA-axis functions (for review, see Lutgendorf et al., 2010). Together, the findings reported here provide support for the hypothesis that CBSM effects on the leukocyte transcriptome reflecting CTRA may potentially contribute to the salutary effects of CBSM on the clinical course of breast cancer.
These findings identified specific health implications for women with breast cancer, and this is the first study of its kind to provide preliminary support for the down-regulation of leukocyte CTRA gene expression as a potential immunological mechanism for the impact of psychosocial interventions such as CBSM on breast cancer recurrence. Within the CTRA alterations, down-regulation of pro-inflammatory genes is notable because chronic inflammation is implicated in breast cancer progression and recurrence (Cole, 2009; Pierce et al., 2009). Further, enhanced expression of type I interferon-related genes - also encompassed in the CTRA composite - is associated with reduced breast cancer progression (Andersen et al., 2010; Recchia et al., 2009; Seth et al., 2003). It is plausible that other psychosocial interventions known to have positive effects on breast cancer disease recurrence may operate via similar mechanisms, though this requires empirical testing. This work may also have implications for other cancers, given recent evidence that greater CTRA also predicts increased relapse risk and decreased leukemia-free survival in recipients of hematopoietic stem cell transplant for acute myelogenous leukemia (Knight et al., 2015).
4.1. Strengths and limitations
Results should be interpreted with caution, as DFS was not a primary endpoint at time of study planning. In addition, the CTRA outcome could not have been hypothesized at time of study planning and recruitment, as the crucial publications describing methods for studying CTRA were not published until study planning was completed (i.e., Fredrickson et al., 2013, 2015). Sample size was low due to a relatively small proportion of the parent study participants providing blood data for longitudinal gene expression analyses. Findings therefore are considered preliminary and require replication in a larger sample. Full mediation analyses were not conducted in the current study due to the limited sample available for analyses of gene expression and the relatively small number of events (i.e., recurrences). It will be important for future research to test this model in larger samples. Despite the statistical similarity between participants who did and did not provide blood samples, the current subgroup cannot be presumed to be identical to the total study sample. Thus the current findings are exploratory and require future replication.
Given the observed non-significant trend at baseline toward elevated CTRA gene expression values among participants randomized to CBSM relative to controls, it is possible that group differences at baseline may have affected the observed changes in CTRA gene expression over time (although groups did not differ significantly on any of the demographic, biological, or treatment-related variables in Table 1). Nonetheless, group differences in CTRA gene expression change over time were significant in both unadjusted analyses and in analyses that controlled for multiple covariates including baseline differences in gene expression. Participants were not selected into CBSM vs. control conditions based on baseline gene expression values (or any proxy of that), nor did groups converge to a common intermediate level at follow-up, so the group differences in change over time cannot be attributed to statistical regression to the mean (Nesselroade & Stigler, 1980). Nonetheless, the present finding should be regarded as preliminary until future research in larger samples with more completely balanced randomization can confirm and clarify the associations currently observed.
The study used the entire leukocyte population in the PBMCs, and we therefore cannot attribute individual gene expression patterns to specific cell populations. Future work should focus on separated cell studies. The study lacked complete data pertaining to important prognostic indicators (i.e., HER2/neu, BMI) and additional clinicopathological variables to further distinguish cancer subtypes (e.g., luminal a, luminal b, histology, grade). Thus, to preserve the limited sample size, analyses could not control for such variables; future studies of the relationship between CTRA gene expression and DFS in breast cancer patients should include such variables, as they may affect findings. The study was limited by factors such as academic study setting, geographical location, and inclusion criteria. The sample was predominantly highly educated women potentially motivated to participate in health-related research, thus limiting the generalizability of findings. This study’s relatively small and comparatively advantaged sample may also explain why we were unable to detect any variations in DFS as a function of baseline CTRA expression such as previously observed by Knight et al. (2015); the significance of any such association may have been attenuated in this sample due to limited power and/or limited variation in socioeconomic status.
Strengths of this study include that approximately one quarter of the sample identified as an ethnic minority, either Hispanic or African American. The study used an a priori chosen set of indicator genes that have been used in other research (Fredrickson et al., 2013, 2015; Knight et al., 2015). In addition, the current study used an a priori list of covariates that was theoretically based and manually entered into the model to minimize overfitting (Babyak, 2004). This approach optimizes the reproducibility of the findings.
4.2. Future research
Future studies should evaluate the long-term effects and underlying mechanisms of cognitive-behavioral interventions on clinical disease outcomes in larger samples of non-metastatic breast cancer patients. This is an area in need of further exploration with clinical endpoints as primary outcomes and more rigorous study designs. Although the CBSM intervention used here involved a moderate-length intervention compared to some in the field, it is critical to learn what is the minimal dose necessary to bring about these transcriptional changes to a degree that could influence disease progression. We learned here that a 10-week program was associated with a buffering of leukocyte CTRA changes out to 12 months of primary treatment, and that the quiescence of this 53-gene ensemble during this period of treatment was associated with a longer period of DFS. However, since this intervention length is possibly too long to be manageable in clinical oncology practice, there may be practical limits to widespread uptake in cancer centers.
