Abstract
Tributyltin (TBT), a toxic environmental contaminant, has been widely utilized for various industrial, agricultural and household purposes. Its usage has led to a global contamination and its bioaccumulation in aquatic organisms and terrestrial mammals. Previous studies suggest that TBT has debilitating effects on the overall immune function of animals, rendering them more vulnerable to diseases. TBT (at concentrations that have been detected in human blood) alters secretion of inflammatory cytokines from human lymphocytes ex vivo. Thus, it is important to determine if specified levels of TBT can alter levels of cytokines in an in vivo system. Mice were exposed to biologically relevant concentrations of TBT (200, 100 or 25 nM final concentrations). The quantitative determination of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL2, IL5, IL7, IL12βp40, IL13, IL15, KC, MIP1β, MIP2 and RANTES was performed in mouse sera by MAGPIX analysis and Western blot. Results indicated alterations (both decreases and increases) in several cytokines. The pro-inflammatory cytokines IFNγ, TNFα, IL-1β, IL-2, IL5, IL12βp40, and IL-15 were altered as were the chemokines MIP-1 and RANTES and the anti-inflammatory cytokine IL-13. Increases in IFNγ and TNFα were seen in serum of mice exposed to TBT for less than 24 hr. IL1-β, IL-12βp40, IL-5 and IL-15 were also modulated in mouse serum depending on the specific experiment and the exposure concentration. IL-2 was consistently decreased in mouse serum when animals were exposed to TBT. There were also TBT-induced increases in MIP-1β, RANTES, and IL-13. These results from human and murine samples clearly suggest that TBT exposures modulate the secretion inflammatory cytokines.
Keywords: Tributylin, cytokines, serum, mouse
Introduction
Butyltins, such as tributyltin (TBT), are toxic chemicals that have had been used in various industrial, agricultural and domestic applications (Tanabe et al. 1998; Tanabe 1999; Loganathan et al. 2000; Hoch 2001). TBT is the most toxic butyltin and affects most taxa (WHO 1999). It was heavily utilized in antifouling paints for boats and ships (IPCS 1999) as well as in disinfectants, polishes, cleansing products, catalysts, and pesticides (Kannan et al. 1999; Takahashi et al. 1999). Due to its many uses, TBT is a widespread environmental pollutant (Kimbrough 1976; Kannan et al. 1995; Sidiki et al. 1996; Takahashi et al. 1999; Gipperth 2009) accumulating in non-target organisms including humans (Alzieu 2000; Bryan et al. 1989; Smialowicz et al. 1989, van Loveren 1990; Whalen et al, 1999; Kannan et al., 1999). Its use has been regulated since 2008 by the International Maritime Organization (IMO), however, it continues to be used and thus to contaminate the environment. Human exposures to TBT are thought to mainly arise from consumption of TBT-contaminated meat, dairy and fish products (WHO 1990; Kannan et al. 1995) but they also occur due to dermal or pulmonary exposures. TBT is found in human blood at levels as high as 261 nM (85 ng/ml) (Kannan et al. 1999; Whalen et al. 1999) and has also been detected in organs, such as the heart, liver, kidney, and stomach (Gui-Bin et al. 2000).
Studies show an increase in the occurrences of tumors and decreased immune cell function in TBT-exposed mammals. It affects the function of human natural killer (NK) lymphocytes (Smialowicz et al. 1989; van Loveren 1990; Ghoneum 1990) including interference with the ability of human NK cells to bind and lyse target cells (Whalen et al. 1999; Dudimah et al. 2007; Whalen et al. 2002; Thomas et al. 2004). Additionally, it has been shown to alter the secretion of the pro-inflammatory cytokines interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β from human immune cells ex vivo (Hurt et al. 2013; Lawrence et al. 2015; Brown and Whalen 2015).
Cytokines create the communication network for the immune response to infection (and cancers) as well as being responsible for chronic inflammation (a known risk factor in cancer progression) (Moudgil 2015). Any alteration in secretion of a particular cytokine could diminish immune functions including cytotoxic T-cell and NK cell-mediated destruction of cancer cells. An increase in the secretion of pro-inflammatory cytokines such as IL-1β, IFNγ, and TNFα has the potential to assist in the progression of tumors (Grivennikov et al. 2010). Studies show that TBT moderates the immune system in vivo in rodents including altering the proliferation of B- and T-lymphocytes (Smialowicz et al. 1989; van Loveren 1990; Ghoneum 1990). There have been no known studies of the effects of known blood levels of TBT on serum cytokine levels in an in vivo setting.
The present study investigated the effects of exposures to TBT on the secretion of 13 cytokines/chemokines in the serum of BALB/C mice. Since alteration of cytokine levels by TBT exposures have been seen in the ex vivo human system (Hurt et al. 2013; Lawrence et al. 2015; Brown and Whalen 2015), it is important to determine if TBT-induced alterations occur in the more complex and dynamic in vivo setting, using the mouse model.
Materials and methods
Chemical preparation
TBT chloride was purchased from Sigma (St. Louis, MO). The TBT was a neat standard, dissolved initially in deionized water as a 1 mM solution. Biologically-relevant concentrations of TBT (25, 100, or 200 nM) were prepared by dilution of the stock into sterile phosphate buffered saline solution (PBS, pH 7.4).
Mice injections
All mice utilized in these experiments were BALB/c mice from Harlan Laboratories (Indianapolis, IN). Mice were ≈ 6–8-wk-old and 25–30 g at time of use. Mice were housed in filter-topped cages under specific pathogen-free conditions in animal facilities maintained at 22°C with a 45–65% relative humidity and with a 12-hr light:dark cycle at Meharry Medical College (Nashville, TN). All mice had ad libitum access to standard rodent chow and filtered water throughout the experiments. All mice were treated in accordance with Meharry Medical College guidelines approved by the Institutional Animal Care and Use Committee (IACUC).
