Abstract
Integrins are heterodimeric cell surface molecules that mediate cell-extracellular matrix (ECM) adhesion, ECM assembly, and regulation of both ECM and growth factor induced signaling. However, the developmental context of these diverse functions is not clear. Loss of β1-integrin from the lens vesicle (mouse E10.5) results in abnormal exit of anterior lens epithelial cells (LECs) from the cell cycle and their aberrant elongation toward the presumptive cornea by E12.5. These cells lose expression of LEC markers and initiate expression of the Maf (also known as c-Maf) and Prox1 transcription factors as well as other lens fiber cell markers, β1-integrin null LECs also upregulate the ERK, AKT and Smad1/5/8 phosphorylation indicative of BMP and FGF signaling. By E14.5, β1-integrin null lenses have undergone a complete conversion of all lens epithelial cells into fiber cells. These data suggest that shortly after lens vesicle closure, β1-integrin blocks inappropriate differentiation of the lens epithelium into fibers, potentially by inhibiting BMP and/or FGF receptor activation. Thus, β1-integrin has an important role in fine-tuning the response of the early lens to the gradient of growth factors that regulate lens fiber cell differentiation.
Keywords: lens fiber cell, growth factor, BMP, FGF, integrin
Introduction
The cell fate decisions critical for tissue development are often controlled by transcription factors that regulate tissue specific gene expression. While the expression and function of these transcription factors are in turn regulated by cell surface receptors that detect a complex set of extracellular cues, the mechanisms by which a complex cellular environment is interpreted by a cell, resulting in a particular cell fate, is less understood (Zhou and Huang, 2011). Integrins are heterodimeric cell adhesion molecules critically important for development, a function usually attributed to their ability to detect changes in ECM composition (van der Flier and Sonnenberg, 2001). However, integrins can differentially influence cell migration (Jacques et al., 1998; Zou et al., 2012), survival (Samuelsson et al., 2007; Simirskii et al., 2007) and cell fate specification (Tate et al., 2004; Velleman and McFarland, 2004) in different cellular contexts, even when the ECM environment is unchanged. While this was initially attributed to the ability of integrins to change their affinity for matrix via inside out signaling, in recent years, the known role of integrins has expanded to include the regulation of growth factor receptor signaling (Ivaska and Heino, 2011). Despite these insights, the significance of integrin modulation of growth factor receptor pathways during normal development is less clear.
The ocular lens is an ideal model to study the molecular mechanisms by which integrins influence development. The lens is derived from the surface ectoderm and has a relatively simple morphology consisting of only two major cell types- the cuboidal lens epithelium and highly elongated lens fibers. In addition, epithelial to fiber cell differentiation in the lens is geographically restricted; in early development starting in the posterior compartment of the lens vesicle to form primary fibers, and in later stages, at the equatorial zone, where epithelial cells exit the cell cycle to form secondary fibers. Several growth and transcription factors that regulate the epithelial to fiber cell fate decision have been identified (Cvekl et al., 2015; de Iongh and Duncan, 2014) although their cross talk is an active field of investigation (Audette et al., 2016; Xie et al., 2016).
The lens expresses numerous integrins, the vast majority of which share the common β1-integrin subunit (Menko and Philip, 1995; Walker and Menko, 2009). Prior studies suggest that the functions of β1-integrins in the lens are complex, with distinct roles in lens cell/lens capsule adhesion, lens cell survival (Samuelsson et al., 2007; Simirskii et al., 2007), lens fiber cell differentiation and fiber morphology (Hayes et al., 2012; Scheiblin et al., 2014), as well as maintenance of the lens epithelial cell phenotype (Simirskii et al., 2007) and its pathological transition to a myofibroblast fate (Barbour et al., 2004; Hay and Zuk, 1995). However, the function of β1-integrins during the early stages of lens development, when the invaginating lens placode forms a lens vesicle, has not been previously reported. Here, we delete β1-integrin from the lens vesicle and identified an unexpected function for integrins in preventing early anterior lens epithelial cells from prematurely initiating the lens fiber cell differentiation pathway – likely by antagonizing BMP and/or FGF signaling.
Materials and methods
Animals
All experiments conform to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Delaware Institutional Animal Care and Use Committee under protocol 1039. B2:129-Itgb1tm1Efu/J mice in which exon 3 of the β1-integrin gene is flanked by LoxP sites (β1 F/F) were obtained from The Jackson Laboratory (Bar Harbor, Maine) (Raghavan et al., 2000). FVB/N mice hemizygous for the Le-Cre transgene (LE mice) were obtained from Dr. Richard Lang’s laboratory at Cincinnati Children’s Hospital with permission from Ruth Ashery-Padan, Tel Aviv University (Ashery-Padan et al., 2000). β1 F/F mice were bred with Le-Cre mice to obtain mice β1 F/F and hemizygous for Le-Cre (β1LE) or β1 F/F and carrying no Cre (Control) (Figure 1A). Mice carrying β1 F/F and homozygous for MLR10-Cre (β1MLR10) have been previously generated in the lab (Simirskii et al., 2007). Embryos were staged designating the day that the vaginal plug was observed as embryonic day (E) 0.5. All mice were maintained at the University of Delaware animal facility in specific pathogen free conditions under a 14/10 hour light/dark cycle.
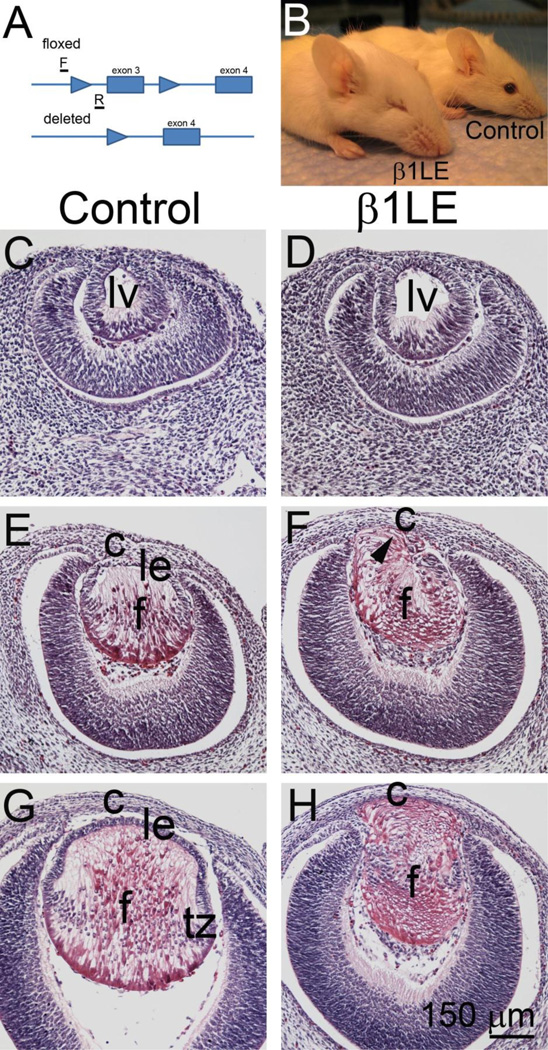
Figure 1. Mice homozygous for a floxed allele of β1-integrin and carrying one LE-Cre allele (β1LE) are microphthalmic as adults.
(A) Diagram of a portion of the β1-integrin locus showing the location of the loxP sites (arrowheads) found in the floxed allele, the structure of the deleted allele, and the location of the PCR primers (F and R) used to genotype the mice. (B) Exterior appearance of adult β1LE and control (homozygous for the β1-integrin flox allele, not carrying Cre) littermates. (C, E, G) Hematoxylin and eosin stained paraffin sections of the eye of control animals (C- E11.5; E- E12.5; G- E14.5), (D, F, H) Hematoxylin and eosin stained paraffin sections from the eyes of β1LE animals (D- E11.5; F- E12.5; H- E14.5). At E11.5, lens vesicles of β1LE mice (D) look similar to controls (C). At E12.5, lenses from β1LE mice (F) show some loss of the anterior epithelium and the eosinophilic staining indicative of lens fiber cells (arrowhead) extends to the cornea as compared to controls (E). This loss of anterior epithelium is very pronounced at E14.5 with complete anterior epithelium loss (H) in β1LE mice as compared to controls (G). All staining was performed on a minimum of three biological replicates.
Abbreviations: lv - lens vesicle, le - lens epithelium; f – lens fiber cells; c – cornea; tz – transition zone. Scale bar panels C-H- 150 µm.
DNA was isolated from tail biopsies or embryos using the PureGene Tissue and Mouse Tail kit (Qiagen Sciences, Germantown, Maryland). Mice were genotyped for the presence of the floxed β1-integrin allele by using primers F 5′-CGGCTCAAAGCAGAGTGTCAGTC-3′ and R 5′-CCACAACTTTCCCAGTTAGCTCTC-3′ (Figure 1A). Mice were genotyped for the presence of the Le-Cre transgene by using primers 5’-ATGCCCAAGAAGAAGAGCGT-3’ and 5’-GAAATCAGTGCGTTCGAACGCTAGA-3’.
Histological analysis and immunohistochemistry
Tissue was fixed in Pen-Fix (Richard Allan Scientific, Kalamazoo, Michigan) for four hours, and stored in 70% ethanol prior to paraffin embedding. Six-micrometer thick sections were stained by hematoxylin and eosin using standard methods. The crystallin expression in the lens was determined by incubating deparafinized sections with rabbit anti-bovine β-crystallin and rabbit anti-bovine γ-crystallin (gifts of Dr. Samuel Zigler, The Wilmer Eye Institute, The Johns Hopkins School of Medicine) followed by detection with an anti-rabbit Dako Envision horseradish peroxidase kit (Dako Laboratories, Carpinteria, CA) using diaminobenzidine as a substrate. pERK and pAKT levels in the lens was detected using the Catalyzed Signal Amplification (CSA) System. (Dako Laboratories, Carpinteria, CA, K150011-2) with Biotinylated Link Antibody (Tris diluent); CSA II Rabbit Link (K150180-2). Briefly, antigen retrieval was performed by double boiling deparafinized sections in 10mM sodium citrate, pH 6 in a slide chamber placed within a rice cooker for 30 minutes. Sections were then cooled to room temperature, briefly rinsed in 1X TBST (Tris buffered saline with Triton X 100), followed by blocking endogenous peroxidase activity. Sections were washed with 1X TBST, blocked, and incubated for two hours at room temperature in a 1:50 dilution of primary antibody (see Table 1). Signal was detected by treating sections with anti-rabbit immunoglobulin-HRP amplification reagent and anti fluorescein-HRP followed by incubation with 3,3′-Diaminobenzidine (DAB) substrate. All experiments were performed on a minimum of three biological replicates (ie samples derived from three independent animals) .
