Abstract
Chronic electric activation of the carotid baroreflex produces sustained reductions in sympathetic activity and arterial pressure and is currently being evaluated for therapy in patients with resistant hypertension. However, patients with significant impairment of renal function have been largely excluded from clinical trials. Thus, there is little information on blood pressure and renal responses to baroreflex activation in subjects with advanced chronic kidney disease, which is common in resistant hypertension. Changes in arterial pressure and glomerular filtration rate were determined in 5 dogs after combined unilateral nephrectomy and surgical excision of the poles of the remaining kidney to produce ~ a 70% reduction in renal mass. After control measurements, sodium intake was increased from ~45 to 450 mol/day. While maintained on high salt, animals experienced increases in mean arterial pressure from 102±4 to 121±6 mmHg and GFR from 40±2 to 45±2 mL/min. During 7 days of baroreflex activation the hypertension induced by high salt was abolished (103±6 mmHg) along with striking suppression of plasma norepinephrine concentration from 139±21 to 81±9 pg/mL, but despite pronounced blood pressure lowering there were no significant changes in GFR (43±2mL/min). All variables returned to pre-stimulation values during a recovery period. These findings indicate that after appreciable nephron loss, chronic suppression of central sympathetic outflow by baroreflex activation abolishes hypertension induced by high salt intake. The sustained antihypertensive effects of baroreflex activation occur without significantly compromising glomerular filtration rate in remnant nephrons.
Keywords: hypertension, blood pressure, baroreflex, sympathetic nervous system, glomerular filtration rate, chronic kidney disease
Introduction
Despite pharmacological interventions, blood pressure control in patients with resistant hypertension (RHT) remains inadequate.1,2 To address this therapeutic need, recent technology using chronic electrical activation of the carotid baroreflex (BA) and renal nerve ablation have been investigated for the treatment of RHT.2–8 Clinical results from these technologies, however, have been inconsistent with some of this inconsistency likely due to the fact that conditions predictive of a favorable antihypertensive response to these device-based therapies are not fully understood. One area where data are particularly limited is the effect of renal function on the blood pressure response to these forms of global and renal-specific sympathoinhibition seen with BA and renal nerve ablation, respectively. This is due in large part to the fact that despite the prevalence of chronic kidney disease (CKD) in patients with RHT,1,9–14 most clinical trials using device-based therapy have been limited to patients with little or no impairment in kidney function.6–8 These patients have been excluded from clinical trials for fear of worsening renal injury due to reductions in renal perfusion pressure and/or theoretical safety concerns regarding damage to the renal artery in the case of renal nerve ablation. The paucity of data from these patients may be especially significant because the presence of CKD is not only often associated with RHT but is also a strong predictor of RHT and is a risk marker for adverse cardiovascular and renal outcomes.1,9–14 Although optimal blood pressure control is likely critical in reducing the development and progression of renal disease in patients with RHT, it remains unclear whether device-based therapies can effectively lower arterial pressure without further exacerbating renal injury in patients with RHT and coexistent CKD.
Remnant kidney models have been used to investigate the pathogenic mechanisms that contribute to the progression of CKD and hypertension.15–21 In the dog, surgical reduction of kidney mass leads to minimal hypertension and renal injury in surviving nephrons during the initial weeks after reducing renal mass as long as sodium intake is normal.20,21 However, during high salt intake (HS), hypertension is clearly manifested. Although not specifically evaluated for resistance to pharmacological treatment of hypertension, this experimental model shares several characteristics present in many patients with RHT including reduced total GFR, volume expansion, suppression of the renin-angiotensin system, excessive salt intake and arterial pressure that is salt-sensitive.1,2,22 At the same time, this model is devoid of the confounding conditions, including obesity, sleep apnea, and hypersecretion of aldosterone, that contribute in variable degrees to the pathogenesis of RHT. Thus, a potential advantage of this model is that it provides an untainted understanding of the fundamental impact of reduced baseline renal function on blood pressure lowering and the attendant changes in renal function during device-based therapies that suppress sympathetic activity.