Wild-type Balb/c mice were used in four separate MAGPIX experiments. For each MAGPIX experiment, mice were injected intravenously (IV) with 100 μl PBS (control), 25, 100, or 200 nM TBT [dissolved in PBS] at n = 2 mice/group (total of 8 mice/experiment). The final concentration was based on average mouse blood volume such that initial exposure concentrations in the mouse bloodstream were relatively accurately determined. After 24 hr, the mice were euthanized by cervical dislocation and blood collected by cardiac puncture. From each sample, serum was isolated and analyzed using a MAGPIX Array (EMD Millipore, Billerica, MA) to measure the presence of 32 cytokines and chemokines. This initial analysis was used to screen for cytokines that showed the most consistent alterations after TBT exposure.
A kinetic study of effect of a single level of TBT (100 nM) was then carried out; three separate kinetic experiments were carried out. Wild-type BALB/c mice were injected IV with 100 μl PBS (control) or 100 nM TBT (3 mice/treatment group and length of exposure, 24 mice/each experiment). After 6, 12, 24 and 48 hr, the mice were euthanized and serum harvested and analyzed by Western blot.
All animals used in this research project were cared for and used humanely according to the following policies: The U.S. Public Health Service Policy on Humane Care and Use of Animals (2000); the Guide for the Care and Use of Laboratory Animals (1996); and the U.S. Government Principles for Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training (1985).
MAGPIX Array
Serum samples were prepared and protein levels quantified using a BCA Protein Assay Kit (Pierce, Waltham, MA). For the assay, 250 μg protein was equalized and used per sample in the cytokine/chemokine magnetic bead panel plate. The MILLIPLEX MAP (MAGPIX) Mouse Cytokine/Chemokine Magnetic Bead kit was used for the simultaneous quantification of 32 murine cytokines and chemokines. Standards and controls were allocated to the appropriate wells and the appropriate matrix solution was added to the background, standards, and control wells. Samples were added to the appropriate wells. Pre-mixed magnetic cytokine/chemokine detection beads were then added to all wells. The plate was sealed and covered with foil and allowed to incubate with agitation on a plate shaker overnight at 4°C. Following washes (two per plate) with sodium dodecyl sulfate (SDS) kit-supplied wash buffer, detection antibodies were added to all wells and allowed to incubate with agitation for 1 hr at room temperature. Kit-provided Streptavidin-Phycoerythrin solution was then added to each well containing the detection antibodies and the plates incubated with agitation for 30 min at room temperature. Following two more plate washes, the premixed beads were re-suspended on a plate shaker for 5 min and the plate then analyzed in the MAGPIX instrument (Millipore) utilizing Millipore xPONENT software. The median fluorescent intensity (MFI) data was saved and analyzed using a 5-parameter logistic or spline curve-fitting method for calculating cytokine/chemokines concentrations in samples.
Western blot
Serum samples (6 μg protein/lane) were resolved over 4–12% NuPAGE Bis-Tris Mini Gels (Thermo Fisher, Waltham, MA) and transferred to PVDF (polyvinylidene difluoride) membranes using an iBlot system (Thermo Fisher). Each PVDF membrane was then blocked with a 5% non-fat milk solution in Tris-buffered saline (TBS) containing 0.1% Tween 20 and then treated with specific primary antibodies (against murine IFNγ, TNFα, IL1β, IL2, IL5, IL7, IL12βp40, IL13, IL15, KC (aka Chemokine [C-X-C motif] ligand-1 [CXCL1]), macrophage inflammatory protein (MIP)-1β (aka Chemokine [C-C motif] ligand 4 [CCL4]), MIP2 (aka CXCL2), and RANTES (Regulated on Activation Normal T-cell-Expressed and Secreted, aka CCL5) obtained from Santa Cruz Biotechnology (Dallas, TX). Each individual primary antibody did not cross react with other cytokines. Following incubation with the primary antibody, the blot was washed and an appropriate secondary antibody (anti-mouse or anti-rabbit) conjugated to horseradish peroxidase was applied to the blot for 1 hr at room temperature. Antibody binding was then visualized using an ECL chemiluminescent detection system (Amersham, Piscataway, NJ) and a UVP Image Station (UVP, Upland, CA). Densitometric analysis using UVP software was conducted to determine the intensity of each protein band. Differences in protein expression were determined relative to the internal control. Measures of β-Actin were used as loading controls.
Statistical analysis
Data was analyzed by pair-wise analysis of control vs. exposed host outcomes in a Student’s t test. A p-value < 0.05 was considered significant.
Results
TBT exposure modulates cytokine levels in mouse serum (MAGPIX analysis)
MAGPIX analysis was used to determine if alterations in serum cytokine levels occurred in TBT-exposed mice after a 24 h exposure. Mice were exposed to 200, 100, 25 nM TBT or PBS (control). Results are shown for 13 cytokines: IL-2, IL-5, IL-7, TNFα, IFNγ, IL-1β, 1L-12βp40, IL-13, IL-15, KC, MIP-1β, MIP-2, and RANTES from an initial screening of 32 cytokines. These 13 were chosen based on alterations in serum levels across the four experiments.
In the first experiment (Figure 1A), mice were exposed for 24 hr to 200 nM or 100 nM TBT or PBS (control). Serum levels of the inflammatory cytokine IL-5 and chemokine KC were decreased in serum by 88% and 59%, respectively (p < 0.05) following exposure to 200 nM TBT as compared to levels in control mouse sera. Exposure to 100 nM TBT also led to significant measureable decreases in IL-1β (87%), IL-5 (100%), KC (70%), and TNFα (100%) (p < 0.05).