Table 1.
Dilutions, product numbers, and protocols used for the antibodies utilized in this study
| Gene | Company | Product # | Fixation | Blocking | Secondary | Dilution |
|---|---|---|---|---|---|---|
| DRAQ5 | Biostatus Limited | DR50200 | acetone rmethanol or 4% PFA in 1xPBS |
2% BSA or 5% goat serum |
1:2000 | |
| αSMA | SigmaAldrich | F3777 | acetone rmethanol or 4% PFA in 1xPBS |
2% BSA or 5% goat serum |
FITC conjugated |
1:250 |
| E-cadherin | Cell Signaling | 4065 | 4% PFA in 1xPBS | 5% goat serum | anti-rabbit | 1:100 |
| Aquaporin 0 | Millipore | ab3071 | acetone rmethanol | 2% BSA | anti-rabbit | 1:200 |
| Pax6 | Millipore | AB2237 | 4% PFA in 1xPBS | 5% goat serum | anti-rabbit | 1:200 |
| c-Maf | Santa Cruz Biotechnology | sc7866 | acetone rmethanol | 2% BSA | anti-rabbit | 1:100 |
| Prox1 | Belecky-Adams et al., 1997; Duncan et al., 2002 |
acetone rmethanol | 2% BSA | anti-rabbit | 1:500 | |
| pErk | Cell signaling | scl0572 | acetone rmethanol | 2% BSA | anti-goat | 1:200 |
| pAkt | Cell signaling | sc48789 | 4% PFA in 1xPBS | 5% goat serum | anti-rabbit | 1:100 |
| Collagen IV | Abeam | AB6586 | acetone rmethanol | 2% BSA | anti-rabbit | 1:200 |
| β1-integrin | Millipore | MAB1997 | acetone rmethanol | 2% BSA | anti-rat | 1:200 |
| Laminin | Abcam | 11575250 | acetone rmethanol | 2% BSA | anti-rabbit | 1:200 |
| p27kip1 | Santa Cruz Biotechnology | sc528 | paraffin sections | 10% BSA | anti-rabbit | 1:50 |
| p57kip2 | Santa Cruz Biotechnology | scl039 | paraffin sections | 10% BSA | anti-rabbit | 1:50 |
| β-crystallin | Gift from Dr. Samuel Zigler | paraffin sections | 5% goat serum | anti-rabbit | 1:100 | |
| γ-crystallin | Santa Cruz Biotechnology | sc22415 | paraffin sections/ 4% PFA fixation |
5% goat serum | anti-goat | 1:100 |
| Jagged 1 | Santa Cruz Biotechnology | sc8303 | 4% PFA fixation | 5% goat serum | anti-rabbit | 1:100 |
| Foxe3 | Santa Cruz Biotechnology | scl34536 | 4% PFA fixation | 5% goat serum | anti-rabbit | 1:100 |
| Hes1 | Santa Cruz Biotechnology | sc25392 | 4% PFA fixation | 2% BSA and 5% goat serum |
anti-rabbit | 1:100 |
| pSmad 1/5/8 | Santa Cruz Biotechnology | scl2353- R |
4% PFA fixation | 1% BSA and 3% goat serum |
anti-rabbit | 1:100 |
| pFRS2α-Y436 | R&D Systems | AF5126 | 4% PFA fixation | 2% BSA and 5% goat serum |
anti-rabbit | 1:100 |
Immunofluorescence
Tissue was excised, sixteen micrometer thick frozen sections prepared, and mounted on ColorFrost plus slides (Fisher Scientific, Hampton, New Hampshire). Slides were immersion fixed in 1:1 acetone–methanol at −20°C for 20 minutes or 4% paraformaldehyde at room temperature for 20 minutes depending on the antibody (Table 1). Sections fixed with acetone-methanol were air-dried, while paraformaldehyde fixed sections were washed twice in 1X PBS for ten minutes each, then blocked for one hour at room temperature. This was followed by incubation with primary antibody diluted in appropriate blocking buffer (see Table 1). Following washes either in 1X PBS (phosphate buffered saline) or 1X TBS (Tris buffered saline), primary antibodies were detected with the appropriate AlexaFluor 568 or 488 labeled secondary antibody (Invitrogen, Grand Island, NY) diluted 1:200 in blocking buffer containing a 1:2000 dilution of the nucleic acid stain Draq-5 (Biostatus Limited, Leicestershire, United Kingdom). Sections were washed again in 1X PBS or 1X TBS and then mounted (Reed et al., 2001). All experiments were performed on a minimum of three biological replicates.
TUNEL labeling
Tissue was excised, fixed for two hours at room temperature in 4% paraformaldehyde, and transferred to 70% ethanol prior to paraffin embedding. Six micron thick sections were prepared, and nuclear DNA fragmentation was detected by TUNEL staining using the In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics, Indianapolis, IN, catalog # 11684795910) following the manufacturer’s directions. Slides were counterstained with 1:2000 Draq-5 in 1X PBS to visualize cell nuclei. Following two, five minutes 1X PBS washes, slides were mounted in mounting media.
Proliferation assays
The number of lens epithelial cells that are in S phase was determined using 5-ethynyl-2’-deoxyuridine (EdU) Click-it proliferation assays (Invitrogen, Grand Island, NY, USA). Pregnant mice were injected intraperitoneally with 8 µg/mouse of EdU dissolved in 100 µl of normal saline. Two hours later, the dam was sacrificed and embryos were removed. Fetal heads were embedded in OCT; and 16 µm frozen sections were mounted on charged glass slides. Slides were stored at −80°C or immediately fixed by 1:1 ice-cold acetone-methanol at −20°C. Sections were allowed to air dry, and the EdU Click-it reaction was carried out following the manufacturer’s instructions. Sections were counterstained and mounted as described above. All experiments were performed on a minimum of three biological replicates.
Confocal image collection and analysis
Slides were stored at −20°C until they were visualized with a Zeiss LSM 780 confocal microscope configured with 405 nm, 458 nm, 488 nm, 514 nm, 561 nm and 633 nm excitation lines (Carl Zeiss Inc, Göttingen, Germany). All comparisons of staining intensity between specimens were done on sections stained simultaneously and the imaging for each antibody was performed using identical laser power and software settings on a minimum of three biological replicates to ensure validity of intensity comparisons. In some cases, brightness and/or contrast of images were adjusted in Adobe Photoshop for optimum viewing on diverse computer screens. However, in all cases, adjustments were applied equally to both experimental and control images to retain the validity of comparison.
Results
Prior work on integrin function in the lens demonstrated that β1-integrins are required for both lens epithelial cell (LEC) phenotype/survival (Simirskii et al., 2007) as well as lens fiber cell structure at later stages of lens development (Scheiblin et al., 2014). However, the previous studies did not address the role of β1-integrins during early lens development. Therefore we employed the mouse transgenic line, Le-Cre (Ashery-Padan et al., 2000; Yoshimoto et al., 2005), which first expresses Cre recombinase in the lens placode, to achieve deletion of β1-integrin during lens morphogenesis (β1LE).
Loss of β1-integrin from the lens vesicle reveals temporal complexity in β1-integrin function during lens development
β1LE mice are severely microphthalmic/anophthalmic as adults while neither mice homozygous for the β1-integrin floxed allele (control) (Figure 1B) nor heterozygous for both the floxed β1-integrin allele and LE-Cre (data not shown) had apparent lens abnormalities, even into adulthood. At E11.5, both controls (Figure 1C) and β1LE (Figure 1D) mice show normal lens vesicle morphology. At E12.5, control lenses show a normal lens epithelium anteriorly and newly differentiated lens fibers posteriorly (Figure 1E). In contrast, E12.5 β1LE lenses lack a morphologically distinct lens epithelium. Instead these cells appear to have transitioned to elongated eosinophilic cells extending beyond the normal anterior anatomical boundary of the lens and encroaching upon the developing cornea (Figure IF, arrowhead). By E14.5, while control lenses show a hematoxylin stained anterior epithelium, a well-established transition zone, and eosinophilic fiber cells posteriorly (Figure 1G), a hematoxylin stained anterior epithelium is completely absent in β1LE lenses and is replaced by eosinophilic elongated cells extending into the developing corneal stroma which obliterates the anterior chamber (Figure 1H).