In strategizing next steps for compressing this intervention approach into a shorter period we have been guided by secondary analyses of this CBSM trial wherein we learned that women attending 5 sessions of this CBSM intervention showed similar reductions in adversity to those who attended 8–10 sessions. In a recently completed trial we found that a 5-week version of stress management intervention decreases psychological adversity indicators compared to the 5-week attention control in women with non-metastatic breast cancer (Gudenkauf et al., 2015). These findings open the door to the possibility that brief stress management interventions delivered during primary treatment for breast cancer may have similar biological and health effects as the longer CBSM intervention reported here. Analyses of immunological changes for this 5-week trial are underway and the cohort is being followed for longer-term health effects. As we probe for transcriptional changes in this trial we are also seeking to extend knowledge of the specific mechanisms underlying this intervention’s effects on CTRA-related processes by isolating the cellular components (monocytes, lymphocytes) responsible for the transcriptional changes.
5. Conclusions
This study showed that a 10-week group-based stress management intervention designed to mitigate adversity delivered during the initial year of primary breast cancer treatment buffered increases in CTRA gene expression, a specific profile previously observed in people confronting significant life adversity (e.g., Cole et al., 2012; Irwin and Cole, 2011). In turn, mitigating CTRA gene expression during the first year of primary treatment predicted longer DFS up to 15 years later. Thus, CBSM when offered early in breast cancer treatment may improve adversity-related gene programs in ways that promote better long-term health outcomes. These preliminary findings provide a biological framework for understanding the effect of behaviorally-targeted interventions on immunologic processes and long term clinical outcomes, and identify biobehavioral oncology pathways to target in future work.
Highlights.
Conserved transcriptional response to adversity (CTRA) rises with cancer treatment.
Cognitive behavioral stress management (CBSM) buffers CTRA during treatment.
Less CTRA rise during cancer treatment predicts longer 11-yr disease-free survival.
CBSM modulates disease relevant biobehavioral processes in breast cancer treatment.
Acknowledgments
This work was supported by the National Cancer Institute (NCI), National Institutes of Health [Contract No. HHSN261200800001E] and by the NCI grant [R01CA064710].
Role of Funding Sources
The funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registration #: NCT01422551, full trial protocol can be accessed at clinicaltrials.gov
Conflicts of Interest
Dr. Antoni reports receiving publication royalties from a book he co-authored on cognitive-behavioral stress management.
Contributors
All authors of this research paper have made substantial contributions in: (1) the conception and design of the study, or acquisition of data, or analyses and interpretation of the data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version of the manuscript submitted herein.
Specifically, MHA, CSC, BBB and SCL contributed to all of these areas; LCB, JMJ, DRJ, and LMG contributed to acquisition, analysis and interpretation of data, and revising and providing final approval for the article; SWC contributed to analyses and interpretation of the data, drafting, revising and approving the article; and SL and ML contributed to interpretation of the data analyses, and revising and approving the article.
References
- Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE., III Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J. Clin. Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Thornton LM, Shapiro CL, Farrar WB, Mundy BL, Yang HC, Carson WE., III Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clin. Cancer Res. 2010;16:3270–3278. doi: 10.1158/1078-0432.CCR-10-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Yang H, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, Young DC, Carson WE., III Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH. Stress Management Intervention for Women with Breast Cancer. Washington, DC: 2003. [Google Scholar]
- Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain Behav. Immun. 2013;30(Suppl):S88–S98. doi: 10.1016/j.bbi.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lechner S, Diaz A, Vargas S, Holley H, Phillips K, McGregor B, Carver CS, Blomberg B. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav. Immun. 2009;23:580–591. doi: 10.1016/j.bbi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lechner SC, Kazi A, Wimberly SR, Sifre T, Urcuyo KR, Phillips K, Gluck W, Carver CS. How stress management improves quality of life after treatment for breast cancer. J. Consult. Clin. Psych. 2006a;74:1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Dtagl J, Arevalo JM, Cole SW. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol. Psychiat. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf S, Cole S, Dhabhar F, Sephton S, McDonald P, Stefanek M, Sood AK. The influence of biobehavioral factors on tumor biology: Pathways and mechanisms. Nat. Rev. Cancer. 2006c;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Wimberly S, Lechner S, Kazi A, Sifre T, Urcuyo K, Phillips K, Smith RG, Petronis VM, Guellati S, Wells KA, Blomberg B, Carver CS. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am. J. Psychiat. 2006b;163:1791–1797. doi: 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaiz-Pena GN, Lutgendorf SK, Cole SW, Sood AK. Neuroendocrine modulation of cancer progression. Brain Behav. Immun. 2009;23:10–15. doi: 10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyak MA. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom. Med. 2004;6:411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol. Psychiatr. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GS, Walker HA, Arevalo JM, Sung CY, Cole SW. Genome-wide transcriptional profiling linked to social class in asthma. Thorax. 2009;64:38–43. doi: 10.1136/thx.2007.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Social regulation of human gene expression. Curr. Dir. Psychol. Sci. 2009;18:132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Elevating the perspective on human stress genomics. Psychoneuroendocrino. 2010;35:955–962. doi: 10.1016/j.psyneuen.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Social regulation of human gene expression: Mechanisms and implications for public health. Am. J. Public Health. 2013;103:S84–S92. doi: 10.2105/AJPH.2012.301183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Human social genomics. PLOS Genet. 2014;10:e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ. Transcriptional modulation of the developing immune system by early life social adversity. P. Natl. Acad. Sci. USA. 2012;109:20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. P. Natl. Acad. Sci. USA. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Nagaraja A, Lutgendorf S, Green P, Sood A. Sympathetic nervous system regulation of the tumor microenvironment. Nat. Rev. Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JC, Stefanek M, Palmer SC. Psychotherapy and survival in cancer: The conflict between hope and evidence. Psychol. Bull. 2007;133:367–394. doi: 10.1037/0033-2909.133.3.367. [DOI] [PubMed] [Google Scholar]
- Daniels J, Kissane DW. Psychosocial interventions for cancer patients. Curr. Opin. Oncol. 2008;20:367–371. doi: 10.1097/CCO.0b013e3283021658. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Grewen KM, Algoe SB, Firestine AM, Arevalo JMG, Ma J, Cole SW. Psychological well-being and the human conserved transcriptional response to adversity. PLOS One. 2015;10:e0121839. doi: 10.1371/journal.pone.0121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Grewen KM, Coffee KA, Algoe SB, Firestine AM, Arevalo JMG, Ma J, Cole SW. A functional genomic perspective on human well-being. P. Natl. Acad. Sci. USA. 2013;110:13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudenkauf LM, Antoni MH, Stagl JM, Lechner SC, Jutagir DR, Bouchard LC, Blomberg BB, Gluck S, Derhagopian RP, Giron GL, Avisar E, Torres-Salichs MA, Carver CS. Brief cognitive-behavioral and relaxation training interventions for breast cancer: A randomized controlled trial. J. Consult. Clin. Psych. 2015;83:677–688. doi: 10.1037/ccp0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JM, Rizzo JD, Logan B, Wang T, Arevalo J, Ma J, Cole SW. Low socioeconomic status, adverse gene expression profiles, and clinical outcomes in hematopoietic stem cell transplant recipients. Clin. Cancer Res. 2015;22:69–78. doi: 10.1158/1078-0432.CCR-15-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, Goodheart M, Buekers TE, Mendez L, Krueger G, Clevenger L, Lubaroff DM, Sood AK, Cole SW. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav. Immun. 2011;25:250–255. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Degeest K, Sung CY, Arevalo JM, Penedo F, Lucci J, III, Goodheart M, Lubaroff D, Farley DM, Sood AK, Cole SW. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav. Immun. 2009;23:176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Johnson EL, Cooper B, Anderson B, Sorosky JI, Buller RE, Sood AK. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JM, Penedo F, DeGeest K, Langley RR, Lucci JA, III, Cole SW, Lubaroff DM. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin. Cancer Res. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: Biobehavioral signaling pathways and interventions. J. Clin. Oncol. 2010;28:4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annu. Rev. Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JMG, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-κB signaling. Biol. Psychiat. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Murphy MLM, Cashman R, Ma R, Ma J, Arevalo JMG, Kobor MS, Cole SW. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav. Immun. 2014;41:191–199. doi: 10.1016/j.bbi.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesselroade JR, Stigler SM, Baltes PB. Regression toward the mean and the study of change. Psychol Bull. 1980;88:622–637. [Google Scholar]
- Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J. Natl. Cancer I. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- Phillips KM, Antoni MH, Lechner SC, Blomberg BB, Llabre MM, Avisar E, Glück S, DerHagopian R, Carver CS. Stress management intervention reduces serum cortisol and increases relaxation during treatment for nonmetastatic breast cancer. Psychosom. Med. 2008;70:1044–1049. doi: 10.1097/PSY.0b013e318186fb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgertner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. P. Natl. Acad. Sci. USA. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchia F, Sica G, Candeloro G, Zecozione S, Bisegna R, Bratta M, Rea S. Beta-interferon, retinoids and tamoxifen in metastatic breast cancer: long-term follow-up of a phase II study. Oncol. Rep. 2009;21:1011–1016. doi: 10.3892/or_00000317. [DOI] [PubMed] [Google Scholar]
- Seth A, Kitching R, Landberg G, Xu J, Zubovits J, Burger AM. Gene expression profiling of ductal carcinomas in situ and invasive breast tumors. Anticancer Res. 2003;23:2043–2051. [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagl JM, Lechner SC, Carver CS, Bouchard LC, Gudenkauf LM, Jutagir DR, Diaz A, Yu Q, Blomberg BB, Ironson G, Gluck S, Antoni MH. A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up. Breast Cancer Res. Tr. 2015;154:319–328. doi: 10.1007/s10549-015-3626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas S, Antoni MH, Carver CS, Lechner SC, Wohlgemuth W, Llabre M, Blomberg BB, Gluck S, DerHagopian RP. Sleep quality and fatigue after a stress management intervention for women with early-stage breast cancer in southern Florida. Int. J. Behav. Med. 2014;21:971–981. doi: 10.1007/s12529-013-9374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]