Figure 1.
MAGPIX Analysis of effects at 24 hr post-exposure to TBT on presence in serum of cytokine: IL-2, IL-5, IL-7, TNFα, IFNγ, IL-1β, 1L-12βp40, IL-13, IL-15, KC, MIP-1β, MIP-2 and RANTES. BALB/C mice were exposed to: (A) PBS (control), 100 or 200 nM TBT (Experiment 1); (B) PBS, 25, 100, or 200 nM TBT (Experiment 2); (C) PBS, 25, 100, or 200 nM TBT (Experiment 3); or (D) PBS, 25, 100, or 200 nM TBT (Experiment 4). *Significant change vs. control, p < 0.05.
Figure 1B shows the results from a second experiment where mice were exposed to 25, 100, or 200 nM TBT or PBS and then evaluated after 24 hr. In this experiment, decreases in several cytokines were again seen at each level of exposure. However, the specific pro-inflammatory and chemotactic cytokines that were altered differed from those noted in the first experiment. Specifically, exposure to 200 nM TBT led to significantly (p < 0.05) decreased serum levels of the inflammatory/immunostimulatory cytokines IL-2 (90%), IL-7 (58%), and IL-15 (62%) compared to control mouse sera levels. Decreases in IL-7, IL-12βp40, and chemokine MIP-2 were seen in sera after host exposure to 100 nM TBT and in IL-1β, IL-2, IL-5, IL-12βp40, IL-15 and KC after the 25 nM exposure. Serum levels of IL-2, Il-12βp40, IL-15, and KC were all decreased by > 80% compared to control, while IL-1β and IL-5 were decreased by 56% and 21%, respectively.
In the third experiment, mice were exposed to each regimen as above (Figure 1C). As in Experiment 2, after 24 hr, serum levels of IL-2 and IL-7 had decreased 75% and 90%, respectively, compared to control values (p < 0.05) due to exposure to 200 nM TBT. Exposure to 100 nM TBT caused no significant changes in any of the cytokines. In contrast, after 24 hr, exposure to 25 nM TBT had caused increases in serum levels of pro-inflammatory cytokines IFNγ, IL-15, and TNFα. IFNγ levels were increased 4-fold and IL-15 4.9 fold above the levels in unexposed mice and TNFα levels went from undetectable to 8.2 pg/ml. Immunostimulatory IL-7 levels were increased 18-fold above control levels 24 hr after host exposure to 25 nM TBT (p < 0.05).
In the fourth experiment, mice were exposed to each regimen as in the second and third experiments (Figure 1D). Here, after 24 hr, mice that had been exposed to 200 nM TBT showed a significant (p < 0.05) increase in serum levels of pro-inflammatory IL-12βp40 compared to values in sera of control mice; IL-12βp40 was undetectable in control serum and 18.3 pg/ml in serum from 200 nM TBT-treated mice. On the other hand, TNFα levels were decreased 100% in the serum of mice treated with 200 nM TBT as compared to control values (p < 0.05%). The 100 nM TBT exposure caused decreases in levels of anti-inflammatory IL-13 (49%) compared to control values (p < 0.05). As in the third experiment, several pro-inflammatory cytokines were again increased in serum by exposure to 25 nM TBT. Levels of IL-2, IL-5, and IL-15 were increased 5.0-, 1.6-, and 3.2-fold, respectively, relative to control values (p < 0.05). Levels of immunostimulatory IL-7 were increased from undetectable to 9.5 pg/ml. Levels of TNFα decreased 100% due to the 25 nM TBT exposure.
Time-course analysis of cytokine/chemokine levels in mice (Western blot analysis)
Due to varied TBT-induced alterations across the four initial experiments using MAGPIX analysis of cytokine levels at the 24-hr post-exposure timepoint, a time-curve was generated to determine if cytokine levels were possibly being altered earlier or later than 24 hr. For this, mice were exposed to 100 nM TBT and serum isolated at 6, 12, 24, and 48 hr post-exposure; the materials then underwent Western blot analyses to determine levels of given cytokines/chemokines. Three separate kinetic experiments were performed.
Alterations of pro-inflammatory/immunostimulatory cytokine levels with TBT exposure
IFNγ
An increase in IFNγ serum levels was observed at one or more timepoint in all the experiments (Figure 2A). There was an increase in IFNγ levels (ranging from 1.07–3.04-fold) observed at 12 hr post-exposure to TBT in all three experiments.
Figure 2.
Time course of changes in inflammatory and immunostimulatory cytokines in mouse serum. Effects at 6, 12, 24 and 48 hr post-exposure to 100 nM TBT on serum levels of (A) IFNγ, (B) TNFα, (C) IL-1β, (D) IL-2, (E) IL-5, (F) IL-7, (G) 1L-12βp40, and (H) IL-15. Results from three separate experiments. Primary antibodies for each of these cytokines were specific to the particular mouse cytokine and did not cross react with other mouse cytokines.
TNFα
TNFα levels were increased in the serum of mice at 6 hr post-exposure to TBT in all three experiments (ranging from 1.15–4.27-fold) (Figure 2B). Two of the three experiments also showed increases in TNFα levels at 12 hr post-exposure.
IL-1β
Effects of TBT exposure on IL-1β levels varied from experiment to experiment (Figure 2C). A decrease was observed in all three experiments at 48 hr post-exposure (6%-93% decrease). In two of the three experiments there were increases in IL-1β levels after 12 hr and in one experiment there were increases seen at 6 hr, 12 hr, and 24 hr.
