Abstract
Objective
17β-Estradiol (17βE) regulates glucose homeostasis in part by centrally mediated mechanisms. In female rodents, the influence of the ovarian cycle on hypoglycemia counterregulation and glucose tolerance is unclear. We found previously that in prepubertal females, 17βE modulates glucose sensing in nonadapting glucose-inhibited (GI) and adapting GI (AdGI) neurons within the ventrolateral portion of the ventromedial nucleus (VL-VMN). Nonadapting GI neurons persistently decrease their activity as glucose increases while AdGI neurons transiently respond to a glucose increase. To begin to understand if endogenous fluctuations in estrogen levels across the estrous cycle impact hypothalamic glucose sensing and glucose homeostasis, we assessed whether hypoglycemia counterregulation and glucose tolerance differed across the phases of the estrous cycle. We hypothesized that the response to insulin-induced hypoglycemia (IIH) and/or glucose tolerance would vary throughout the estrous cycle according to changes in 17βE availability. Moreover, that these changes would correlate with estrous-dependent changes in the glucose sensitivity of VL-VMN glucose-sensing neurons (GSNs).
Methods
These hypotheses were tested in female mice by measuring the response to IIH, glucose tolerance and the glucose sensitivity of VL-VMN GSNs during each phase of the estrous cycle. Furthermore, a physiological brain concentration of 17βE seen during proestrus was acutely applied to brain slices isolated on the day of diestrous and the response to low glucose in VL-VMN GSNs was assayed.
Results
The response to IIH was strongest during diestrous. The response of nonadapting GI and AdGI neurons to a glucose decrease from 2.5 to 0.5mM also peaked during diestrous; an effect which was blunted by the addition of 17βE. In contrast, the glucose sensitivity of the subpopulation of GSNs which are excited by glucose (GE) was not affected by estrous phase or exogenous 17βE application.
Conclusion
These data suggest that physiological fluctuations in circulating 17βE levels across the estrous cycle lead to changes in hypothalamic glucose sensing and the response to IIH.
Keywords: estrogen, glucose-excited neurons, glucose-inhibited neurons, hypoglycemia counterregulation, glucose tolerance
1. INTRODUCTION
The counterregulatory response to hypoglycemia is a sympathoadrenal and hormonal mechanism that restores euglycemia by decreasing glucose utilization and stimulating hepatic glycogenolysis and gluconeogenesis [1]. Sex differences in hypoglycemia counterregulation, which are well documented in humans and rodents (recently reviewed by Lopez et al. [2]), suggest a role for gonadal hormones in glucose homeostasis. In both sexes, 17β-estradiol (17βE) acts centrally to regulate energy and glucose homeostasis [3]. However, the impact of fluctuating endogenous ovarian hormones on glucose homeostasis is unclear. This issue is rarely considered in studies involving female subjects, and when it is, conflicting results of the impact of the follicular phase (i.e., high circulating 17βE) on insulin sensitivity are reported [4,5]. Conflicting data in both humans and rodents has also been generated concerning glucose homeostasis across the female cycle [6,7,8,9,10,11,12,13]. Additionally, we have found only one study conducted in rodents where the authors report impaired glucose tolerance in diestrus and improved glucose tolerance in ovariectomized females following 17βE replacement [12]. Some variability in the literature may be due to different experimental protocols (i.e., hypoglycemic clamps versus bolus glucose/insulin injections), endpoints (i.e., homeostatic model assessment for insulin resistance [HOMA-IR] versus glucose tolerance tests [GTT]), sample sizes, cycle phase determination or animal strains utilized in these studies. In particular, the gold standard method used to assess hypoglycemia counterregulation, hyperinsulinemic-hypoglycemic clamp, may confound the effects of circulating 17βE due to the prolonged hypoglycemic and hyperinsulinemic conditions required for this technique [9,14].
In the brain, ventromedial hypothalamic (VMH) estrogen receptors (ER) are particularly important for 17βE regulation of food intake [15,16], energy expenditure [16,17] and reproduction [18,19]. The VMH, which is comprised of the arcuate (ARC) and ventromedial nuclei (VMN), is also critical for glucose homeostasis. The VMH contains glucose sensing neurons that either increase (glucose-excited or GE), or decrease (glucose-inhibited or GI) their activity as glucose increases [20]. VMH GI neurons can be further subdivided depending on whether their response to low glucose is sustained (nonadapting) or reverses (adapting or AdGI) prior to return to baseline glucose levels [21]. In studies using male rats, local VMH glucopenia [22] or glucose infusion during systemic hypoglycemia [23] initiates or attenuates the peripheral release of counterregulatory hormones, respectively. We have previously shown that male VMN GI neurons play a role in hypoglycemia detection and counterregulation [24,25]. While the basic hormonal counterregulatory response is similar in males and females, the magnitude of the response is blunted in females [26]. Interestingly, we have recently shown that in prepubertal females 17βE blunts the ability of nonadapting GI and AdGI neurons within the ventrolateral (VL)-VMN to respond to decreased glucose [21]. This suggests that the blunted response of female nonadapting GI and AdGI neurons to low glucose may contribute to their attenuated counterregulatory response compared to males.
In this study, we hypothesized that hypoglycemia counterregulation and glucose tolerance vary across the estrous cycle and are correlated with changes in the glucose sensitivity of VL-VMN nonadapting GI and AdGI neurons. Thus, we evaluated the response to insulin-induced hypoglycemia (IIH), glucose tolerance, and the response of VL-VMN glucose sensing neurons to low glucose during each phase of the mouse estrous cycle. We found that the recovery from IIH was strongest during diestrous when 17βE levels are lowest. Similarly, the response of VL-VMN nonadapting and AdGI neurons to low glucose also peaked during diestrous. Consistent with these findings, exogenous 17βE blunted the response of VL-VMN nonadaptive and AdGI neurons to low glucose during diestrous. These data suggest that 17βE regulation of hypothalamic glucose sensing may contribute to cyclic changes in the response to IIH.