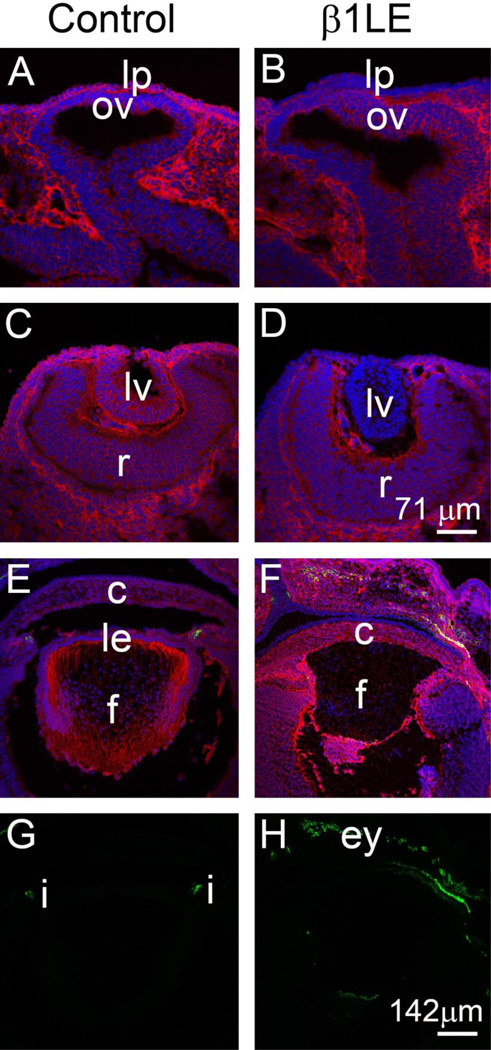
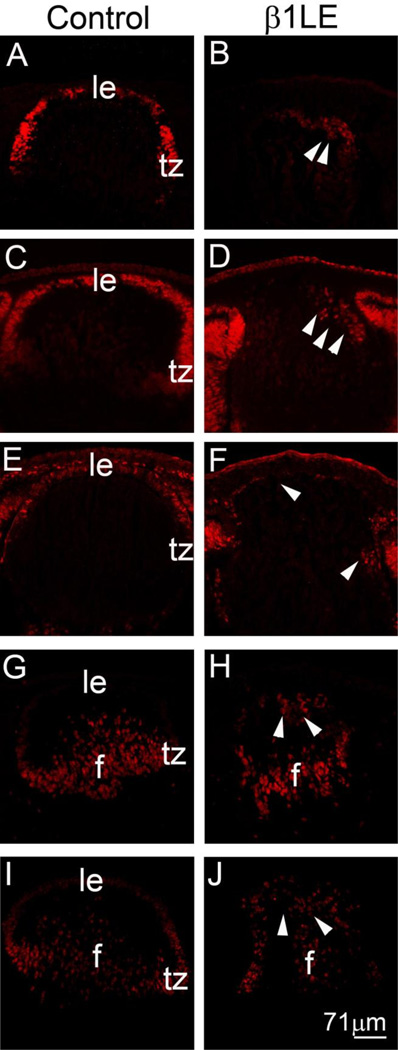
While Le-Cre activity is first detected in the lens placode (Ashery-Padan et al., 2000), β1 integrin protein is known to have a long half-life in the lens and other tissues (Li et al., 2005; Raghavan et al., 2000; Scheiblin et al., 2014; Simirskii et al., 2007). Thus, the timing of β1-integrin protein loss in β1LE mice was determined by immunofluorescence (Figure 2A–F). At E9.5, β1-integrin protein (red) is detectable throughout the lens placode in controls (Figure 2A) while β1-integrin protein levels were lower, but still detectable in the lens placode of β1LE mice (Figure 2B). At E10.5, β1-integrin protein is detectable in all cells of the developing lens vesicle in controls (Figure 2C) while β1-integrin protein was not detected in the lens vesicle of β1LE mice at this age (Figure 2D). Consistent with this, E16.5 control lenses express β1-integrin protein in all lens cells (Figure 2E), while β1LE lenses lack detectable β1 integrin protein (Figure 2F).
Figure 2. β1LE mice lose β1-integrin protein from the developing lens vesicle by E10.5.
Immunofluorescent confocal microscopy showing β1-integrin protein (red) expression in control (A, C, E) and β1LE (B, D, F) lenses at E9.5 (A, B), E10.5 (C, D) and E16.5 (E, F). At E9.5, β1-integrin protein expression is reduced in the β1LE lens placode (B), compared to control (A). By E10.5, β1-integrin protein is detected in all cells of the lens vesicle of control mice (C) whereas β1-integrin protein levels fall beyond the level of detection in β1LE lenses (D). At E16.5, β1-integrin is still detectable in all cells of control lenses (E) while, consistent with the result at E1 0.5, no β1-integrin was detected in β1LE lenses at this age (F). Co-staining of the E16.5 sections shown in panels E and F for αSMA did not reveal any αSMA signal (green) within the boundary of the lens in either in control (E, see panel G for αSMA channel only) or β1LE lenses (F, see panel H for αSMA channel only). All staining was performed on a minimum of three biological replicates.
Red - β1-integrin, Green - αSMA, Blue - DNA. Abbreviations: lp - lens placode, lv - lens vesicle, le - lens epithelium, c - cornea, f - lens fiber cells, ov - optic vesicle, r - retina, i - iris, ey -eyelids. Scale bars- Panels A, B, C, D-71µm; Panels E, F, G, H- 142 µm
β1-integrin loss from the E10.5 lens vesicle resulted in LEC elongation initiating between E12.5-E13.5 (see Figure 1) while loss of β1-integrin from all lens cells at E11.5-E12.5 (β1MLR10) did not (Simirskii et al., 2007). Thus, we investigated whether the underlying molecular phenotype was distinct as well by investigating alpha smooth muscle actin (αSMA) expression in β1LE lenses. Consistent with their histological appearance, immunolocalization studies of β1LE lenses between E11.5–16.5 showed no up-regulation of αSMA (see Figure 2F, H for E16.5 data, other ages are not shown) as compared to the controls (see Figure 2E, G for E16.5 data, other ages are not shown), suggesting a temporal complexity in the function of β1-integrins during lens development.
β1LE lenses lose their anterior lens capsule
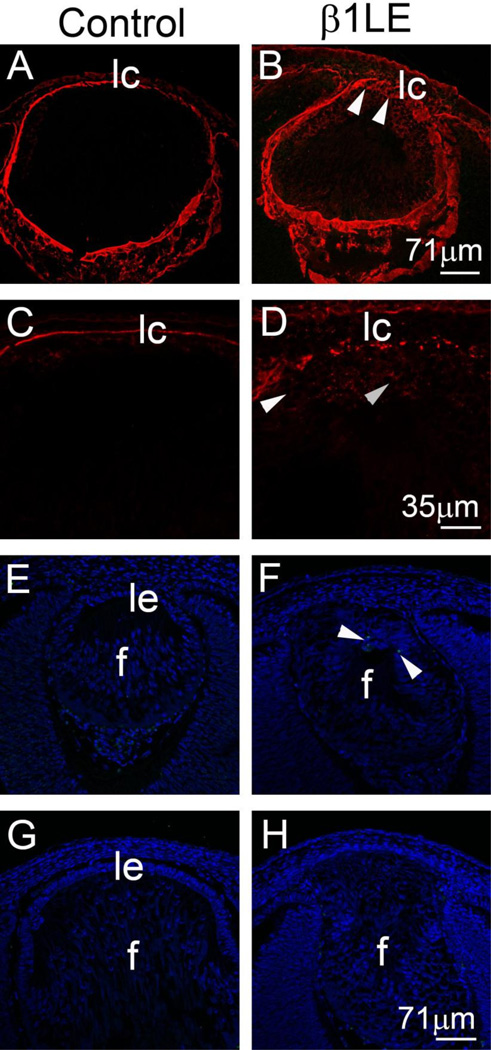
The presence of lens cells outside of the normal anterior anatomical boundary of the lens suggested that the lens capsule, which completely surrounds the lens and sequesters it from other ocular tissues (Danysh and Duncan, 2009), might be disrupted in β1LE mice. Confocal immunofluorescence using antibodies against the known lens capsule components- laminin (Figure 3A, B), collagen IV (data not shown), and perlecan (data not shown), show the expected intact lens capsule in E12.5 control lenses (Figure 3A), while defects in the anterior lens capsule are seen in E12.5 β1LE lenses (Figure 3B, arrowheads). At higher magnification, all of the laminin associated with the E12.5 lens is confined to the lens capsule in controls (Figure 3C), while E12.5 β1LE lenses (Figure 3D) exhibit intracellular laminin immuno-reactivity (arrowheads) suggestive of intracellular retention of newly synthesized laminin. Similar results were also obtained for collagen IV (not shown).
Figure 3. β1LE lenses show defects in anterior lens capsule starting at E12.5 without massive lens cell apoptosis.
Confocal immunofluorescence showing the staining pattern of laminin in the lenses of control (A, C) and β1LE (B, D) mice at E12.5. Control lenses (A) exhibit continuous laminin staining around the lens at E12.5, while this pattern is interrupted, particularly on the anterior lens surface (arrowheads), in β1LE lenses (B). At higher magnification, E12.5 control lenses (C) only exhibit laminin staining associated with the lens capsule, while β1LE lenses (D) show intracellular laminin immuno-reactivity at this age (D- arrowheads). TUNEL assay for apoptosis (green), in controls (E- E12.5, G- E13.5) and β1LE lenses (F- E12.5, H- E13.5). Control lenses do not show anterior lens epithelium apoptosis as measured by TUNEL during normal lens development both at E12.5 (E) and E13.5 (G), β1LE lenses only show occasional TUNEL positive cells (F, arrowheads) but this is not consistently detected in every section (H, data not shown). . All staining was performed on a minimum of three biological replicates.
Red (panels A, B, C, D)- Laminin; Green (panels E, F, G, H) - TUNEL; Blue - DNA. Abbreviations: f - lens fiber cells, lc - lens capsule, le - lens epithelium. Scale bars Panels A, B, E, F, G, H - 71µm, Panels C, D - 35µm
Cell adhesion to extracellular matrices via β1-integrins has long been proposed to protect cells from apoptosis/anoikis by signaling to cell survival pathways (Raghavan et al., 2000). Therefore, the loss of lens capsule in β1LE lenses suggested that anoikis might be responsible for the loss of lens epithelium observed in these lenses. At E11.5, only sporadic TUNEL positive cells were observed in both control and β1LE lens vesicles (not shown), consistent with the role of apoptosis in the separation of lens vesicle from the head ectoderm (Vecino and Acera, 2015). Later, few to no TUNEL positive cells were observed in E12.5 (Figure 3E) or E13.5 control lenses (Figure 3G) while only sporadic TUNEL signals were detected in β1LE lenses at E12.5 (Figure 3F, arrowheads)). TUNEL positive cells were generally not detected at E13.5 (Figure 3H). These data suggest that the absence of an anterior lens epithelium in β1LE lenses after E13.5 is unlikely to be primarily attributable to LEC apoptosis.