IL-2
Levels of IL-2 were decreased in serum of TBT-exposed mice in three separate experiments at one or more post-exposure timepoint (Figure 2D). The point at which decreases occurred varied among the experiments. A decrease was observed in 2 of 3 experiments at the 12 and 48 hr timepoints. However, a consistent decrease in IL-2 levels was observed at both 6 and 24 hr post-exposure to TBT in all three experiments.
IL-5
Exposure to 100 nM TBT increased serum levels of IL-5 in two of the three experiments at the 12 hr post-exposure timepoint (Figure 2E). Increases in IL-5 levels were seen in one experiment (experiment 3) at each length of exposure and ranged from 1.68–3.03 fold above the control. Additionally, significant decreases in IL-5 were seen in both experiments 1 and 2 at 24 hr post exposure.
IL-7
The effects of exposure to 100 nM TBT on IL-7 levels varied across the three experiments with no consistent increases or decreases at a given timepoint (Figure 2F).
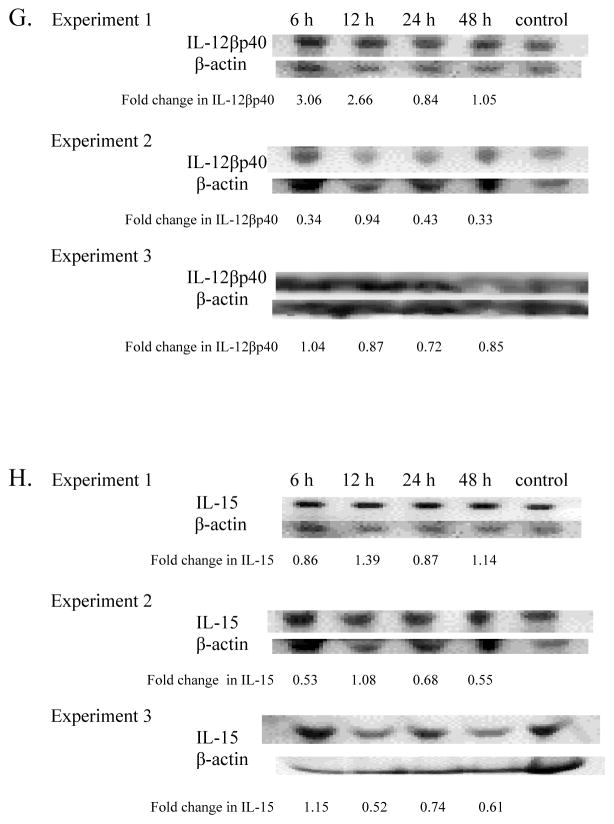
IL-12βp40
Analysis of IL-12βp40 in TBT-exposed mice sera showed a general increase in levels of the cytokine at the 6 and 12 hr exposures in experiment 1 (Figure 2G). All three experiments showed decreased IL-12βp40 24 hr post exposure. The decreases were 16%, 57%, and 28% in experiments 1, 2, and 3, respectively.
IL-15
Serum levels of IL-15 did not show consistent increases after a given length of exposure across the three experiments (Figure 2H). However, a decrease in IL-15 was observed in each of 3 experiments at 24 hr post-exposure.
Alterations in chemotactic cytokine (chemokine) levels after TBT exposure
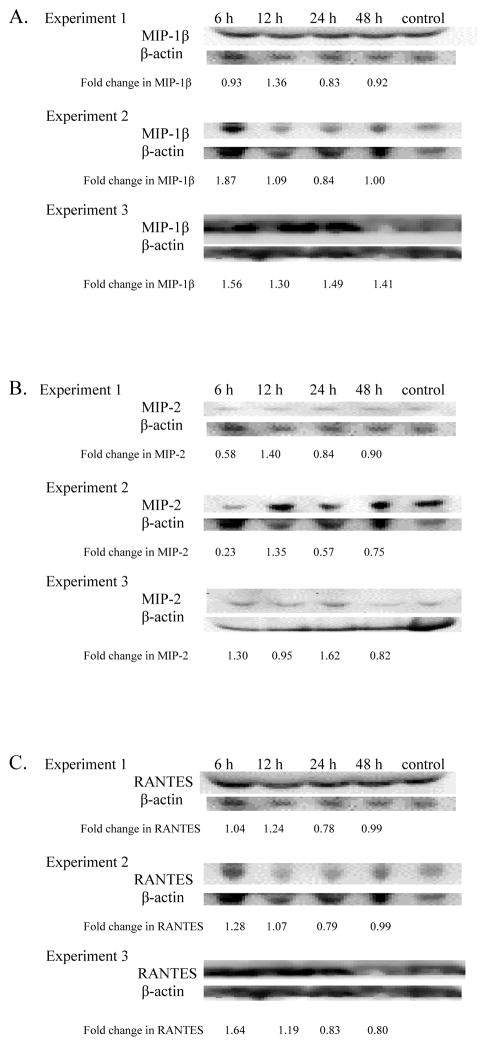
MIP-1β
MIP-1β levels in serum of TBT-exposed mice were increased in each of the three experiments at 12 h post exposure (1.36, 1.09, and 1.30 fold in experiments 1, 2, and 3, respectively) (Figure 3A).
Figure 3.
Time-course of changes in chemotactic cytokines in mouse serum. Effects of 6, 12, 24 and 48 hr post-exposure to TBT on (A) MIP-1β, (B) MIP-2, and (C) RANTES in mouse serum. Results from three separate experiments. Primary antibodies for each of these cytokines were specific to the particular mouse cytokine and did not cross react with other mouse cytokines.