The major goals of this study were to determine the arterial pressure and GFR responses to BA in canines with hypertension secondary to surgical reduction of kidney mass and HS. High baseline levels of sympathetic activity, which are common in both RHT and CKD,23–27 would be expected to favor a robust fall in blood pressure in response to BA-induced sympathoinhibition. However, it is unclear whether this consideration is relevant to the present study as sympathetic activity has not been reported previously in the canine model of reduced kidney mass-salt-induced hypertension. Nonetheless, because BA chronically suppresses central sympathetic outflow and lowers arterial pressure in normotensive dogs and in hypertensive dogs with low, normal, and high baseline levels of sympathetic activity,28–33 it is reasonable to hypothesize that BA will also suppress sympathetic activity in this model of CKD and, as a result, attenuate the severity of the salt-sensitive hypertension. On the other hand, despite suppression of sympathetic activity, blood pressure lowering could be appreciably diminished in the face of the substantially impaired renal function associated with loss of functioning nephrons. The basis of this concern is that because renal autoregulation is impaired in patients with hypertension and renal insufficiency,34 and more specifically to the present study in remnant nephrons after reduction of renal mass in experimental animals,17,18 excessive reductions in glomerular pressure and GFR, in response to blood pressure lowering, may promote salt and water retention and thus counteract any sustained antihypertensive effects of BA. To this point, we have previously reported that along with normalizing arterial pressure, BA markedly reduces the glomerular hyperfiltration in hypertensive obese dogs.31 Although total GFR is elevated in obese dogs and depressed after surgical reduction of renal mass, single nephron filtration rate is elevated under both conditions. Therefore, because of a blunted ability of the preglomerular vasculature to dilate further in response to a fall in arterial pressure (impaired renal autoregulation), GFR may also decrease substantially during BA in dogs after loss of nephrons. Thus, a final goal of this study was to determine whether blood pressure lowering during BA leads to further, possibly excessive, reductions in GFR in dogs with reduced renal mass and hyperfiltering remnant nephrons.
Methods
Animal Preparation
All procedures were performed in accordance with National Institutes of Health (NIH) Guidelines and approved by the Institutional Animal Care and Use Committee. Surgical procedures were conducted under isoflurane anesthesia (1.5–2.0%) after pre-medication with acepromazine (0.15 mg/kg, sq) and induction with thiopental (10mg/kg, sq). Carprofen (Rimadyl), 4mg/kg, was administered for 3 days postoperatively for analgesia. The specific surgical procedures for implantation of catheters in the aorta and vena cava, and stimulating electrodes (first-generation, Rheos) around each carotid sinus have been described previously.28 More specific information on the surgical procedures, including establishing reduced renal mass, are described in the online-only Data Supplement. General methods for maintenance and experimentation are also described in the on-line Data Supplement. Experiments were conducted in 5 male dogs weighing 21–27 kg.
Experimental Protocol
During the last 2 days of the normal salt intake (NS) control period and on the last 2 days of each protocol (see below), blood samples (~10 ml) were taken from one of the two arterial catheters at 11 AM prior to feeding. In addition, a single determination of GFR was made at end of each protocol. Arterial pressure and heart rate were sampled continuously, 24-hours/day from an arterial catheter.28 The daily values for mean arterial pressure (MAP) and heart rate were averaged for the 20-hour period between 11:30 AM and 7:30 AM. As detailed in the on-line Data Supplement, NS (~45 mmol/day) and HS (~450 mmol/day) were achieved by feeding the dogs a low sodium diet and by adjusting the continuous infusion rate of isotonic saline.
Summary of Protocols
NS control
Days 1–7, HS
Days 8–14, HS + BA
Days 15–21, HS
Days 22–23, NS recovery
Analytical Methods
The plasma levels of hormones and norepinephrine (NE) were measured by radioimmunoassay and high-performance liquid chromatography with electrochemical detection, respectively, in the Departmental Core facility.29 Standard techniques were used to measure hematocrit and the plasma concentrations of sodium, potassium, and protein.28,29 GFR and sodium iothalamate space (an index of extracellular fluid volume) were determined from the clearance of 125I-iothalamate (Glofil, Isotex Diagnostics, Friendwood, TX).33
Statistical Analyses
Results are expressed as mean ± SE. One-way, repeated-measures ANOVA followed by either the Dunnett or Bonferroni post hoc t-tests for multiple comparisons were used to compare responses to appropriate controls during HS, HS+BA, and NS recovery. Two way ANOVA followed by Sidak’s multiple comparison test was used to assess the interaction between kidney mass and salt intake in the steady-state relationships between arterial pressure and sodium intake/excretion in dogs under control conditions37 and after reduced kidney mass. Statistical significance was considered to be P<0.05.