β1LE lens epithelial cells show reduced proliferation
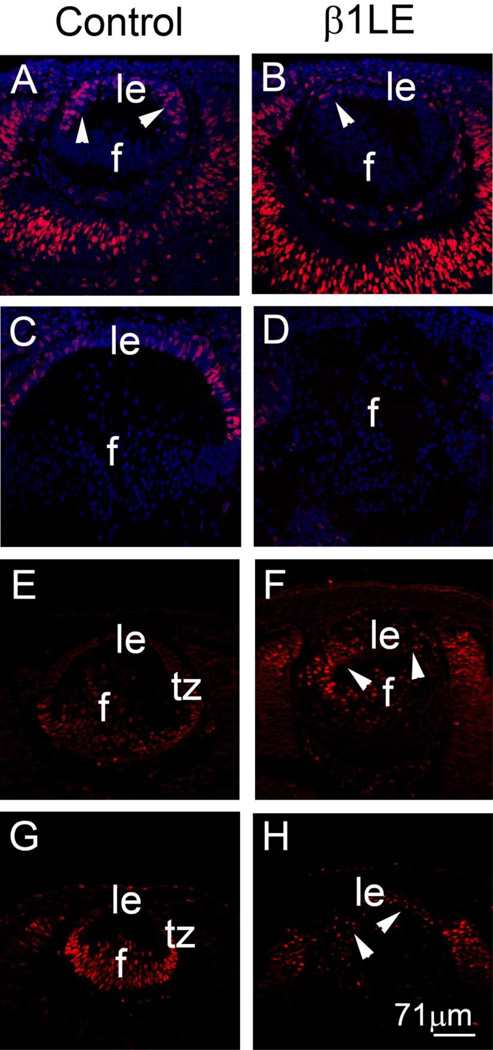
We then investigated if proliferation defects contribute to the loss of anterior epithelium in β1LE lenses. At E12.5, the anterior LECs of control lenses are actively synthesizing DNA (Figure 4A – arrowheads) as measured by EdU incorporation, while only a few EdU positive cells are seen in the anterior epithelium of E12.5 β1LE lenses (Figure 4B – arrowhead). At E13.5, control lenses (Figure 4C) continue to exhibit proliferating cells that are actively synthesizing DNA in the anterior epithelium, whereas we detected no cells actively synthesizing DNA in E13.5 β1LE lenses (Figure 4D). In the normal lens, LECs up-regulate the cyclin-dependent kinase inhibitors p27Kip1 (Figure 4E) and p57Kip2 (Figure 4G) (Antosova et al., 2013; Jia et al., 2007; Rowan et al., 2008; Saravanamuthu et al., 2009; Saravanamuthu et al., 2012), during cell cycle exit and initiation of fiber cell differentiation in the posterior region of the lens vesicle in early development. In contrast, abnormal up-regulation of both p27Kip1 (Figure 4 F – arrowheads) and p57Kip2 (Figure 4 H – arrowheads) was seen in the LECs of E12.5 β1LE mice although the levels of both proteins may be lower in β1LE fibers compared to control.
Figure 4. β1LE lenses exhibit decreased LEC proliferation coincident with the up regulation of cell cycle exit markers.
EdU cell proliferation assays (A–D) comparing control (A- E12.5; C-E13.5) with β1LE lenses (B- E12.5; D- E13.5). A decrease in the number of LECs actively synthesizing DNA is seen starting at E12.5 in β1LE lenses (B) as compared to controls (A). By E13.5, β1LE lenses (D) show complete loss of cells actively synthesizing DNA as compared to controls (C) which maintain cell proliferation in the lens epithelium. Confocal immunofluorescence showing the expression pattern of cell cycle exit markers in controls (E- p27Kip1 ; G- p57Kip2 ) versus β1LE lenses (F- p27Kip1 and H- p57Kip2). Control lenses show little to no p27Kip1 (E) as well as p57Kip2 (G) in LECs at E12.5, while β1LE lenses show large numbers of cells exiting the cell cycle as compared to controls, shown by both p27Kip1 (F- arrowheads) staining and p57Kip2 staining (H-arrowheads). . All staining was performed on a minimum of three biological replicates.
Red (panels A, B, C, D)- Sites of active DNA synthesis, (panels E, F)- p27Kip1, (panels G, H)-p57Kip2 ; Blue- DNA. Abbreviations: f - lens fiber cells, le, lens epithelium, tz - transition zone. Scale bar - 71µm.
β1LE lenses lose E-cadherin expression and show fiber specific marker expression in the entire tissue
The loss of proliferation and up regulation of cell cycle exit markers in the anterior LECs of the β1LE mice, along with expanded domain of eosinophilic staining (diagnostic of cells expressing high concentrations of protein), suggested that the lens epithelium was differentiating inappropriately into lens fibers. To characterize this finding further, the expression pattern of the epithelium specific marker E-cadherin was analyzed. In E13.5 control lenses (Figure 5A, C), E-cadherin staining is observed throughout the anterior LECs, while it disappears coincident with fiber differentiation, as visualized by γ-crystallin immunoreactivity at the transition zone (Figure 5A). In contrast, we observed a reduced number of cells expressing E-cadherin starting at E12.5 in β1LE lenses (not shown) and by E13.5, much of the anterior lens has lost E-cadherin, and instead is immunoreactive for γ-crystallin (Figure 5B, arrowhead). This was much more evident at E14.5, where control lenses express E-cadherin in the lens epithelium and γ-crystallin in fibers as expected, while β1LE mice lose E-cadherin positive cells from the lens, while γ-crystallin immunoreactivity is detected at the boundary between the lens and cornea (arrowheads; Figure 5D).
Figure 5. β1LE LECs down-regulate E-cadherin while exhibiting aberrant fiber cell marker staining.
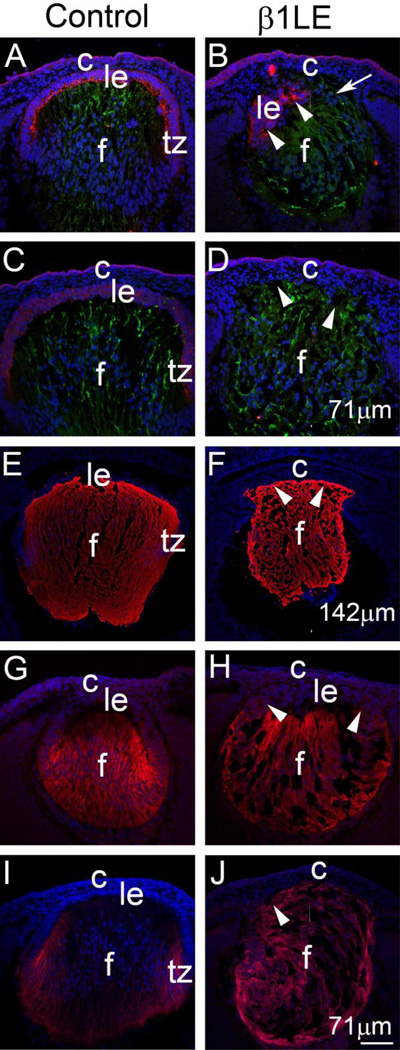
Panels (A to D) show co-immunolocalization of E-cadherin (red) and γ-crystallin (green) at E13.5 (A,B) and E14.5 (C,D). Control E13.5 (A) lenses show staining for E-cadherin (red) only in anterior LECs, with γ-crystallin (green) restricted only to fiber cells. In contrast, β1LE lenses at E13.5 (B) show γ-crystallin staining extending up-to the cornea (arrow) in cells that exhibit cell nuclei on the anterior aspect of the lens. Only a few lens epithelial cells expressing E-cadherin (arrowheads) are still observed in this lens region. E14.5 control lenses (C) show uniform E-cadherin staining in the anterior LECs while γ-crystallin expression is confined to the lens fiber cells. In contrast, a complete loss of E-cadherin expression is seen in E14.5 β1LE lenses, with all lens cells positive for γ-crystallin (D - arrowheads). At E16.5, Aquaporin0 staining is restricted to lens fiber cells in control lenses (E), while β1LE lenses exhibit Aquaporin0 (F) staining in almost all lens cells (arrowheads). At E12.5 (G), Jagged1 levels up-regulate at the transition zone of control lenses, while β1LE lenses show an anterior shift in Jagged1 expression (H - arrowheads). At E13.5, Jagged1 is predominately located in the newly differentiated lens fibers of control lenses (I), whereas in β1LE lenses, all lens cells are positive for Jagged1 (J-arrowheads). All staining was performed on a minimum of three biological replicates.
Red (panels A–D) – E-Cadherin, (panels E–F)– Aquaporin0, (panels G-J)- Jagged1; Green (panels A–D)- γ-crystallin, Blue - DNA. Abbreviations: le – lens epithelium, c- cornea, f - lens fiber cells, tz – transition zone. Scale bar Panels A, B, C, D, G, H, I, J = 71 µm; Panels E, F =142µm.
In order to support this evidence, we investigated whether other established fiber cells markers, β-crystallin (Lampi et al., 2014), Aquaporin 0 (Chepelinsky, 2003), and Jagged1 (Saravanamuthu et al., 2009; Saravanamuthu et al., 2012) were expressed inappropriately in β1LE lenses. Similar to γ-crystallin (Figure 5A–D), β-crystallin staining was confined to the lens fiber cells in controls at E12.5 and E13.5, while the anterior cells of β1LE lenses show β-crystallin (data not shown) staining, mirroring the domain of eosinophilic staining seen in Figure 1H. Aquaporin 0 staining is confined only to the lens fiber cells both at E14.5 (data not shown) and E16.5 (Figure 5E) in controls (Figure 5E), while in the β1LE lenses, the anterior-most lens cells express Aquaporin 0 (E14.5 not shown; E16.5, Figure 5F). Similarly, Jagged1, a membrane protein important for Notch signaling in lens, is confined to the fiber cells at E12.5 in control lenses (Figure 5G), whereas this expression domain shifts anteriorly in β1LE lenses (Figure 5H - arrowheads). By E13.5, Jagged1 expression is restricted to the newly formed fiber cells in the transition zone of control lenses (Figure 5I), while E13.5 β1LE lenses exhibit Jagged1 expression in all lens cells (Figure 5J - arrowheads).
β1LE LECs down regulate the expression of transcription factors important for LEC phenotype
In order to confirm that β1LE LECs were losing their lens epithelial identity, we examined the expression of the transcription factors Foxe3 (Blixt et al., 2000), Pax6 (Ashery-Padan et al., 2000; Duncan et al., 2004), and Hes1 (Lee et al., 2005) that regulate lens epithelial cell identity. Consistent with their function in LECs, Foxe3 (Figure 6A), Pax6 (Figure 6C) and Hes1 (Figure 6E) staining were largely restricted to anterior LECs of E13.5 control lenses, while only a few cells in the anterior region of E13.5 β1LE lenses expressed these proteins (Figure 6B–Foxe3, arrowheads; Figure 6D- Pax6, arrowheads; Figure 6F Hes1, arrowheads).
Figure 6. β1LE lenses down regulate the expression of LEC preferred transcription factors and show ectopic fiber preferred transcription factor expression at E13.5.