2. MATERIALS AND METHODS
2.1. Animals and Tissue Preparation
Female C57Bl/6 mice (3–4 weeks and 6–7 weeks old) were maintained on a 12:12 light:dark cycle (7am-lights on; 7pm-lights off) with standard rodent chow and water ad libitum. Animal procedures were approved by the Institutional Animal Care and Use Committee at Rutgers, The State University of New Jersey, RBHS-New Jersey Medical School. Estrous phase was determined in 6–7 week old females by examining vaginal cytology via light microscopy. Vaginal smears were collected within 2 hours following the onset of the lights (7–9am). Brain slices were collected by 11am on each day of the estrous cycle. This allowed for initiation of the 17βE surge in the slices collected on proestrous [27]. The following vaginal cytology descriptions were used: Diestrous-circular leukocytes, Proestrous-nucleated epithelial cells, Estrous-cornified squamous epithelial cells, and Metestrous-clustered cornified squamous epithelial cells and polynucleated leukocytes [28]. We established that all mice cycled regularly (3–5 day cycles), and on the day of the experiments estrous phase was re-assessed. All 3–4 week old female mice were only used prior to vaginal opening, a visual marker of pubertal transition [28].
2.2. Electrophysiology
Compared to isolated primary cell or cell-line cultures, patch-clamp analysis in brain slice preparations allows for the study of neuronal behavior in an environment that more closely mimics that seen in-vivo [29]. Therefore, following transcardial perfusion with 4°C oxygenated (95%O2/5%CO2) perfusion buffer (composition in mM: 2.5 KCl, 7 MgCl2, 1.25 NaH2PO4, 28 NaHCO3, 0.5 CaCl2, 7 glucose, 1 ascorbate, 3 pyruvate; ~300mOsm, pH 7.4), brains were quickly removed and placed in 4°C oxygenated perfusion buffer. The hypothalamus was dissected and 250–300µm coronal slices were made on a vibratome (Vibroslice, Camden Instruments). VMH containing slices (3 per animal) were maintained in oxygenated artificial cerebrospinal fluid (aCSF, in mM: 126 NaCl, 1.9 KCl, 1.2KH2PO4, 26 NaHCO3, 2.5 glucose, 1.3 MgCl2, 2.4 CaCl2; ~300mOsm, pH 7.4) for at least one hour prior to recording. Standard whole-cell current clamp recordings were made as previously described [30]. Briefly, borosilicate pipettes (4–7MΩ; Sutter Instruments) were filled with an intracellular solution containing (in mM): 128 K-gluconate, 10 KCl, 4 KOH, 10 HEPES, 4 MgCl2, 0.5 CaCl2, 5 EGTA, 2 Na2ATP, 0.4 Na2GTP; ~300 mOsm, pH 7.2. Pipette-solution junction potential was nulled prior to formation a GΩ seal, membranes were ruptured with mild suction followed by whole cell capacitance compensation. Cells were deemed viable for recording if action potentials crossed 0 mV and access resistance was less than 30 MΩ following membrane rupturing. Input resistance was calculated from hyperpolarizing current pulses given every three seconds (500ms; −10, −15 or −20pA).
2.3. Slice treatments
Slices were exposed to the various treatment solutions for ten minutes each based on previous studies showing this exposure time was sufficient to determine glucose and hormone responses in VMH neurons [20,31,32,33,34,35]. Moreover, based on years of study and published data, we have concluded that the duration of exposure to the media is not enough to alter the basal physiology and thus in vivo changes in physiological state do indeed translate to measureable changes in glucose sensing within hypothalamic slices which persist during slicing and several hours of subsequent recording [14, 25, 33, 34]. Additionally, by using a media which is free of exogenous hormones we specifically ‘leveled the playing field so that regardless of the day of cycle, all cells were exposed to the same media. All recordings were performed between 12pm and 7pm following cycle determination. Glucose sensing was evaluated in response to a glucose decrease from 2.5mM to 0.1mM or 0.5mM. These glucose concentrations represent those typically seen in the brain during peripheral euglycemia (2.5mM) and moderate (0.5mM) or severe (0.1mM) hypoglycemia [36,37]. The tissue concentration of 17βE in the VMH during each phase of the estrous cycle has yet to be determined, therefore, the 17βE concentration utilized in this study was selected based on hippocampal studies [38]. Thus, to determine the effect of 17βE on hypothalamic glucose sensing, glucose was lowered in the presence of 4nM 17βE, the hippocampal 17βE concentration during proestrus [38], in brain slices collected during diestrus.
On average 4 neurons were recorded per slice (for approximately 1.5 hours each), but no slice was exposed to 17βE for >30 minutes. In some cases, DMSO was used as the vehicle for drug treatments, but the final DMSO concentration for all treatments was ≤0.1%. At this concentration no significant changes in basal neuronal activity are observed [21].
2.4. Glucose Tolerance Test (GTT)
On the morning of of GTT, mice were phased, weighed and food was subsequently removed approximately 2 hours after lights on. Five hours later mice were given an intraperitoneal (IP) injection of sterile D-glucose (1g/kg; Sigma) and blood glucose was measured at t=0, 20, 40, 60, 90, and 120 minutes post injection using a FreeStyle Lite glucose meter [39]. All blood sampling was collected via the tail snip method and less than 10µL of blood was collected at each time point. All testing was completed prior to 3pm; these mice were only used for GTT. In total, glucose tolerance was evaluated in 8, 6, 9, and 9 mice in diestrous, proestrous, estrous and metestrous, respectively.
2.5. Insulin-Induced Hypoglycemia (IIH)
On the morning of IIH, mice were phased, weighed and food was subsequently removed approximately 2 hours after lights on. Five hours later mice were given a SC injection of sterile Humulin®-R (2U/kg, Lilly Pharmaceuticals) and blood glucose was measured at t=−30, 0, 15, 30, 45, 60, 75, 90, 105 and 120 minutes post injection using a FreeStyle Lite glucose meter. All blood sampling was collected via the tail snip method and less than 10µL of blood was collected at each time point. All testing was completed prior to 3pm; these mice were only used for ITT. Two mice exhibited signs of severe hypoglycemia during the course of this study and were euthanized and excluded from analysis. In total, IIH was evaluated in 8, 7, 8, and 8 animals in diestrous, proestrous, estrous and metestrous, respectively.