MIP-2
No consistent changes in the serum levels of MIP2 were seen across the three experiments (Figure 3B).
RANTES
Serum RANTES levels were increased by exposure to 100 nM TBT when examined 6 and 12 hr post exposure (Figure 3C). The increases at 6 hr were 1.04 fold (experiment 1), 1.28 fold (experiment 2), and 1.64 fold (experiment 3). After 12 h the increases were 1.24, 1.07, and 1.19 fold in experiments 1, 2, and 3 respectively.
KC
The levels of KC were undetectable in all three experiments analyzed using Western blot.
Anti-inflammatory cytokine levels showed alterations after TBT exposure
IL-13
Mice exposed to 100 nM TBT showed increases at 6 and 12 hr post-exposure in each of the three experiments (Figure 4). At 6 hr post exposure there were increases of 1.35 (experiment 1), 1.39 (experiment 2), and 2.67 (experiment 3) fold compared to the control.
Figure 4.

Time course of changes in anti-inflammatory IL-13 in mouse serum. Results from three separate experiments. The primary antibody for Il-13 was specific to mouse IL-13 and did not cross react with other mouse cytokines.
Discussion
The present study evaluated the effects of tributyltin (TBT) exposures on the cytokine signaling network in mouse serum and reveals significant dysregulation of cytokine production by the compound. Due to the importance of cytokines in regulating general immune response and other cellular functions, it is crucial to determine whether TBT, an environmental contaminant found in human blood (Kannan et al. 1999; Whalen et al. 1999) and shown to alter certain cytokines in an ex vivo human system (Hurt et al. 2013; Brown and Whalen 2015; Lawrence et al. 2015), affects cytokine levels in serum in vivo.
Persistent compound-induced alterations of cytokine levels may prompt irregular inflammatory responses that have been implicated in a number of diseases, including cancer (Coussens and Werb 2002). It is not possible to carry out in vivo studies of TBT-induced alterations in humans. Thus, the present study examined the effects of TBT exposures (at levels that have been measured in human blood [Kanan et al. 1999; Whalen et al. 1999]) on cytokine levels in the serum of mice. This model will indicate if alterations in cytokine levels are being triggered by exposure to TBT in a similar and dynamic in vivo system such as would be seen in the human body. Three general categories of cytokine were examined: pro-inflammatory and/or immunostimulatory cytokines; anti-inflammatory cytokines; and chemotactic cytokines. Alterations of each of the categories were seen here in the serum of mice after exposure to TBT.
The pro-inflammatory cytokines that showed alteration either in the MAGPIX analyses at 24 hr after exposure to either 25, 100, or 200 nM TBT, or in the kinetic studies/Western blot analyses after a 6, 12, 24, or 48 hr after exposure to 100 nM TBT were: IFNγ, TNFα, IL-1β, IL-2, IL-5, IL-7, IL12p40, and IL15. Pro-inflammatory cytokines are produced by a variety of leukocytes including lymphocytes, monocytes/macrophages, and granulocytes (Dinarello 2000) and are important for mounting innate and adaptive immune responses during pathogen infection and lead to mutual cytokine induction. Thus the changes induced by TBT may cause unwanted inflammation in the case of increases (Moudgil, 2015) or lead to dysregulation of host immune responses to infection and trauma if they are decreased.
Both IL-1β and IL-2 tended to be decreased in mouse serum after exposure to TBT. This was seen in both in the 24h MAGPIX experiments and in the western blot time course experiments. IL-1β is involved in mediating initial inflammatory processes (Gabay et al. 2010) and is thus essential to protection from pathogens. However, elevated levels of IL-1β can stimulate the development of tumors and metastasis (Konishi et al. 2005). IL-2 is vital in the regulation of T-cell and NK cell responses (Fehniger et al. 2002). In contrast, each of the other pro-inflammatory cytokines showed both increases and decreases in their levels dependent on the length of exposure and concentration of TBT. For instance, IL-5 tended to be decreased at 24 hr post-exposure here; when the time-course of alterations was examined, there were increases at 12 hr post-exposure. IL-5 induces cell proliferation, survival and differentiation and is linked to allergic inflammation (Ogata et al. 1998). IL-15 was decreased in mouse serum by 100 nM TBT 24 h post exposure in both the MAGPIX and western blot experiments. IL-15 is structurally similar to IL-2 (which is also decreased by TBT) and like IL-2 induces the proliferation of NK cells (Steel et al. 2012). Serum levels of IFNγ and TNFα tended to be increased in mice that had been exposed to TBT for < 24 h. Consistent increases occurred at 12 hr for IFNγ and, at 6 h for TNFα.
Pro-inflammatory cytokine IFNγ - secreted by T-cells, NK cells, and also by myeloid lineage cells like macrophages (Darwich et al. 2008; Billiau and Matthys 2009) - is able to control production of itself as well as of TNFα. IFNγ regulates T-helper (TH)-1 cells as well as recruitment of innate immune cells to sites of infection or tumor (Zaidi and Merlino 2011). IFNγ and TNFα have an ability to cause chronic inflammation; thus, their elevation induced by TBT has a potential to result in pathologies associated with elevated levels of pro-inflammatory cytokines, such as gastrointestinal cancer (Macarthur et al. 2004; Gee et al. 2009). Previous ex vivo studies in human lymphocytes showed increased levels of IFNγ, TNFα, and IL-1β in response to 2.5 to 100 nM TBT (Figure 5, Hurt et al. 2013; Brown and Whalen 2015; Lawrence et al. 2015). Changes in the levels of IFNγ and TNFα in the serum of the mice here might be as a result of over-stimulation of T-cells and NK cells by TBT.