Immunolocalization of Foxe3 (A, B), Pax6 (C, D) and Hes1 (E, F) in E13.5 lenses reveals that these three proteins are found in all LECs of control lenses (A, C, E), while all of these factors are restricted to just a few cells at the anterior surface of E13.5 β1LE lenses (B, D, F; arrowheads). (G, H) Immunolocalization of Maf and (I, J) Prox1. Control lenses express Maf (G) in the fiber cell compartment, while the anterior lens cells of E13.5 β1LE lenses (H- arrowheads) ectopically express c-Maf. In controls, Prox1 (I) is normally expressed in all lens cells, but its levels upregulate at the transition zone coincident with fiber cell differentiation. In contrast, almost all lens cells of E13.5 β1LE lenses (J-arrowheads) express high levels of Prox1 protein. All staining was performed on a minimum of three biological replicates.
Red –(panels A,B)- Foxe3; (panels C, D)- Pax6; (panels E, F)- Hes1; (panels G-H)- Maf; (panels I-J)- Prox1. Abbreviations: le - lens epithelium; tz - transition zone, f - lens fiber cells. Scale bar −71µm.
β1LE LECs upregulate the expression of transcription factors important for lens fiber cell differentiation
In the normal lens, as LEC-expressed negative regulators of fiber differentiation down-regulate at the transition zone, positive regulators of lens fiber differentiation such as Prox1 (Audette et al., 2016; Wigle et al., 1999b) and Maf (Kawauchi et al., 1999) up regulate. Expectedly, E13.5 control lenses exhibit strong Maf (Figure 6G) and Prox1 (Figure 6I) immunoreactivity in the lens fiber cells. In contrast, both Maf (Figure 6H – arrowheads) and Prox1 (Figure 6J – arrowheads) signals are robust in the cell nuclei found at the anterior aspect of E13.5 β1LE lenses, consistent with the proposition that these cells are undergoing inappropriate fiber cell differentiation.
β1LE lenses up-regulate downstream effectors of pathways influencing lens fiber differentiation
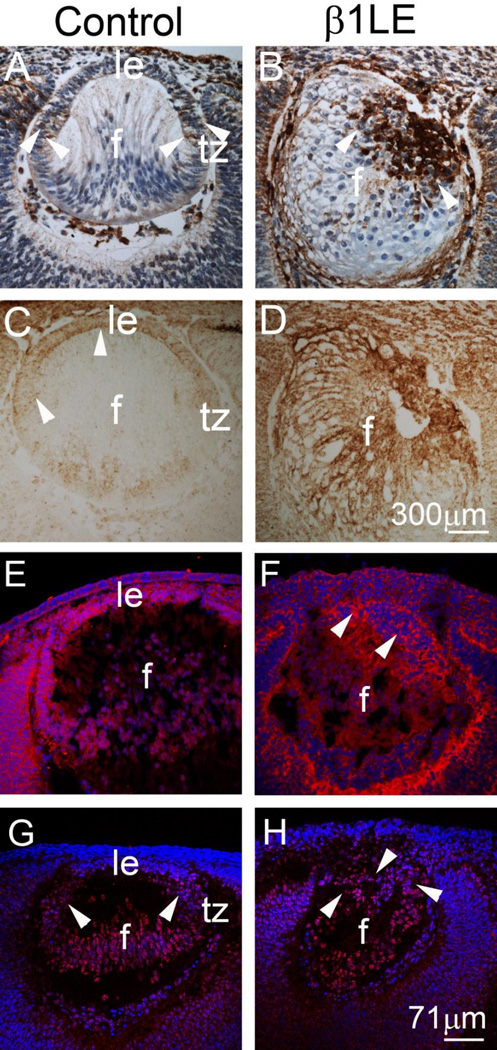
Activation of FGF induced MAPK/ERK1/2 and PI3K–AKT signaling is required for differentiation of lens epithelial cells to lens fibers (Robinson, 2006; Wang et al., 2009; Weber and Menko, 2006), while lower levels of pERK activity are essential for LEC proliferation (Chandrasekher and Sailaja, 2003; Iyengar et al., 2006). Thus, we tested whether β1-integrin deletion in the early lens influences the distribution and level of ERK1/2 and AKT phosphorylation at E12.5 (Figure 7), using immunohistochemistry. In the normal E12.5 lens, pERK1/2 is detectable by immunohistochemistry only in cells undergoing fiber differentiation at the transition zone (Figure 7A – arrowheads) (Madakashira et al., 2012), whereas high levels of pERK1/2 was detected in a large number of E12.5 β1LE anterior LECs (Figure 7B – arrowheads). During normal lens development, pAKT is detectable by immunohistochemistry (Figure 7C) in E12.5 anterior LECs as well as in newly differentiating cells at the transition zone, whereas no staining is detectable in the differentiated lens fibers (Li et al., 2014). In contrast, all lens cells of E12.5 β1LE lenses (Figure 7D – arrowheads) strongly stain for pAKT, including the cells of the posterior lens exhibiting the morphological characteristics of lens fibers. We next tested whether these elevations in pERK and pAKT could be mediated by FGF receptor (FGFR) signaling by staining lenses for pFRS2α, a downstream effector of FGF signaling (Li et al., 2014; Madakashira et al., 2012; Teo et al., 2014). At E13.5, control lenses exhibited pFRS2α staining throughout the lens epithelium into the transition zone (Figure 7E), while β1LE lenses exhibited intense pFRS2α staining in the cells of the anterior lens at this stage (Figure 7F, arrowheads).
Figure 7. Abnormal distribution of pERK1/2, pAKT, pFRS2α, and pSmad 1/5/8 in β1LE lenses at E12.5.
Immunolocalization of pERK1/2 at E12.5 in control (A) and β1LE (B) lenses. E12.5 control lenses (A) exhibit pERK1/2 in the actively differentiating lens fibers found at the transition zone (arrowheads), while little to no signal is detectable by this method in the lens epithelium. In contrast, β1LE lenses exhibit intense pERK1/2 staining in the anterior lens cells (B-arrowheads). Immunohistochemical localization of pAKT at E1 2.5 in control (C) and β1LE lenses (D). At E12.5, pAKT is detected in both the lens epithelium and newly differentiated lens fiber cells at the transition zone in controls (C– arrowheads), whereas it is up-regulated in almost all cells of β1LE lenses, (D). At E13.5, pFRS2α is localized throughout the lens epithelium and transition zone of control lenses (E) while intense pFRS2α staining was observed in the anterior cells of E13.5 β1LE lenses (arrowheads) in the process of differentiating into lens fibers. pSmad 1/5/8 localization in E12.5 controls (Figure 7G) reveals nuclear pSmad 1/5/8 staining in cells at the transition zone and new lens fibers, while little signal is detected elsewhere in the lens. However, E12.5 β1LE lenses exhibit staining for pSmad 1/5/8 extending into the anterior LECs (H – arrowheads). All staining was performed on a minimum of three biological replicates.
Brown- (Panels A, B), pERK1/2; (Panels C, D), pAKT; Red– (panels E, F)- pFRS2α, (panels G-H)-pSmad 1/5/8; Blue – (panels E-H)- DNA Abbreviations; le - lens epithelium; f – lens fiber cells; tz - transition zone. Scale bar Panels A,B,C,D - 300µm, Panels E-H - 71µm
While FGF receptor (FGFR) signaling is essential for lens fiber cell differentiation, it is not sufficient (Lovicu et al., 2011). For instance, while bone morphogenetic protein (BMP) signaling is essential for lens induction, it is also required for both primary and secondary fiber differentiation (Belecky-Adams et al., 2002; Boswell et al., 2008a; Boswell et al., 2008b; Pandit et al., 2011). Further BMP and FGF crosstalk is essential for regulating proliferation and differentiation in the developing lens (Boswell et al., 2008a; Boswell and Musil, 2015a; Jarrin et al., 2012). Thus, the changes in FGF signaling upon β1 integrin deletion prompted us to investigate if BMP signaling was also affected. In control lenses, the BMP mediator pSmad1/5/8 (Beebe et al., 2004), is detected in the nuclei of lens fibers at E12.5 while it is absent from the lens epithelium (Figure 7G – arrowhead). In contrast, robust pSmad1/5/8 staining extends into the anterior LECs in E12.5 β1LE lenses (Figure 7H – arrowheads).
Discussion
β1-integrins play diverse functions in the lens including mediation of LEC-capsule interactions, fiber cell structure, and lens development (Bassnett et al., 1999; Scheiblin et al., 2013 ; Simirskii et al., 2007; Walker and Menko, 2009). Further, β1-integrin expression up-regulates during epithelial-mesenchymal transition (EMT) of lens cells to myofibroblasts following lens injury (de Iongh et al., 2005; Mamuya et al., 2014; Zuk and Hay, 1994), and blockade of β1-integrin function can prevent myofibroblast migration (Zuk and Hay, 1994). Despite this, the in vivo function of the β1-integrins expressed by LECs, particularly their role in early lens development, was not clear.
Previously, we characterized mice lacking β1-integrins from the lens beginning at E11.5 (β1MLR10) (Simirskii et al., 2007). In these mice, early lens growth proceeds normally up to E15.5, however, later in development, the lens epithelial cells (LECs) become spindle shaped, and begin expressing the mesenchymal marker, αSMA, as well as some lens fiber cell markers showing that β1MLR10 LECs lose their epithelial identity. By birth, β1MLR10 LECs undergo apoptosis, leading to microphthalmia in adulthood (Simirskii et al., 2007). In contrast, in the present study, lenses that lose β1-integrin at E10.5 (β1LE), just one-two days earlier than β1MLR10 mice, show a distinctly different phenotype with the exit of LECs from the cell cycle, and their elongation into highly eosinophilic cells which do not express αSMA. Deletion of β1-integrin from lens fibers alone (β1MLR39) results in destabilization of the F-actin cytoskeleton of lens fibers which results in a progressive destabilization of lens fiber structure during postnatal life (Scheiblin et al., 2014). These data indicate that β1-integrins have multiple distinct functions in the lens which change as development proceeds.