2.6. Data Analysis & Statistics
All data are expressed as average ± standard error. For GTT and IIH, area under the curve from time 0 to 120 min was calculated using baseline values to 90mg/dL and 40mg/dL, respectively. These baseline values were selected because they encompassed the standard deviation of the curves while minimizing background contribution. For electrophysiological recordings, treatment effects were quantified using the percent changes in input resistance (IR) and membrane potential (Vm). IR was calculated from the membrane voltage responses to hyperpolarizing pulses according to Ohm’s law (Resistance =Voltage/Current) within the last minute of treatment application. For AdGI neurons, IR in low glucose was calculated for one minute once maximal activation of these neurons occurred (~5–6min after solution change). Percent change was calculated relative to 2.5mM glucose. Data outliers, if any, were identified using studentized residuals and excluded from any supplemental statistical analyses. Data was compared using paired student t-tests, standard one-way ANOVAs with Tukey posthoc tests or repeated two-way ANOVAs with Bonferonni posthoc tests as specified in the figure legends. Bartlett’s test for equality of variances was used to ensure validity of ANOVA tests. For t-tests, *p<0.05, +p<0.01. For ANOVAs, columns with different symbols indicate significant differences.
3. RESULTS
3.1. Glucose Tolerance Tends to be Impaired During Proestrus
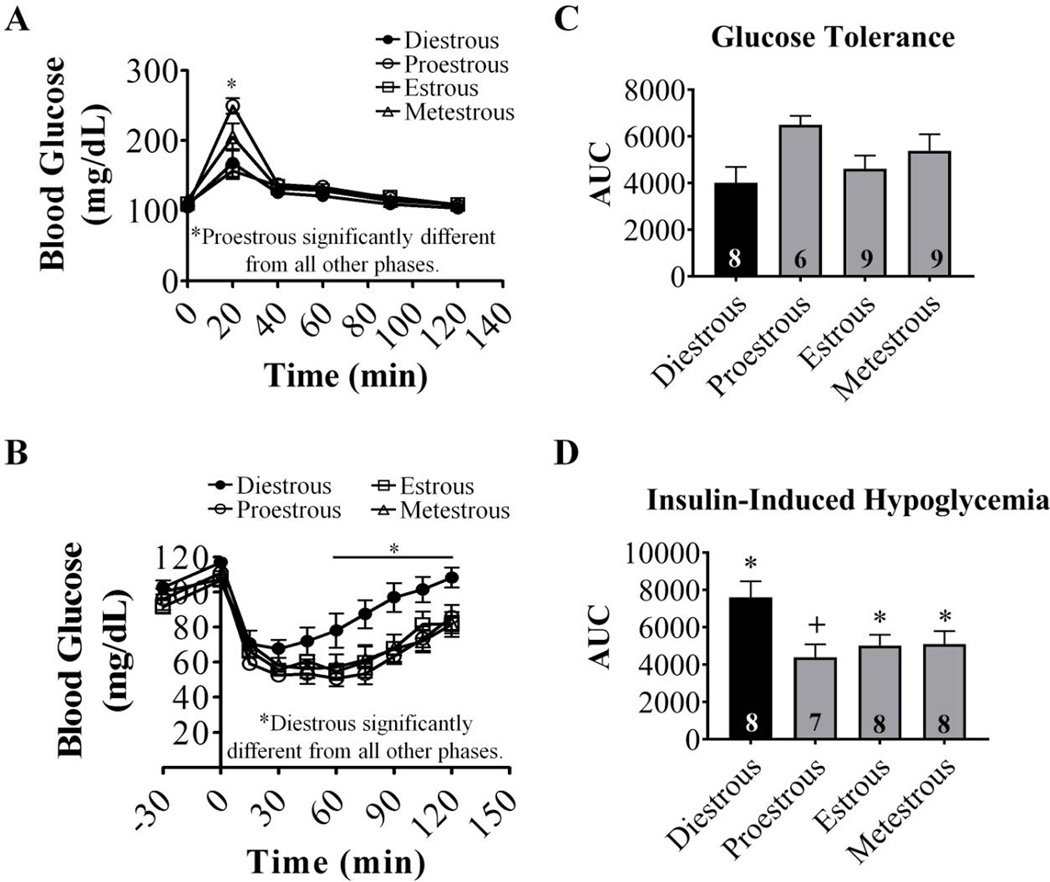
Fasting blood glucose levels were similar in all phases (diestrous: 105±4 (n=16), proestrous: 101±5 (n=13), estrous: 102±4 (n=17) and metestrous: 108±3 (n=17) mg/dL). Blood glucose levels peaked at 20 minutes in all groups following D-glucose injection (Figure 1A). Peak blood glucose levels in proestrous were significantly higher than peak levels in all other phases (proestrous: 250±5 mg/dL; diestrous: 168±7; estrous: 157±4; metestrous: 205±6; P<0.05). However, blood glucose levels returned to baseline by 40 min in all phases. A similar trend (p=0.07) was observed in area under the curve (AUC) for GTT, but no statistical inter-phase differences were observed.
Figure 1. Glucose tolerance and the response to insulin-induced hypoglycemia vary with estrous phase.
(A, B) Blood glucose levels in response to injection of D-glucose (A, 1 g/kg IP) or insulin (B, 2 U/kg, SC) in cycling females. Data sets marked with an asterisk (*) are significantly different according to repeated measures two-way ANOVA followed by Bonferoni post-hoc tests. (C, D) Quantification of AUC for glycemia. Baseline was set at 90mg/dL and 40mg/dL for GTT and IIH curves, respectively. Numbers within columns indicate the n number for each group. Columns which share the same symbols are not significantly different from each other via standard one-way ANOVA followed by Tukey post-hoc tests. AUC: area under curve, IP: intraperitoneal, SC: subcutaneous, n.s: not significantly different.
3.2. Recovery from IIH is Fastest During Diestrous
Blood glucose levels fell below 70mg/dL in all groups following insulin injection (Figure 1B). However, in diestrous blood glucose fell to its lowest point at 30 minutes post-insulin injection and returned to baseline within 90 minutes. In all other phases, blood glucose reached its nadir at 60 minutes post-insulin injection and did not return to baseline within the 120 minute monitoring period. AUC was also significantly higher during diestrus compared to proestrous (Figure 1D). This suggests that the counterregulatory response to hypoglycemia varies during the estrous cycle, with the strongest recovery occurring during diestrous.
3.3. Glucose Sensitivity of VL-VMN Nonadapting GI and AdGI, but not GE, Neurons Varies Throughout the Estrous Cycle
Membrane capacitance (Cm), a measure of cell size, resting membrane potential (Vm) and input resistance (IR) were statistically similar in all phases for nonadapting GI, AdGI and GE neurons (Supplemental Figure 1). However, Bartlett’s test for unequal variance revealed significant intragroup differences for resting IR in nonadapting GI neurons (p=0.01).