Figure 5.
Effects at 24 and 48 exposure to TBT on (A) TNFα, (B) IFNγ, and (C) IL-1β production by human monocyte-depleted PBMC (MD-PBMC) from an individual healthy donor. *Indicates significant change in secretion compared to control [p < 0.05]. Data taken from (A) Hurt et al. [2013] (B) Lawrence et al. [2015], and (C) Brown and Whalen [2015].
The immunostimulatory cytokine IL-7 was seen here to undergo to both increases and decreases in expression in the serum of the exposed mice, depending on the TBT concentration. Mice exposed to 200 or 100 nM TBT tended to show decreases in the serum levels of IL-7, while those exposed to 25 nM showed significant increases. IL-7 functions in T- and B-cell development, acting as a growth factor (Bikker et al. 2012). It is produced by non-hematopoietic stromal cells, rather than leukocytes such as T-cells, B-cells and NK cells. That changes occurred in IL-7 levels that in some cases were in line with those for IFNγ, IL-1β, and/or TNFα would be in keeping with what is known about cross-regulating effects of each of these cytokines upon one another. It is well-established that IL-7 levels can be up-regulated by IFNγ, IL-1β, and/or TNFα (Jin et al. 2013; Jana et al. 2014; Hou et al. 2015) and that, conversely, IL-7 can impact on formation of each of the three cytokines (Alderson et al. 1991; Toraldo et al. 2003; Yuan et al. 2014; Zhou et al. 2015) by a variety of cell types. How precisely the IL-7 might be being affected by the TBT here - whether directly or by some interruption in these cross-regulatory pathways – remains to be determined.
Anti-inflammatory cytokines decrease levels/effectiveness of pro-inflammatory cytokines like IL-1 and TNFα (De Waal Malefyt et al. 1993). IL-13 decreases synthesis of IL-1 and TNFα and increases synthesis of IL-1 receptor antagonist (De Waal Malefyt et al. 1993;Yangawa et al. 1995). Here, IL-13 levels showed a general increase with exposure to 100 nM TBT in the kinetic experiments. The TBT-induced increases like those of the pro-inflammatory cytokines, IFNγ and TNFα occurred 6 and 12 hrs post exposure. This suggests that the overall effect that might occur in vivo will be dependent upon the relative balance of changes in pro-inflammatory versus anti-inflammatory cytokines.
MIP-1β and RANTES are both members of the CC chemokine family and appear to be synthesized by a variety of cells, including monocytes, macrophages, and T-cells (Song et al. 2000; Luo et al. 2004). MIP-1β is a potent lymphocyte chemo-attractant, attracting both T-cells and NK cells. Both of these chemokines showed increases in the serum of mice exposed to TBT here. In contrast, the two CXC cytokines KC and MIP-2 were decreased significantly in one or more of the MAGPIX experiments. KC is involved in the chemotaxis and cell activation of neutrophils (Moser et al. 1990), while MIP-2 is responsible for attracting neutrophils and hematopoeitic stem cells (Pelus and Fukuda 2006). Since chemokine networks are involved in inflammation, the presence of TBT may dysregulate their ability to act as chemo-attractants to guide the migration of cells in inflammatory responses. Based on the current result, there is some indication that the CXC chemotactic cytokines may be significantly affected by the presence of the contaminant TBT in exposed individuals.
Analysis of effects of TBT on cytokine levels in mouse serum revealed a similar increase in the levels of the pro-inflammatory cytokines IFNγ and TNFα as was observed when secretion from human lymphocytes was measured following an ex vivo exposure to TBT (Hurt et al., 2013; Lawrence et al. 2015). Additionally, the in vivo data showed a decrease in IL-1β in the serum of TBT-exposed mice; a decrease in IL-1β secretion was also seen when human lymphocytes were exposed to higher concentrations of TBT (Brown and Whalen 2015). The results of these studies are important for understanding the possible toxic effects of TBT on the cytokine signaling network in complex biological systems. This initial study indicates similarities (such as those discussed above) between the in vivo mouse studies and the studies on human lymphocytes. Thus the mouse model may be reliable in assessing potential in vivo effects of TBT exposures. There is a need for further studies using the mouse model to examine additional parameters and longer time courses to determine both short term and longer term effects of TBT exposure on cytokine levels in serum as well as other tissues. Additionally the effects on other acute inflammatory molecules such as C-reactive protein, serum amyloid A protein, and fibrinogen could be monitored as could the effect on tumor progression and metastasis.
Conclusions
TBT exposures in the mouse in vivo system led to changes in cytokine and chemokine levels. Exposures were able to increase IFNγ TNFα, MIP-1β, RANTES and IL-13 in serum of mice at one or more length of exposure to TBT. IL-5 and IL-15 were both increased and decreased in mouse serum depending on the specific experiment and exposure concentration. IL-1β and IL-2 levels were decreased in mouse serum when the animals were exposed to TBT. The increases seen in IFNγ and TNFα in this in vivo system are in agreement with changes seen in secretion of these two cytokines from human lymphocytes (ex vivo) exposed to concentrations of TBT of 2.5–100 nM. This initial study indicates that mouse may provide a reliable model for future studies assessing potential in vivo effects of TBT exposures.
Acknowledgments
These studies were funded by Grant U54CA163066 from the National Institutes of Health.
Footnotes
Declaration of interest
The Authors report no conflicts of interest. The Authors are alone responsible for the content and writing of the paper.