β1-integrins are necessary for lens capsule assembly
Although β1MLR10 lenses lose most if not all LECs by birth, their lens capsule is still largely intact (Simirskii et al., 2007). In contrast, β1LE lenses exhibit discontinuities in the anterior lens capsule by E13.5, along with the presence of laminin and collagen IV immunopositive intracellular aggregates. Laminin is the first ECM component laid down during development, and β1-integrin dependent assembly of the laminin heterotrimer is required for its secretion to form the primary basement membrane (Aumailley et al., 2000; Lohikangas et al., 2001). Collagen IV is also ubiquitous in BMs including the lens capsule (Danysh and Duncan, 2009; Kelley et al., 2002), integrating with the initial laminin scaffold to provide stability and strength to the basement membrane (Halfter et al., 2015). Notably, laminin a1 mutant zebrafish which do not efficiently form laminin 111, also do not form an organized collagen IV network in the lens capsule; instead, collagen IV was detected in aggregates throughout the lens (Pathania et al., 2014). This suggests that the lens, like the early embryo (Aumailley et al., 2000; Lohikangas et al., 2001), requires β1-integrins for the secretion and assembly of the lens capsule basement membrane. However, once the early lens capsule is formed, β1-integrins are less crucial for this process, as deletion of β1-integrins later in lens development does not result in obvious lens capsule defects (Simirskii et al., 2007). This could reflect a requirement for integrins in the assembly of the early lens capsule during its rapid thickening during lens morphogenesis (Danysh and Duncan, 2009), while integrins are less necessary once the capsule is established.
β1-integrin regulates cell fate decisions early in lens development
The transition of LECs into elongated eosinophilic cells is consistent with the hypothesis that these cells are inappropriately differentiating into post-mitotic lens fibers. This was supported by the observation that the expression of the LEC marker, E-cadherin, is downregulated in these cells while the expression of numerous lens fiber cell markers initiates in the aberrantly elongating anterior LECs of β1LE lenses. Further, these elongating LECs are leaving the cell cycle, as measured by a decrease in the number of S phase cells, coupled with an up-regulation of the cyclin dependent kinase inhibitors, p27kip1 and p57kip2. This result is similar to that observed in skin keratinocytes (Raghavan et al., 2000), hair follicles (Brakebusch et al., 2000) or luminal mammary epithelial cells (Li et al., 2005; Naylor et al., 2005), as none of these cell types undergo apoptosis upon deletion of the β1-integrin gene even following cell detachment from the underlying basement membrane. In all of these cases, these epithelial cells instead exhibit reduced proliferation, similar to β1LE lenses.
These observations are also consistent with the assertion that anterior LECs undergo inappropriate fiber differentiation upon loss of β1 integrin from the lens vesicle as p27kip1 and p57kip2 are critical regulators of the cell cycle exit of LECs and their differentiation into lens fibers (Zhang et al., 1998). The modest reduction of p27kip and p57kip staining in lens fibers compared to control at E12.5 may suggest that fiber cell differentiation in the posterior lens vesicle is also accelerated as the expression of both proteins usually downregulates later in lens fiber cell differentiation (Zhang et al., 1998). Alternatively, the downregulation of p27kip1 and p57kip2 levels could be secondary to the upregulation of AKT observed in β1LE lens fibers (Figure 7D), as activation of the AKT pathway in cancer cells is known to result in both p27kip1 (Alkarain and Slingerland, 2004) and p57kip2 (Zhao et al., 2013) relocalization from the nucleus to the cytoplasm, and their subsequent degradation.
The transcription factors Maf and Prox1 are first expressed in the lens vesicle but upregulate in differentiating fiber cells (Duncan et al., 2002; Duncan et al., 2004). This upregulated expression is critical since both Maf and Prox1 null mutants fail to form primary fiber cells (Audette et al., 2016; Kawauchi et al., 1999; Kim et al., 1999; Wigle et al., 1999a). In contrast, Pax6 and Foxe3 are predominately expressed in LECs and their over expression in the posterior lens vesicle disrupts fiber differentiation (Duncan et al., 2004; Landgren et al., 2008). Thus, the up-regulation of Prox1 and Maf coupled with down regulation of Pax6 and Foxe3 in β1LE LECs indicated that β1-integrins plays a crucial role in lens cell fate determination by impinging upon lens fiber cell differentiation pathways. Notably, overexpression of Pax6 protein in lens fibers leads to cellular abnormalities and cataracts associated with elevated expression of β1-integrin (Chauhan et al., 2002; Duncan et al., 2000), while the present data show that Pax6 expression in lost from the β1LE lens, suggesting that Pax6 and β1-integrin participate in a feed forward loop playing an important role in maintaining the lens epithelium.
Currently, we believe that the structural abnormalities observed in the β1LE lens fiber cells are an indirect effect of the loss of the lens epithelium. It has been shown that signaling initiated by the lens epithelium is a major regulator of lens fiber architecture during normal lens development (McAvoy et al., 2016; Sugiyama et al., 2011), while loss of β1-integrin from lens fibers (at least in adulthood) did not result in obvious lens epithelial defects (Scheiblin et al., 2014).
β1-integrins may negatively regulate growth factor signaling required for early stage lens fiber cell differentiation
Growth factor induced cell signaling is critical for lens determination, LEC proliferation and the differentiation of LECs into lens fiber cells (Gunhaga, 2011; Lovicu et al., 2011). Low levels of FGF induced ERK phosphorylation induces LEC proliferation (Iyengar et al., 2009) while increased FGF induced ERK phosphorylation drives fiber cell differentiation (Lovicu and McAvoy, 2001), at least in part via upregulation of Maf and Prox1 expression (Audette et al., 2016; Xie et al., 2016; Zhao et al., 2008). In the eye, the selective differentiation of lens fiber cells at the lens equator has been attributed to a gradient of FGF that is produced in the posterior compartment of the eye (Wu et al., 2014). However, the entire anterior lens epithelium of the early lens exhibits FGF signaling as measured by phosphorylation of the FGF receptor effector, FRS2α, (Madakashira et al., 2012; Teo et al., 2014) (see Figure 7). Overall, the mechanisms that fine-tune the differential response of the anterior and posterior lens vesicle to FGF in vivo are less well understood
Notably though, while FGF signaling is necessary for lens fiber cell differentiation, it is not sufficient, as BMP signaling is required for the exit of LECs from the cell cycle in response to FGF (Boswell et al., 2008a; Boswell et al., 2008b; Jarrin et al., 2012). Further, BMP signaling maintains the posterior lens vesicle in an optimal FGF responsive state, at least in part, by selectively increasing FGFR expression (Boswell and Musil, 2015b; Hayashi et al., 2003). Since β1LE LECs upregulate both FGF signaling as measured by pERK/pAKT/pFRS2α levels and BMP signaling, as measured by nuclear pSMAD 1/5/8 levels, in the early anterior lens coincident with the upregulation of p27Kip1, p57Kip2, Maf, and Prox1 expression; we propose that β1 integrins are important negative regulators of the cooperative FGF/BMP signaling necessary for primary lens fiber cell differentiation (see figure 8 for model). Loss of this negative regulation results in conversion of anterior lens cells of the early lens to a lens fiber fate. Notably, we also consistently see that this fate conversion is apparently asymmetric with one side of the lens epithelium entering the fiber cell differentiation pathway prior to the other (See figure 1F, 5B, 6B, 6D, 7B, 7D). This may further support a role for β1-integrins in the negative regulation of BMP signaling as it has been previously proposed that the effect of BMP signaling on primary lens fiber differentiation is asymmetric (Faber et al., 2002). However, as our data do not definitively prove that the elevated pERK/pAKT levels observed in β1LE lenses comes from elevated FGFR signaling, it is still possible that β1-integrin is negatively regulating ERK/AKT signaling in the early lens via other, unknown, mechanisms.
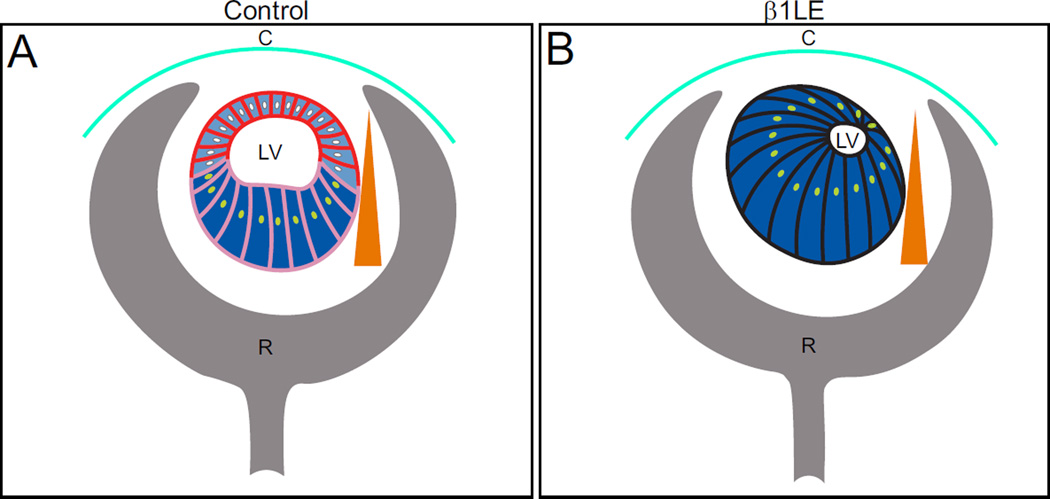
Figure 8. Model describing the potential crosstalk between β1-integrin and FGF/BMP signaling in the early lens.
A) In control lenses, β1-integrins (red) are found at the cell membrane on all lens cells, with lower expression (pink) in the posterior lens. ERK signaling (light blue) is weakly active in the anterior lens, but upregulates in the posterior lens (blue) in response to the FGF gradient (orange triangle). BMP signaling (yellow nuclei) (Faber et al., 2002) and fiber cell differentiation is confined to the posterior lens . B) In β1LE lenses which lack β1-integrins (black), BMP (yellow nuclei) and ERK signaling (blue) are activated in all lens cells, leading to inappropriate differentiation of anterior LECs into lens fibers. C, cornea; LV, lens vesicle; R, retina
In the lens, the effect of β1-integrin on the BMP pathway is likely to be integrin-signaling independent since the loss of integrin signaling in the lens epithelium due to mutation of Crim1 neither affects BMP pathway activation nor results in inappropriate LEC differentiation to lens fibers (Zhang et al., 2016). Interestingly, it has been reported that β1-integrins can suppress BMP signaling by sequestering BMP receptors away from the lipid raft environment, while deletion of β1-integrins from ependymal cells of the spinal cord leads to inappropriate activation of BMP signaling (North et al., 2015), similar to the situation we report here in in β1LE lenses, suggesting a possible molecular mechanism for our observations.