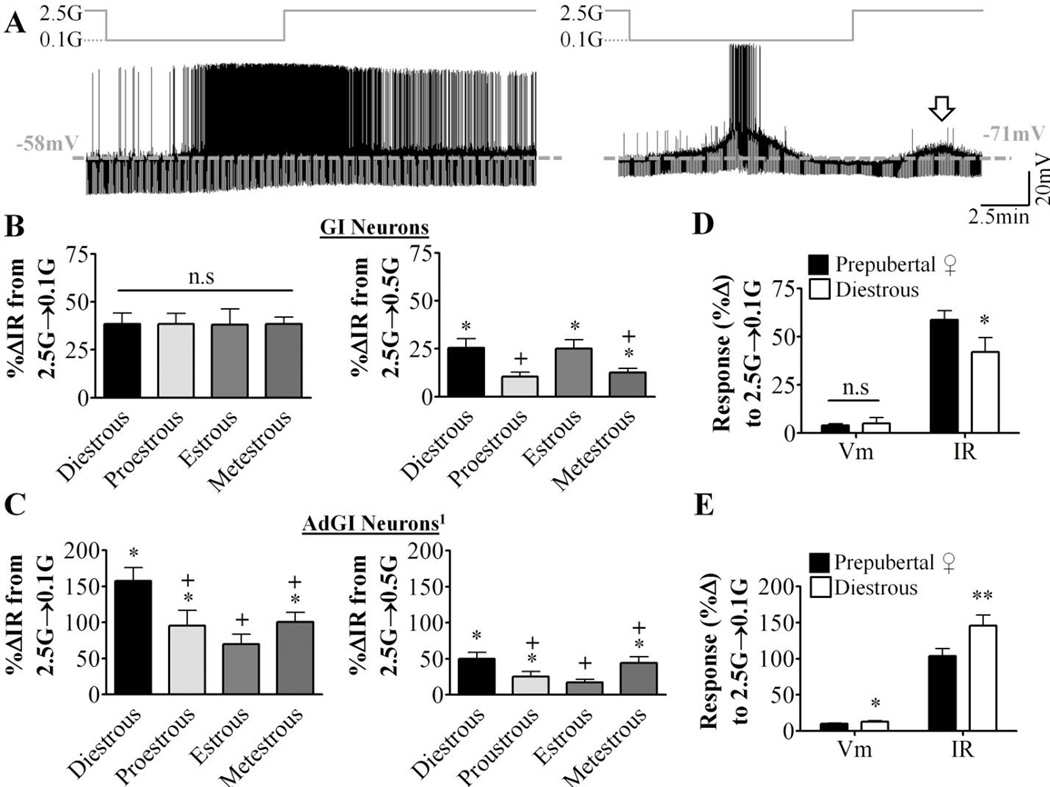
Representative current-clamp recordings for nonadapting GI and AdGI neurons are shown in Figure 2A. Nonadapting GI neurons depolarized and increased their action potential frequency and IR in low glucose (Figure 2A, left). AdGI neurons behaved similarly, but their response to low glucose reversed prior to return to 2.5mM glucose (Figure 2A, right). As seen in prepubertal females [21], a subpopulation of AdGI neurons showed a smaller amplitude transient depolarization in response to a subsequent glucose challenge from 0.1mM to 2.5mM. In total, 65% (15 of 23), 33% (4 of 12), 10% (1 of 10) and 10% (1 of 10) of AdGI neurons displayed this secondary glucose response in diestrous, proestrous, estrous and metestrous respectively (Figure 2A, arrow). The magnitude, but not the duration or time to peak of this response was significantly larger in diestrous than in proestrous (p<0.05; data not shown). IR changes in nonadapting GI neurons in response to a glucose decrease from 2.5mM to 0.5mM, but not to 0.1mM, fluctuated across the estrous cycle peaking in diestrus and reached a nadir at proestrous (Figure 2B). The IR response of AdGI neurons to both 0.5 and 0.1mM also peaked in diestrus but reached its nadir during estrous (Figure 2C). Nonadapting GI neurons from diestrous females increased their IR to a lesser degree in response to a glucose decrease from 2.5 to 0.1mM compared to nonadapting GI neurons from prepubertal females (Figure 2D). The converse was true for AdGI neurons (Figure 2E). In all cases the degree of depolarization in response to low glucose was similar. Finally, Bartlett’s test for unequal variance revealed significant intergroup differences in AdGI neurons for the percent change in IR in response to 0.1mM glucose (p=0.03).
Figure 2. Glucose sensing in VL-VMN nonadapting GI and adapting GI neurons varies throughout the estrous cycle.
Glucose sensing was evaluated in hypothalamic slices taken at each phase of the estrous cycle. (A) Representative whole cell current-clamp recordings of a nonadapting GI (left) and adapting GI (AdGI, right) neuron. Glucose changes are schematically displayed above each recording; dashed grey line represents resting Vm. (B, C) Quantification of %ΔVm and %ΔIR in response to decreasing glucose from 2.5 mM to 0.1mM (2.5G→0.1G) or 0.5mM (2.5G→0.5G) in nonadapting GI (B) and AdGI neurons (C). For nonadapting GI, n=7, 6, 5, 8 at 0.1mM glucose and n=5, 5, 5, 7 at 0.5mM glucose. For AdGI, n=23, 12, 10, 10 at 0.1mM and n=6, 7, 9, 7 at 0.5mM respectively. n# indicated in this figure legend is for diestrous, proestrous, estrous and metestrous, respectively. (D, E) Quantification of %ΔVm and %ΔIR in response to decreasing glucose from 2.5 mM to 0.1mM (2.5G→0.1G) or 0.5 mM (2.5G→0.5G) in nonadapting GI (D; prepubertal n=18 and diestrous n=8) and AdGI neurons (E; prepubertal n=37 and diestrous n=23) from prepubertal and diestrous females. *p<0.05, **<p<0.01 via unpaired student t-tests. Columns which share the same symbols are not significantly different from each other via standard one-way ANOVA and Tukey posthoc tests. G: mM glucose, IR: input resistance, n.s: not statistically different, Vm: membrane potential. 1For AdGI neurons only, Bartlett’s test for unequal variances determined the variances between phases was significantly different (p=0.03) for %ΔIR in response to 0.1mM glucose.