References
- Alderson M, Tough T, Ziegler S, Grabstein K. Interleukin-7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med. 1991;173:923–930. doi: 10.1084/jem.173.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzieu C. Environmental impact of TBT: The French experience. Sci Total Environ. 2000;258:99–102. doi: 10.1016/s0048-9697(00)00510-6. [DOI] [PubMed] [Google Scholar]
- Billiau A, Matthys P. IFNγ: A historical perspective. Cytokine Growth Factor Rev. 2009;20:97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Bikker A, Hack CE, Lafeber FP, van Roon JA. IL-7: A key mediator in T-cell-driven autoimmunity, inflammation, and tissue destruction. Curr Pharm Des. 2012;18:2347–3456. doi: 10.2174/138161212800165979. [DOI] [PubMed] [Google Scholar]
- Brown S, Whale M. Tributyltin alters secretion of IL-1β from human immune cells. J Appl Toxicol. 2015;35:895–908. doi: 10.1002/jat.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan G, Gibbs P, Huggett R, Curtis L, Bailey D, Dauer D. Effects of tributyltin pollution on the mud snail. Ilyanassa obsoleta, from the York River and Sarah Creek, Chesapeake Bay. Mar Pollut Bull. 1989;20:458–462. [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwich L, Coma G, Pena R, Bellido R, Blanco EJ, Este JA, Borras FE, Clotet B, Ruiz L, Rosell A, et al. Secretion of IFNγ by human macrophages demonstrated at the single-cell level after co-stimulation with IL-12 plus IL-18. Immunology. 2008;126:386–393. doi: 10.1111/j.1365-2567.2008.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waal Malefyt R, Figdor C, Huijbens R, Mohan-Petersen S, Bennett B, Culpepper J, Dang W, Zurawski G, De Vries JE. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes: Comparison with IL-4 and modulation by IFNγ or IL-10. J Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- Dinarello CA. Pro-inflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Dudimah F, Odman-Ghazi S, Hatcher F, Whalen MM. Effect of tributyltin (TBT) on ATP levels in human natural killer cells: Relationship to TBT-induced decreases in NK function. J Appl Toxicol. 2007;27:86–94. doi: 10.1002/jat.1202. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Cooper MA, Caligiuri MA. IL-2 and IL-15: Immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Gee K, Guzzo C, Che Mat N, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;1:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- Ghoneum M, Hussein A, Gill G, Alfred L. Suppression of murine natural killer cell activity by tributyltin: In vivo and in vitro assessment. Environ Res. 1990;52:178–186. doi: 10.1016/s0013-9351(05)80252-x. [DOI] [PubMed] [Google Scholar]
- Gipperth L. The legal design of the international and European Union ban on tributyltin anti-fouling paint: Direct and indirect effects. J Environ Manage. 2009;90:S86–S95. doi: 10.1016/j.jenvman.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Greten F, Karin M. Immunity, inflammation and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui-Bin J, Qun-Fang Z, Bin H. Speciation of organotin compounds, total tin, and major trace metal elements in poisoned human organs by gas chromatography-flame photometric detector and inductively coupled plasma-mass spectrometry. Environ Sci Technol. 2000;34:2697–2705. doi: 10.1007/s0012800125. [DOI] [PubMed] [Google Scholar]
- Hoch M. Organotin compounds in the environment - an overview. Appl GeoChem. 2001;16:719–743. [Google Scholar]
- Hou L, Jie Z, Liang Y, Desai M, Soong L, Sun J. Type 1 IFN-induced IL-7 maintains CD8+ T-cell responses and homeostasis by suppressing PD-1 expression in viral hepatitis. Cell Mol Immunol. 2015;12:213–221. doi: 10.1038/cmi.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt K, Hurd-Brown T, Whalen M. Tributyltin and dibutyltin alter secretion of TNFα from human natural killer (NK) cells and a mixture of T-cells and NK cells. J Appl Toxicol. 2013;33:503–510. doi: 10.1002/jat.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCS. Concise International Chemical Assessment Documents, No 14, Tributyltin Oxide. Geneva: World Health Organization; 1999. [Google Scholar]
- Jana M, Mondal S, Jana A, Pahan K. Interleukin-12 (IL-12), but not IL-23, induces the expression of IL-7 in microglia and macrophages: Implications for multiple sclerosis. Immunology. 2014;141:549–563. doi: 10.1111/imm.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Shinohara Y, Yu Q. Innate immune signaling induces IL-7 production from salivary gland cells and accelerates the development of primary Sjogren’s syndrome in a mouse model. PLoS One. 2013;8:e77605. doi: 10.1371/journal.pone.0077605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy J. Occurrence of butyltin compounds in human blood. Environ Sci Technol. 1999;33:1776–1779. [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R. Occurrence of butyltin residues in certain food stuffs. Bull Environ Contam Toxicol. 1995;55:510–516. doi: 10.1007/BF00196029. [DOI] [PubMed] [Google Scholar]
- Kimbrough RD. Toxicity and health effects of selected organotins compounds: A Review. Environ Health Perspect. 1976;14:51–56. doi: 10.1289/ehp.761451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi N, Miki C, Yoshida T, Tanaka K, Toiyama Y, Kusunoki M. IL-1 receptor antagonist inhibits the expression of vascular endothelial growth factor in colorectal carcinoma. Oncology. 2005;68:138–145. doi: 10.1159/000086768. [DOI] [PubMed] [Google Scholar]