In contrast, deletion of β1-integrins after the lens vesicle stage (i.e. in β1MLR10 mice) does not result in complete conversion of LECs to lens fibers although lens fiber cell marker expression is upregulated in these cells (Simirskii et al., 2007). This is consistent with previous investigations which suggest that the requirement for BMP signaling in lens fiber cell differentiation is less prominent later in lens development (Faber et al., 2002; Pandit et al., 2011). It can be speculated that once the transition zone is established concomitant with the development of a distinct anterior chamber, the FGF gradient is sufficient to regulate the timing of lens fiber cell differentiation. The lower requirement for BMP signaling in fiber cell differentiation later in lens development is attributed to cell fate commitment driven by the upregulation of transcription factors driving lens fiber differentiation (Pandit et al., 2011). The hypothesis that the anterior lens cells of the lens vesicle and early lens are molecularly different from the more mature lens epithelium is supported by our prior report that the transcription factor Sip1 is required to turn off the expression of genes indicative of the head ectoderm in the early lens (Manthey et al., 2014).
It is also possible that different α-integrins complex with β1-integrin at these stages of lens development, changing integrin function. While there is no direct evidence for this, it is known that the relative expression level of integrin subunits changes as lens epithelial cells transition to fibers (Hoang et al., 2014; Walker and Menko, 2009). Alternatively, the differences between the β1MLR10 and β1LE phenotypes could be, at least in part, influenced by a reduction in Pax6 levels in mice harboring LE-Cre due to competition of the Pax6 P0 promoter for factors regulating endogenous Pax6 expression (Dora et al., 2014). In this scenario, the partial differentiation of LECs to lens fibers observed in β1MLR10 lenses (Simirskii et al., 2007) is pushed towards complete fiber cell differentiation due to a reduction in Pax6, which at high levels, negatively regulates lens fiber cell differentiation (Duncan et al., 2004). Alternatively, LE-Cre may drive high enough levels of Cre in the lens epithelium to cause some genotoxic damage to the early lens which could sensitize LECs to enter the fiber cell differentiation pathway, although mice heterozygous for the LE-Cre transgene but carrying one wildtype β1-integrin allele did not exhibit any obvious lens phenotype, even in adulthood (data not shown). Finally, it is also possible that the loss of β 1-integrin from the head ectoderm in β1LE mice (see Figure 2F) changes the milieu of factors secreted from the presumptive corneal epithelium resulting in the loss of LEC identity. However, the surface ectoderm is largely maintaining its phenotype as it still expresses E-cadherin (Figure 5B,D), and Pax6. (Figure 6 D). It also appears to appropriately secrete the signals required for stromal formation (Lwigale, 2015), as the corneal stroma does form based on morphological criteria (Figure 1 F , H).”
Overall, this investigation shows that β1-integrins may limit BMP-FGF crosstalk in the early lens, in order to maintain a balance between BMP and FGF induced LEC proliferation and differentiation. This extra level of control prevents inappropriate LEC differentiation and allows for the precise positioning of the transition zone as the primary patterning of the lens is established.
Summary Statement.
β1-integrins are negative regulators of fiber cell differentiation in the early lens, likely via modulation of BMP signaling.
Acknowledgments
We thank Dr. Salil Lachke, Yichen Wang, Mahbubul Shihan and Ramachandran Balasubramanian for pre-submission comments on the manuscript and Soma Dash for generating the Figure 8 diagram.
Funding:
National Institutes of Health grant-EY015279 to MKD, INBRE program grant P20 RR16472 supported the University of Delaware Core Imaging facility; and 1S10 (RR027273-01) which funded the acquisition of the confocal microscope used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: none
Author contributions:
Mallika Pathania: Performed most of the experiments; prepared figures and primary manuscript drafts; work included as part of University of Delaware doctoral dissertation. Yan Wang: Performed some experiments and provided key technical support. Vladimir N. Simirskii: Created the β1LE mouse strain and performed some initial characterization.
Melinda K. Duncan: Principal investigator of the project; MP’s dissertation advisor, initial project conception; oversaw experiments, edited manuscript and figures.
References
- Alkarain A, Slingerland J. Deregulation of p27 by oncogenic signaling and its prognostic significance in breast cancer. Breast Cancer Res. 2004;6:13–21. doi: 10.1186/bcr722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antosova B, Smolikova J, Borkovcova R, Strnad H, Lachova J, Machon O, Kozmik Z. Ectopic activation of Wnt/beta-catenin signaling in lens fiber cells results in cataract formation and aberrant fiber cell differentiation. PLoS One. 2013;8:e78279. doi: 10.1371/journal.pone.0078279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audette DS, Anand D, So T, Rubenstein TB, Lachke SA, Lovicu FJ, Duncan MK. Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression. Development. 2016;143:318–328. doi: 10.1242/dev.127860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M, Pesch M, Tunggal L, Gaill F, Fassler R. Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J Cell Sci. 2000;(113 Pt 2):259–268. doi: 10.1242/jcs.113.2.259. [DOI] [PubMed] [Google Scholar]
- Barbour W, Saika S, Miyamoto T, Ohkawa K, Utsunomiya H, Ohnishi Y. Expression patterns of beta1-related alpha integrin subunits in murine lens during embryonic development and wound healing. Curr Eye Res. 2004;29:1–10. doi: 10.1080/02713680490513137. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J. Cell. Sci. 1999;112:2155–2165. doi: 10.1242/jcs.112.13.2155. [DOI] [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int J Dev Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Lein PJ, Musil LS. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol Biol Cell. 2008a;19:2631–2641. doi: 10.1091/mbc.E08-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Musil LS. Synergistic Interaction Between the Fibroblast Growth Factor and Bone Morphogenetic Protein Signaling Pathways in Lens Cells. Mol Biol Cell. 2015a doi: 10.1091/mbc.E15-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Musil LS. Synergistic interaction between the fibroblast growth factor and bone morphogenetic protein signaling pathways in lens cells. Mol Biol Cell. 2015b;26:2561–2572. doi: 10.1091/mbc.E15-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Overbeek PA, Musil LS. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008b;324:202–212. doi: 10.1016/j.ydbio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekher G, Sailaja D. Differential activation of phosphatidylinositol 3-kinase signaling during proliferation and differentiation of lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:4400–4411. doi: 10.1167/iovs.03-0136. [DOI] [PubMed] [Google Scholar]
- Chauhan BK, Reed NA, Yang Y, Cermak L, Reneker L, Duncan MK, Cvekl A. A comparative cDNA microarray analysis reveals a spectrum of genes regulated by Pax6 in mouse lens. Genes Cells. 2002;7:1267–1283. doi: 10.1046/j.1365-2443.2002.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepelinsky AB. The ocular lens fiber membrane specific protein MIP/Aquaporin 0. J Exp Zool A Comp Exp Biol. 2003;300:41–46. doi: 10.1002/jez.a.10307. [DOI] [PubMed] [Google Scholar]
- Cvekl A, McGreal R, Liu W. Lens Development and Crystallin Gene Expression. Prog Mol Biol Transl Sci. 2015;134:129–167. doi: 10.1016/bs.pmbts.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Danysh BP, Duncan MK. The lens capsule. Exp Eye Res. 2009;88:151–164. doi: 10.1016/j.exer.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh R, Duncan MK. Growth factor signaling in lens fiber differentiation. In: Saika S, Werner L, Lovicu FJ, editors. Lens epithelium and posterior capsular opacification. Springer Japan; 2014. pp. 81–104. [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Dora NJ, Collinson JM, Hill RE, West JD. Hemizygous Le-Cre transgenic mice have severe eye abnormalities on some genetic backgrounds in the absence of LoxP sites. PLoS One. 2014;9:e109193. doi: 10.1371/journal.pone.0109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MK, Cui W, Oh DJ, Tomarev SI. Prox1 is differentially localized during lens development. Mech Dev. 2002;112:195–198. doi: 10.1016/s0925-4773(01)00645-1. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Kozmik Z, Cveklova K, Piatigorsky J, Cvekl A. Overexpression of PAX6(5a) in lens fiber cells results in cataract and upregulation of (alpha)5(beta)1 integrin expression. J Cell Sci. 2000;113(Pt 18):3173–3185. doi: 10.1242/jcs.113.18.3173. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Xie L, David LL, Robinson ML, Taube JR, Cui W, Reneker LW. Ectopic Pax6 expression disturbs lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2004;45:3589–3598. doi: 10.1167/iovs.04-0151. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Gunhaga L. The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philos Trans R Soc Lond B Biol Sci. 2011;366:1193–1203. doi: 10.1098/rstb.2010.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Oertle P, Monnier CA, Camenzind L, Reyes-Lua M, Hu H, Candiello J, Labilloy A, Balasubramani M, Henrich PB, Plodinec M. New concepts in basement membrane biology. FEBS J. 2015;282:4466–4479. doi: 10.1111/febs.13495. [DOI] [PubMed] [Google Scholar]
- Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995;26:678–690. doi: 10.1016/0272-6386(95)90610-x. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Ishisaki A, Imamura T. Smad mediates BMP-2-induced upregulation of FGF-evoked PC12 cell differentiation. FEBS Lett. 2003;536:30–34. doi: 10.1016/s0014-5793(03)00005-x. [DOI] [PubMed] [Google Scholar]
- Hayes JM, Hartsock A, Clark BS, Napier HR, Link BA, Gross JM. Integrin alpha5/fibronectin1 and focal adhesion kinase are required for lens fiber morphogenesis in zebrafish. Mol Biol Cell. 2012;23:4725–4738. doi: 10.1091/mbc.E12-09-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TV, Kumar PK, Sutharzan S, Tsonis PA, Liang C, Robinson ML. Comparative transcriptome analysis of epithelial and fiber cells in newborn mouse lenses with RNA sequencing. Mol Vis. 2014;20:1491–1517. [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Patkunanathan B, Lynch OT, McAvoy JW, Rasko JE, Lovicu FJ. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp Eye Res. 2006;83:667–678. doi: 10.1016/j.exer.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Patkunanathan B, McAvoy JW, Lovicu FJ. Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors. 2009;27:50–62. doi: 10.1080/08977190802610916. [DOI] [PubMed] [Google Scholar]
- Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli CH, ffrench-Constant C. Neural precursor cell chain migration and division are regulated through different beta1 integrins. Development. 1998;125:3167–3177. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- Jarrin M, Pandit T, Gunhaga L. A balance of FGF and BMP signals regulates cell cycle exit and Equarin expression in lens cells. Mol Biol Cell. 2012;23:3266–3274. doi: 10.1091/mbc.E12-01-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Lin M, Zhang L, York JP, Zhang P. The Notch signaling pathway controls the size of the ocular lens by directly suppressing p57Kip2 expression. Mol Cell Biol. 2007;27:7236–7247. doi: 10.1128/MCB.00780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- Kelley PB, Sado Y, Duncan MK. Expression of Collagen IV subtypes in the developing lens capsule. Matrix Biol. 2002;21:415–423. doi: 10.1016/s0945-053x(02)00014-8. [DOI] [PubMed] [Google Scholar]
- Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci U S A. 1999;96:3781–3785. doi: 10.1073/pnas.96.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Wilmarth PA, Murray MR, David LL. Lens beta-crystallins: The role of deamidation and related modifications in aging and cataract. Progress in biophysics and molecular biology. 2014;115:21–31. doi: 10.1016/j.pbiomolbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren H, Blixt A, Carlsson P. Persistent FoxE3 expression blocks cytoskeletal remodeling and organelle degradation during lens fiber differentiation. Invest Ophthalmol Vis Sci. 2008;49:4269–4277. doi: 10.1167/iovs.08-2243. [DOI] [PubMed] [Google Scholar]
- Lee HY, Wroblewski E, Philips GT, Stair CN, Conley K, Reedy M, Mastick GS, Brown NL. Multiple requirements for Hes 1 during early eye formation. Dev Biol. 2005;284:464–478. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Tao C, Cai Z, Hertzler-Schaefer K, Collins TN, Wang F, Feng GS, Gotoh N, Zhang X. Frs2alpha and Shp2 signal independently of Gab to mediate FGF signaling in lens development. J Cell Sci. 2014;127:571–582. doi: 10.1242/jcs.134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J. 2005;24:1942–1953. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohikangas L, Gullberg D, Johansson S. Assembly of laminin polymers is dependent on beta1-integrins. Exp Cell Res. 2001;265:135–144. doi: 10.1006/excr.2001.5170. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–5084. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW, de Iongh RU. Understanding the role of growth factors in embryonic development: insights from the lens. Philos Trans R Soc Lond B Biol Sci. 2011;366:1204–1218. doi: 10.1098/rstb.2010.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwigale PY. Corneal Development: Different Cells from a Common Progenitor. Prog Mol Biol Transl Sci. 2015;134:43–59. doi: 10.1016/bs.pmbts.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Madakashira BP, Kobrinski DA, Hancher AD, Arneman EC, Wagner BD, Wang F, Shin H, Lovicu FJ, Reneker LW, Robinson ML. Frs2alpha enhances fibroblast growth factor-mediated survival and differentiation in lens development. Development. 2012;139:4601–4612. doi: 10.1242/dev.081737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK. The roles of alphaV integrins in lens EMT and posterior capsular opacification. J Cell Mol Med. 2014;18:656–670. doi: 10.1111/jcmm.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey AL, Lachke SA, FitzGerald PG, Mason RW, Scheiblin DA, McDonald JH, Duncan MK. Loss of Sip1 leads to migration defects and retention of ectodermal markers during lens development. Mech Dev. 2014;131:86–110. doi: 10.1016/j.mod.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy JW, Dawes LJ, Sugiyama Y, Lovicu FJ. Intrinsic and extrinsic regulatory mechanisms are required to form and maintain a lens of the correct size and shape. Exp Eye Res. 2016 doi: 10.1016/j.exer.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Philip NJ. Beta 1 integrins in epithelial tissues: a unique distribution in the lens. Exp Cell Res. 1995;218:516–521. doi: 10.1006/excr.1995.1186. [DOI] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North HA, Pan L, McGuire TL, Brooker S, Kessler JA. beta1-Integrin alters ependymal stem cell BMP receptor localization and attenuates astrogliosis after spinal cord injury. J Neurosci. 2015;35:3725–3733. doi: 10.1523/JNEUROSCI.4546-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit T, Jidigam VK, Gunhaga L. BMP-induced L-Maf regulates subsequent BMP-independent differentiation of primary lens fibre cells. Dev Dyn. 2011;240:1917–1928. doi: 10.1002/dvdy.22692. [DOI] [PubMed] [Google Scholar]
- Pathania M, Semina EV, Duncan MK. Lens extrusion from Laminin alpha 1 mutant zebrafish. ScientificWorldJournal. 2014;2014:524929. doi: 10.1155/2014/524929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Oh DJ, Czymmek KJ, Duncan MK. An immunohistochemical method for the detection of proteins in the vertebrate lens. J Immunol Methods. 2001;253:243–252. doi: 10.1016/s0022-1759(01)00374-x. [DOI] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Conley KW, Le TT, Donner AL, Maas RL, Brown NL. Notch signaling regulates growth and differentiation in the mammalian lens. Dev Biol. 2008;321:111–122. doi: 10.1016/j.ydbio.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AR, Belvindrah R, Wu C, Muller U, Halfter W. Beta1-integrin signaling is essential for lens fiber survival. Gene Regul Syst Bio. 2007;1:177–189. [PMC free article] [PubMed] [Google Scholar]
- Saravanamuthu SS, Gao CY, Zelenka PS. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev Biol. 2009;332:166–176. doi: 10.1016/j.ydbio.2009.05.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanamuthu SS, Le TT, Gao CY, Cojocaru RI, Pandiyan P, Liu C, Zhang J, Zelenka PS, Brown NL. Conditional ablation of the Notch2 receptor in the ocular lens. Dev Biol. 2012;362:219–229. doi: 10.1016/j.ydbio.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiblin DA, Gao J, Caplan JL, Simirskii VN, Czymmek KJ, Mathias RT, Duncan MK. Beta-1 integrin is important for the structural maintenance and homeostasis of differentiating fiber cells Submitted. 2013 doi: 10.1016/j.biocel.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiblin DA, Gao J, Caplan JL, Simirskii VN, Czymmek KJ, Mathias RT, Duncan MK. Beta-1 integrin is important for the structural maintenance and homeostasis of differentiating fiber cells. Int J Biochem Cell Biol. 2014;50:132–145. doi: 10.1016/j.biocel.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simirskii VN, Wang Y, Duncan MK. Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev Biol. 2007;306:658–668. doi: 10.1016/j.ydbio.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Lovicu FJ, McAvoy JW. Planar cell polarity in the mammalian eye lens. Organogenesis. 2011;7:191–201. doi: 10.4161/org.7.3.18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate MC, Garcia AJ, Keselowsky BG, Schumm MA, Archer DR, LaPlaca MC. Specific beta1 integrins mediate adhesion, migration, and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci. 2004;27:22–31. doi: 10.1016/j.mcn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Teo ZL, McQueen-Miscamble L, Turner K, Martinez G, Madakashira B, Dedhar S, Robinson ML, de Iongh RU. Integrin linked kinase (ILK) is required for lens epithelial cell survival, proliferation and differentiation. Exp Eye Res. 2014;121:130–142. doi: 10.1016/j.exer.2014.01.013. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Vecino E, Acera A. Development and programed cell death in the mammalian eye. Int J Dev Biol. 2015;59:63–71. doi: 10.1387/ijdb.150070ev. [DOI] [PubMed] [Google Scholar]
- Velleman SG, McFarland DC. Beta1 integrin mediation of myogenic differentiation: implications for satellite cell differentiation. Poult Sci. 2004;83:245–252. doi: 10.1093/ps/83.2.245. [DOI] [PubMed] [Google Scholar]
- Walker J, Menko AS. Integrins in lens development and disease. Exp Eye Res. 2009;88:216–225. doi: 10.1016/j.exer.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Stump R, McAvoy JW, Lovicu FJ. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp Eye Res. 2009;88:293–306. doi: 10.1016/j.exer.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci. 2006;47:4490–4499. doi: 10.1167/iovs.06-0401. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury k, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 1999a;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999b;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Wu W, Tholozan FM, Goldberg MW, Bowen L, Wu J, Quinlan RA. A gradient of matrix-bound FGF-2 and perlecan is available to lens epithelial cells. Exp Eye Res. 2014;120:10–14. doi: 10.1016/j.exer.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, McGreal R, Harris R, Gao CY, Liu W, Reneker LW, Musil LS, Cvekl A. Regulation of c-Maf and alphaA-Crystallin in Ocular Lens by Fibroblast Growth Factor Signaling. J Biol Chem. 2016;291:3947–3958. doi: 10.1074/jbc.M115.705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto A, Saigou Y, Higashi Y, Kondoh H. Regulation of ocular lens development by Smad-interacting protein 1 involving Foxe3 activation. Development. 2005;132:4437–4448. doi: 10.1242/dev.02022. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes & Development. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fan J, Ho JW, Hu T, Kneeland SC, Fan X, Xi Q, Sellarole MA, de Vries WN, Lu W, Lachke SA, Lang RA, John SW, Maas RL. Crim1 regulates integrin signaling in murine lens development. Development. 2016;143:356–366. doi: 10.1242/dev.125591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Yang HY, Shin J, Phan L, Fang L, Che TF, Su CH, Yeung SC, Lee MH. CDK inhibitor p57 (Kip2) is downregulated by Akt during HER2-mediated tumorigenicity. Cell Cycle. 2013;12:935–943. doi: 10.4161/cc.23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JX, Huang S. Understanding gene circuits at cell-fate branch points for rational cell reprogramming. Trends Genet. 2011;27:55–62. doi: 10.1016/j.tig.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Zou L, Cao S, Kang N, Huebert RC, Shah VH. Fibronectin induces endothelial cell migration through beta1-integrin and Src dependent phosphorylation of fibroblast growth factor receptor-1 at tyrosines 653/654 and 766. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M111.304972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A, Hay ED. Expression of beta 1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev Dyn. 1994;201:378–393. doi: 10.1002/aja.1002010409. [DOI] [PubMed] [Google Scholar]