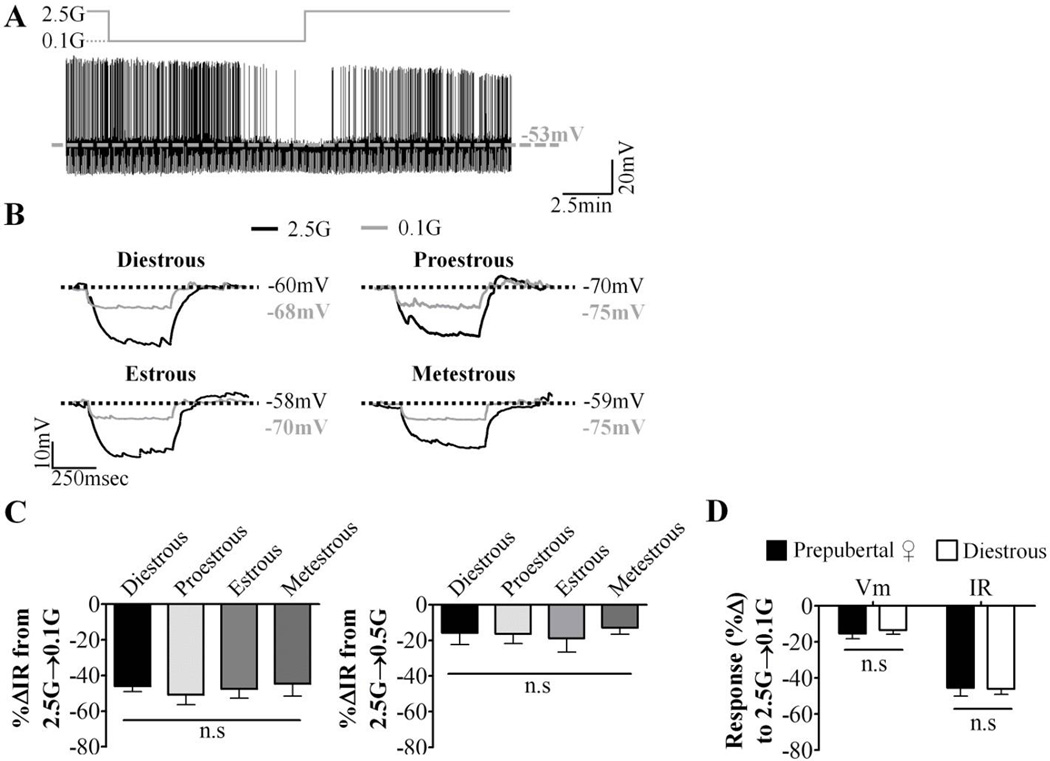
VL-VMN GE neurons hyperpolarized and decreased their IR and action potential frequency in response to low glucose to the same degree in all phases of the estrous cycle (Figure 3A–C). Furthermore, there was no significant difference in the response of GE neurons from diestrous and prepubertal females to decreased glucose (Figure 3D). This suggests that this population of glucose sensing neurons is not sensitive to fluctuations of hormones across the estrous cycle.
Figure 3. Glucose sensing in VL-VMN GE neurons does not vary throughout the estrous cycle.
Glucose sensing was evaluated in hypothalamic slices taken at each phase of the estrous cycle. (A) Representative whole cell current-clamp recording of a GE neuron. Glucose changes are schematically displayed above each recording; dashed grey line represents resting Vm. (B) Representative voltage responses to a hyperpolarizing pulse in response to 0.1mM glucose at each phase of the estrous cycle. Vm was normalized to 2.5mM glucose to emphasize changes in IR (C) Quantification of %ΔVm and %ΔIR in response to decreasing glucose from 2.5 mM to 0.1mM (2.5G→0.1G) (left; n=21, 9, 10, 7) or 0.5mM (2.5G→0.5G) (right; n=6, 4, 5, 5). n# indicated in this figure legend is for diestrous, proestrous, estrous and metestrous, respectively. (D) Quantification of %ΔVm and %ΔIR in response to decreasing glucose from 2.5 mM to 0.1mM (2.5G→0.1G) in GE neurons from prepubertal (n=17) and diestrous (n=7) females. n.s: not statistically different via standard one-way ANOVA and Tukey posthoc test. G: mM glucose, IR: input resistance, Vm: membrane potential.
3.4. 17βE attenuates the response of VL-VMN Nonadapting GI and AdGI, but not GE, neurons to decreased glucose
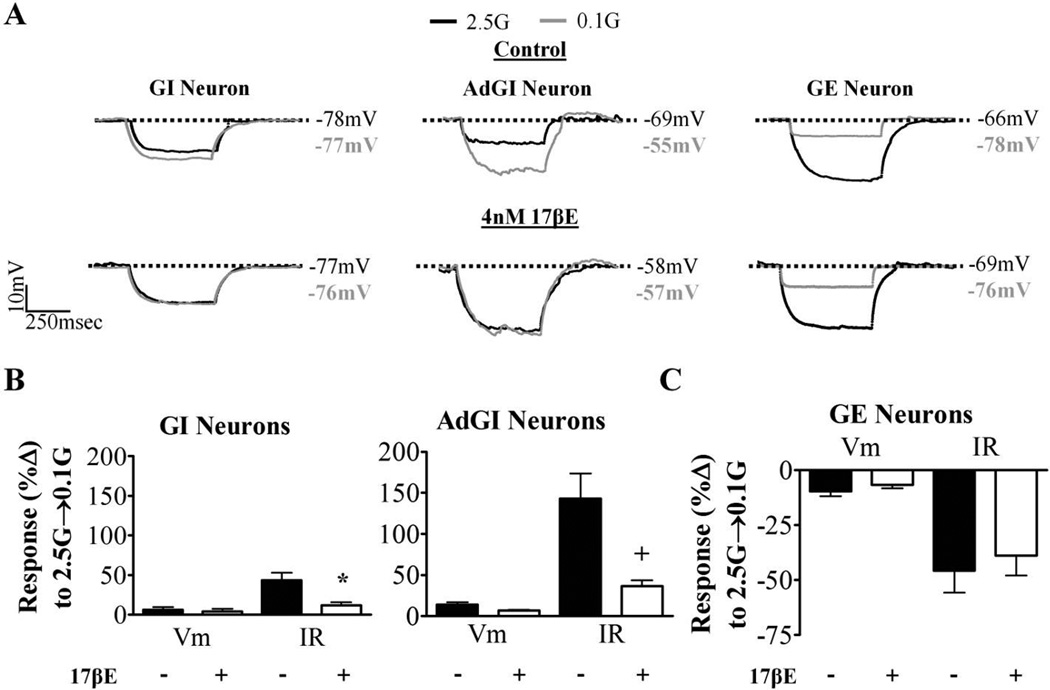
We next tested whether a physiological brain concentration of 17βE, typically seen during proestrous [38], would modulate the glucose sensitivity of VL-VMN glucose sensing neurons from diestrous animals when applied exogenously to isolated brain slices. The response of nonadapting GI and AdGI neurons to reductions in glucose was significantly blunted in the presence of 17βE (Figure 4A, B). However, 17βE had no effect on the glucose sensitivity of GE neurons in diestrous (Figure 4A, C).