- Lawrence S, Reid J, Whalen M. Secretion of IFNγ from human immune cells is altered by exposure to tributyltin and dibutyltin. Environ Toxicol. 2015;30:559–571. doi: 10.1002/tox.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan B, Kannan K, Owen D, Sajwan K. Butyltin compounds in freshwater ecosystems. In: Lipnick RL, Hermens J, Jones K, Muir D, editors. Persistent, Bioaccumulative, and Toxic Chemicals I Fate and Exposure. Washington, DC: Am. Chem. Soc; 2000. [Google Scholar]
- Luo X, Yu Y, Liang A, Xie Y, Liu S, Guo J, Wang W, Qi R, An H, Zhang M, Xu H, Guo Z, Cao X. Intra-tumoral expression of MIP-1β induces anti-tumor responses in pre-established tumor model through chemoattracting T-cells and NK cells. Cell Mol Immunol. 2004;1:199–204. [PubMed] [Google Scholar]
- Macarthur M, Hold G, El-Omar E. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol. 2004;286:G515–G520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990;171:1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil KD. Interplay among cytokines and T-cell subsets in the progression and control of immune-mediated diseases. Cytokine. 2015;74:1–4. doi: 10.1016/j.cyto.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Ogata N, Kouro T, Yamada A, Koike M, Hanai N, Ishikawa T, Takatsu K. JAK2 and JAK1 constitutively associate with an IL-5 receptor α and β subunit, respectively, and are activated upon IL-5 stimulation. Blood. 1998;91:226–234. [PubMed] [Google Scholar]
- Pelus L, Fukuda S. Peripheral blood stem cell mobilization: the CXCR2 ligand GROβ rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34:1010–1020. doi: 10.1016/j.exphem.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Sidiki A, Williams D, Carrier R, Thomas B. Pilot study on the contamination of drinking water by organotin compounds from PVC materials. Chemosphere. 1996;32:2389–2398. doi: 10.1016/0045-6535(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Smialowicz R, Riddle M, Rogers R, Luebke R, Copeland C. Immunotoxicity of tributyltin oxide in rats exposed as adults or pre-weanlings. Toxicology. 1989;57:97–111. doi: 10.1016/0300-483x(89)90037-1. [DOI] [PubMed] [Google Scholar]
- Song A, Nikolcheva T, Krensky A. Transcriptional regulation of RANTES expression in T-lymphocytes. Immunol Rev. 2000;177:236–245. doi: 10.1034/j.1600-065x.2000.17610.x. [DOI] [PubMed] [Google Scholar]
- Steel J, Waldmann T, Morris J. IL-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Tanabe S, Takeuchi I, Miyazaki N. Distribution and specific bioaccumulation of butyltin compounds in a marine ecosystem. Arch Environ Contam Toxicol. 1999;37:50–61. doi: 10.1007/s002449900489. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Prudente M, Mizuno T, Hasegawa J, Iwata H, Miyazaki N. Butyltin contamination in marine mammals from north Pacific and Asian coastal waters. Environ Sci Technol. 1998;32:193–198. [Google Scholar]
- Tanabe S. Butyltin contamination in marine mammals: A review. Mar Marine Pollut Bull. 1999;39:62–72. [Google Scholar]
- Thomas LD, Shah H, Green SA, Bankhurst AD, Whalen MM. Tributyltin exposure causes decreased granzyme B and perforin levels in human natural killer cells. Toxicology. 2004;200:221–233. doi: 10.1016/j.tox.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Toraldo G, Roggia C, Qian W, Pacifici R, Weitzmann M. IL-7 induces bone loss in vivo by induction of receptor activator of NF-κB ligand and TNFα from T-cells. 2003. Proc Natl Acad Sci USA. 100:125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loveren H, Krajnc E, Rombout P, Blummaert F, Vos JG. Effect of ozone, hexa-chlorobenzene and bis(tri-n-butyltin)oxide on natural killer activity in the rat lung. Toxicol Appl Pharmacol. 1990;102:21–33. doi: 10.1016/0041-008x(90)90080-e. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Loganathan B, Kannan K. Immunotoxicity of environmentally relevant concentrations of butyltins on human natural killer cells in vitro. Environ Res. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Green SA, Loganathan BG. Brief butyltin exposure induces irreversible inhibition of the cytotoxic function on human natural killer cells in vitro. Environ Res. 2002;88:19–29. doi: 10.1006/enrs.2001.4318. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Environmental Health Criteria 116. Geneva: WHO; 1990. Tributyltin Compounds. [Google Scholar]
- World Health Organization (WHO) Document 14: Tributyltin oxide. Geneva: WHO; 1999. International Program on Chemical Safety (IPCS) Concise International Chemical Assessment. [Google Scholar]
- Yanagawa H, Sone S, Haku T, Mizuno K, Yano S, Ohmoto Y, Ogura T. Contrasting effect of IL-13 on IL-1 receptor antagonist and pro-inflammatory cytokine production by human alveolar macrophages. Am J Respir Cell Mol Biol. 1995;12:71–76. doi: 10.1165/ajrcmb.12.1.7811472. [DOI] [PubMed] [Google Scholar]
- Yuan C, Yang X, Zhu C, Liu S, Wang B, Wang F. Interleukin-7 enhances the in vivo anti-tumor activity of tumor-reactive CD8+ T cells with induction of IFNγ in a murine breast cancer model. Asian Pac J Cancer Prev. 2014;15:265–271. doi: 10.7314/apjcp.2014.15.1.265. [DOI] [PubMed] [Google Scholar]
- Zaidi MR, Merlino G. The two faces of IFNγ in cancer. Clin Cancer Res. 2011;17:1–7. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Jin J, Du J, Yu Q. Innate immune signaling induces IL-7 production, early inflammatory responses, and Sjogren’s-like dacryoadenitis in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2015;56:7831–7838. doi: 10.1167/iovs.15-17368. [DOI] [PMC free article] [PubMed] [Google Scholar]