Figure 4. 17βE blunts the response of VL-VMN nonadapting GI and AdGI neurons to low glucose.
In this experiment, 17βE was added to hypothalamic slices taken during diestrus only. (A) Representative voltage responses to a hyperpolarizing pulse in response to 0.1mM glucose in the presence and absence of 17βE (4nM, diestrous only). Vm was normalized to 2.5mM glucose to emphasize changes in IR. (B, C) Quantification of %ΔVm and %ΔIR in response to decreasing glucose from 2.5 mM to 0.1mM (2.5G→0.1G) in nonadaptive GI (B left; n=4), AdGI (B right; n=6) and GE (C; n=6) neurons during diestrous in the presence and absence of 17βE (4nM). *p<0.05, +p<0.01 via paired students t-test. G: mM glucose, IR: input resistance, Vm: membrane potential.
4. DISCUSSION
In this study, we demonstrate that glucose homeostasis and the glucose sensitivity of VL-VMN nonadapting GI and AdGI neurons varies across the estrous cycle. In general, the recovery from IIH in vivo and the response of both types of GI neurons to low glucose in vitro fluctuated according to cycle day. Furthermore, exogenous 17βE blunted the response of nonadaptive GI and AdGI neurons to low glucose during diestrous. Glucose tolerance also varied across the cycle although the effects of the estrous cycle on glucose tolerance were much milder than on IIH. Neither estrous phase nor 17βE affected the glucose sensitivity of VL-VMN GE neurons suggesting that these neurons are not sensitive to the effects of fluctuating levels of 17βE. Our data suggest that the preovulatory hormone surge, particularly increased plasma 17βE, affects glucose sensing in VL-VMN nonadapting GI and AdGI neurons and the response to IIH in a similar fashion. Paradoxically, glucose tolerance appears to be greatest when the responses of GI neurons to low glucose are highest. Thus, changes in hypothalamic glucose sensing may contribute, in part, to the changes we observed in the response to IIH across the estrous cycle. This suggests that clinically, insulin therapies may need to be titrated to account for fluctuations in insulin sensitivity over the menstrual cycle.
4.1. Reproductive Cycle and Glucose Tolerance
There is overwhelming evidence that 17βE is a key metabolic hormone. However, the impact of cyclic fluctuations in 17βE levels on glucose homeostasis is still unclear. In euglycemic or hypoglycemic clamps studies involving small cohorts of women (n≤8) insulin sensitivity and hypoglycemia counterregulation are similar throughout the menstrual cycle [7,8,9]. But studies using the minimal glucose model show reduced insulin sensitivity in humans following an intravenous GTT during the follicular phase (i.e., when circulating 17βE peak) [5]. Euglycemic or hypoglycemic clamps are the gold-standard for evaluating glucose homeostasis and they are designed to test small affects in glucose homeostasis. However, the prolonged exposure to insulin (or hypoglycemia) during these clamps is nonphysiologic [40].
Similarly, in two different strains of rats, both improved and impaired glucose tolerance following an oral GTT has been observed during the 17βE surge [12,41]. In our study we used an IP GTT, which increases blood glucose more rapidly than oral administration. We show that peak glucose levels are greater during proestrous, when plasma 17βE peaks in mice compared to the rest of the cycle. Moreover, glucose tolerance, as indicated by a reduced AUC, tends to be greatest during diestrous when 17βE levels are low. The slower rise in glucose following an oral GTT may explain why earlier rodent studies did not observe consistent changes during the estrous cycle. Age may also be a factor in the disparate results as adult rats were used by Bailey et al, and we used adolescent mice in our study [12]. Our choice to use adolescent mice resulted from difficulty in obtaining viable electrophysiological recordings in older animals due to increased connective tissue which reduces visibility and cell viability [42]. Our findings suggest a trend toward improved glucose tolerance in diestrus.
4.2. Reproductive Cycle and Insulin Induced Hypoglycemia
The response to IIH and the response of VL-VMN nonadapting GI and AdGI neurons to low glucose were all enhanced during diestrous when 17βE levels are lowest. Moreover, we found here that a physiological concentration of 17βE typically seen by the brain in proestrus reduced the response of VL-VMN nonadapting GI and AdGI neurons to low glucose when isolated during diestrous. Together, these data suggest that when glucose levels fall during periods of low circulating 17βE levels, maximal hypothalamic glucose sensing occurs allowing for full initiation of a counterregulatory response. However, we did not measure 17βE levels in this study. Thus, we cannot rule out the contribution of other ovarian hormones (e.g., progesterone, androgens). It is also possible that our observed effects result from secondary influences of ovarian hormones on other neuroendocrine or physiological systems.
Our data are consistent with previous reports of attenuated feeding and hyperglycemia responses in female versus male rats following peripheral administration of 2-deoxyglucose, a non-metabolizable glucose analog that initiates counterregulatory processes [43]. That is, in this study, which did not account for estrous status, elevated 17βE levels and reduced hypothalamic glucose sensitivity may have contributed to the observed sex differences reported. One caveat to our interpretation is that we did not measure counterregulatory hormones. Thus, changes in insulin sensitivity may have contributed to our findings. While, the higher insulin dose employed here helps to overcome the potential influence of insulin sensitivity, it may still prove to be a factor if influenced by estrogens or the estrous cycle.
Interestingly, the response to low glucose in both nonadapting GI and AdGI neurons fluctuated with the estrous phase and peaked at diestrous. Furthermore, when these two populations of VL-VMN glucose sensing neurons are considered together the additive response to low glucose is blunted at every phase except diestrous. While direct evidence of a role for VMN glucose sensing neurons in hypoglycemia counterregulation is currently unavailable, we have previously shown a parallel relationship between the glucose sensitivity of nonadapting VMN GI neurons and hypoglycemia counterregulation under a number of conditions [14,24,25]. These data imply that even a slight reduction in the response to low glucose in either nonadapting GI or AdGI neurons is sufficient to blunt the response to IIH. At the present time we can only speculate as to the physiological relevance of adaptation in AdGI neurons. However, it is possible that they enhance sensitivity to slow declines in extracellular glucose. On the other hand, the lack of effect of estrous cycle on GE neurons implies that these glucose sensing neurons, at least those in the vlVMN, may not be linked to hypoglycemia detection.
Our finding that the response to IIH varies with the estrous cycle conflicts with data from earlier hyperinsulinemic-hypoglycemic clamp studies performed in healthy non obese women [7,8]. These clamp studies found no differences in hypoglycemia counterregulation in a small cohort of women during the late follicular phase and the mid-luteal phase. Furthermore, a longitudinal study (n>250 healthy non obese subjects) shows that HOMA-IR, a measure of insulin resistance, also did not differ in these phases [44]. Our data suggest that the response to IIH is similar during these two periods (i.e., during and just after the preovulatory surge). Thus, previous studies may have not detected a change in hypoglycemia counterregulation due to the time points chosen for evaluation and/or age differences in the subjects.
Changes in hypoglycemia counterregulation warrant special attention given the current diabetes epidemic. More women than men are projected to be diagnosed with Type 2 Diabetes and may require more insulin-intensive therapies [45]. In order to avoid hypoglycemia associated autonomic failure, which increases the risk of undetected life-threatening hypoglycemic episodes, insulin therapies may need to be titrated to account for ovarian hormone induced changes in hypothalamic glucose sensing.
In summary, this study describes changes in the response to IIH and hypothalamic glucose sensing that coincide with the preovulatory surge in plasma 17βE. Furthermore, the directional changes in the response to IIH and the glucose sensitivity of VL-VMN nonadapting GI and AdGI neuron were similar. This suggests that physiological elevations in circulating 17βE impairs the ability of VL-VMN glucose sensing neurons to respond to glucose deficit and may blunt the response to IIH. These data have implications for cycling women utilizing intensive insulin therapy in Type 1 and 2 Diabetes Mellitus.
Supplementary Material
Supplemental Figure 1: Basal Cm, Vm, and IR of VL-VMN glucose sensing neurons during each estrous phase. (A–C) Cm (A; GI:n=7, 7, 4, 5, AdGI:n=13, 10, 8, 8, GE:n=18, 7, 6, 5), Vm (B; GI:n=7, 7, 5, 8, AdGI:n=23, 12, 10, 10, GE:n=21, 9, 9, 7) and IR (C; GI:n=7, 7, 5, 8, AdGI:n=23, 12, 10, 10, GE:n=21, 9, 9, 7) in 2.5mM glucose for VL-VMN glucose sensing neurons at each estrous phase. n.s: not statistically significant via standard one-way ANOVA and Tukey post-hoc test. n# indicated in this figure legend is for diestrous, proestrous, estrous and metestrous, respectively. Cm: membrane capacitance, IR: input resistance, Vm: membrane potential. 1For GI neurons only, Bartlett’s test for unequal variances determined the variances between phases was significantly different (p=0.01) for IR.
Highlights.
The response to IIH varied throughout the estrous cycle
Changes in hypothalamic glucose sensing coincided with those seen in the response to IIH
Natural fluctuations in 17βE levels may alter the response to IIH
Acknowledgments
This study was supported by NIH DK081538 (VHR) and NRSA 5R31DK093331 (AMS).
Abbreviations
- 17βE
17β-estradiol
- AMPK
AMP-activated protein kinase
- ARC
arcuate nucleus
- Cm
membrane capacitance
- ER
estrogen receptor
- GE
glucose-excited
- GGT
glucose tolerance test
- GI
glucose-inhibited
- GSNs
glucose-sensing neurons
- HOMA-IR
homeostatic model assessment for insulin resistance
- IIH
insulin-induce hypoglycemia
- IP
intraperitoneal
- IR
input resistance
- NGS
non-glucose sensing
- SC
subcutaneous
- VMH
ventromedial hypothalamus
- VL-VMN
ventrolateral VMN
- Vm
membrane potential
- VMN
ventromedial hypothalamic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sprague JE, Arbeláez AM. Glucose Counterregulatory Responses to Hypoglycemia. Pediatric endocrinology reviews : PER. 2011;9:463–475. [PMC free article] [PubMed] [Google Scholar]
- 2.López M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends in Endocrinology & Metabolism. 2015;26:411–421. doi: 10.1016/j.tem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab. 2010 doi: 10.1016/j.tem.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Ortiz M, Martinez-Abundis E, Lifshitz A. Insulin sensitivity and sex steroid hormone levels during the menstrual cycle in healthy women with non-insulin-dependent diabetic parents. Gynecol Obstet Invest. 1998;46:187–190. doi: 10.1159/000010030. [DOI] [PubMed] [Google Scholar]
- 5.Escalante Pulido JM, Alpizar Salazar M. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30:19–22. doi: 10.1016/s0188-0128(98)00008-6. [DOI] [PubMed] [Google Scholar]
- 6.Bingley CA, Gitau R, Lovegrove JA. Impact of menstrual cycle phase on insulin sensitivity measures and fasting lipids. Horm Metab Res. 2008;40:901–906. doi: 10.1055/s-0028-1082081. [DOI] [PubMed] [Google Scholar]
- 7.Diamond MP, Grainger DA, Rossi G, Connolly-Diamond M, Sherwin RS. Counter-regulatory response to hypoglycemia in the follicular and luteal phases of the menstrual cycle. Fertility and Sterility. 1993;60:988–993. [PubMed] [Google Scholar]
- 8.Yki-Jarvinen H. Insulin sensitivity during the menstrual cycle. J Clin Endocrinol Metab. 1984;59:350–353. doi: 10.1210/jcem-59-2-350. [DOI] [PubMed] [Google Scholar]
- 9.Diamond MP, Jacob R, Connolly-Diamond M, DeFronzo RA. Glucose metabolism during the menstrual cycle. Assessment with the euglycemic, hyperinsulinemic clamp. J Reprod Med. 1993;38:417–421. [PubMed] [Google Scholar]
- 10.Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiolo Endocrinol Metab. 2007;293:E1012–E1020. doi: 10.1152/ajpendo.00649.2006. [DOI] [PubMed] [Google Scholar]
- 11.Drake K, Gateva E, Deutsch J, Cohen WR. Sex differences in the adrenal catecholamine response to hypoglycemia in rats. Metabolism. 1998;47:121–124. doi: 10.1016/s0026-0495(98)90205-0. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CJ, Matty AJ. Glucose Tolerance and Plasma Insulin of the Rat in Relation to the Oestrous Cycle and Sex Hormones. Horm Metab Res. 1972;4:266–270. doi: 10.1055/s-0028-1094063. [DOI] [PubMed] [Google Scholar]
- 13.Adams JM, Legan SJ, Ott CE, Jackson BA. Modulation of hypoglycemia-induced increases in plasma epinephrine by estrogen in the female rat. Journal of Neuroscience Research. 2005;79:360–367. doi: 10.1002/jnr.20369. [DOI] [PubMed] [Google Scholar]
- 14.Fioramonti X, Deak A, Deshpande S, Carneiro L, Zhou C, Sayed N, Orban B, Berlin JR, Penicaud L, Leloup C, Beuve A, Routh VH. Hypothalamic S-nitrosylation contributes to the counter-regulatory response impairment following recurrent hypoglycemia. PLoS One. 2013;8:e68709. doi: 10.1371/journal.pone.0068709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010;99:175–180. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Nedungadi Thekkethil P, Zhu L, Sobhani N, Irani Boman G, Davis Kathryn E, Zhang X, Zou F, Gent Lana M, Hahner Lisa D, Khan Sohaib A, Elias Carol F, Elmquist Joel K, Clegg Deborah J. Distinct Hypothalamic Neurons Mediate Estrogenic Effects on Energy Homeostasis and Reproduction. Cell Metabolism. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker GC, McKee ME, Bishop C, Coscina DV. Whole-body metabolism varies across the estrous cycle in Sprague-Dawley rats. Physiol Behav. 2001;74:399–403. doi: 10.1016/s0031-9384(01)00599-6. [DOI] [PubMed] [Google Scholar]
- 18.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of Pre- and Postsynaptic influcences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- 21.Santiago AM, Clegg DJ, Routh VH. Estrogens modulate ventrolateral ventromedial hypothalamic glucose-inhibited neurons. Mol Metab. 2016 doi: 10.1016/j.molmet.2016.08.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local Ventromedial Hypothalamus Glucopenia Triggers Counterregulatory Hormone Release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 23.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. The Journal of Clinical Investigation. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fioramonti X, Marsollier N, Song Z, Fakira KA, Patel RM, Brown S, Duparc T, Pica-Mendez A, Sanders NM, Knauf C, Valet P, McCrimmon RJ, Beuve A, Magnan C, Routh VH. Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation. Diabetes. 2010;59:519–528. doi: 10.2337/db09-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Z, Routh VH. Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1283–R1287. doi: 10.1152/ajpregu.00148.2006. [DOI] [PubMed] [Google Scholar]
- 26.Amiel SA, Maran A, Powrie JK, Umpleby AM, Macdonald IA. Gender differences in counterregulation to hypoglycaemia. Diabetologia. 1993;36:460–464. doi: 10.1007/BF00402284. [DOI] [PubMed] [Google Scholar]
- 27.Yoshinaga K, Hawkins RA, Stocker JF. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology. 1969;85:103–112. doi: 10.1210/endo-85-1-103. [DOI] [PubMed] [Google Scholar]
- 28.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix. 2009;4 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 31.Cotero VE, Routh VH. Insulin blunts the response of glucose-excited neurons in the ventrolateral-ventromedial hypothalamic nucleus to decreased glucose. Am J Physiol Endocrinol Metab. 2009;296:E1101–E1109. doi: 10.1152/ajpendo.90932.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fioramonti X, Contie S, Song Z, Routh VH, Lorsignol A, Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- 33.Murphy BA, Fioramonti X, Jochnowitz N, Fakira K, Gagen K, Contie S, Lorsignol A, Penicaud L, Martin WJ, Routh VH. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am J Physiol Cell Physiol. 2009;296:C746–C756. doi: 10.1152/ajpcell.00641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng Z, Santiago AM, Thomas MP, Routh VH. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Molecular and Cellular Neuroscience. 2014;62:30–41. doi: 10.1016/j.mcn.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2005;54:15–22. doi: 10.2337/diabetes.54.1.15. [DOI] [PubMed] [Google Scholar]
- 36.Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- 38.Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295:E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 40.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 41.Hsu C, Hsu HK, Lee JN, Yu JY. The influence of estrogen on the hyperglycemic action of alloxan in female rats. Proceedings of the National Science Council, Republic of China. Part B, Life sciences. 1986;10:92–97. [PubMed] [Google Scholar]
- 42.Vazirani RP, Fioramonti X, Routh VH. Membrane Potential Dye Imaging of Ventromedial Hypothalamus Neurons From Adult Mice to Study Glucose Sensing. Journal of Visualized Experiments : JoVE. 2013:50861. doi: 10.3791/50861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandoval DA, Ryan KK, de Kloet AD, Woods SC, Seeley RJ. Female rats are relatively more sensitive to reduced lipid versus reduced carbohydrate availability. Nutrition & Diabetes. 2012;2:e27. doi: 10.1038/nutd.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeung EH, Zhang C, Mumford SL, Ye A, Trevisan M, Chen L, Browne RW, Wactawski-Wende J, Schisterman EF. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab. 2010;95:5435–5442. doi: 10.1210/jc.2010-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Basal Cm, Vm, and IR of VL-VMN glucose sensing neurons during each estrous phase. (A–C) Cm (A; GI:n=7, 7, 4, 5, AdGI:n=13, 10, 8, 8, GE:n=18, 7, 6, 5), Vm (B; GI:n=7, 7, 5, 8, AdGI:n=23, 12, 10, 10, GE:n=21, 9, 9, 7) and IR (C; GI:n=7, 7, 5, 8, AdGI:n=23, 12, 10, 10, GE:n=21, 9, 9, 7) in 2.5mM glucose for VL-VMN glucose sensing neurons at each estrous phase. n.s: not statistically significant via standard one-way ANOVA and Tukey post-hoc test. n# indicated in this figure legend is for diestrous, proestrous, estrous and metestrous, respectively. Cm: membrane capacitance, IR: input resistance, Vm: membrane potential. 1For GI neurons only, Bartlett’s test for unequal variances determined the variances between phases was significantly different (p=0.01) for IR.