Results
Responses to High Salt in Dogs with Reduced Kidney Mass
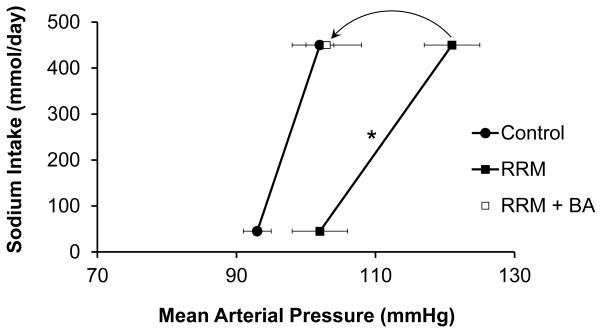
In the present study, MAP (NS control=102±4 mmHg) increased 19±3 mmHg during HS. This increase in MAP is illustrated by the chronic salt-loading renal function curves in Figure 1, which show the steady-state relationships between salt intake/excretion and arterial pressure under different experimental conditions.35,36 Responses to HS in control dogs with intact kidneys are taken from one our recent studies in which dogs were fed NS and HS diets under experimental conditions comparable to those in the present investigation.37 This figure clearly shows the salt-sensitivity of arterial pressure in dogs with reduced nephron number as the increase in MAP in response to HS (19±3 mmHg) was significantly greater than in dogs with intact kidneys (9±2 mmHg; P<0.05 by two way ANOVA). The increase in MAP during HS in the present study was not associated with a statistically significant change in heart rate (NS control=77±5 bpm). On NS, sodium and potassium excretion were 46±1 and 49±2 mmol/day, respectively, reflecting daily intake. During the initial day of HS, there was considerable sodium retention (200±8 mmol) before sodium balance was achieved on subsequent days (day 7, HS= 427±7 mmol/day; Figure 2). There were no significant changes in potassium excretion (day 7, HS=51±1 mmol/day) during HS.
Figure 1.
Steady-state relationships between arterial pressure and sodium intake/excretion in dogs under control conditions37 and after reduced kidney mass. In addition, this figure shows that BA abolishes blood pressure salt sensitivity in dogs with reduced kidney mass. *P<0.05 for interaction between kidney mass and sodium intake/excretion.
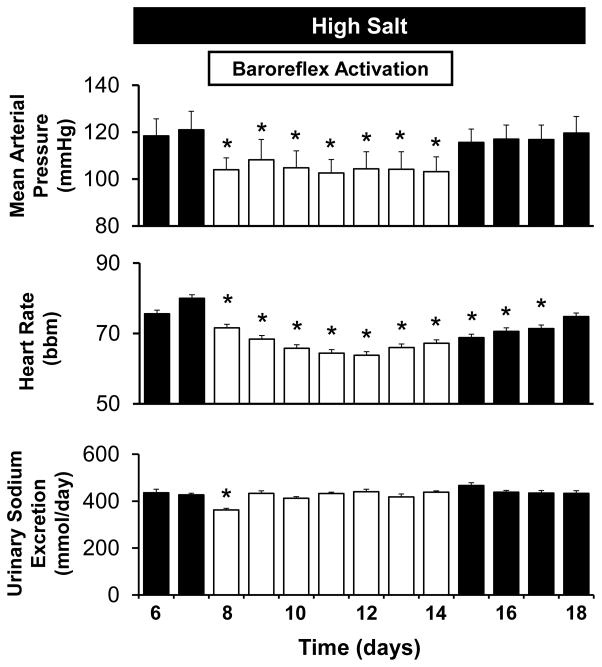
Figure 2.
Mean arterial pressure, heart rate, and sodium excretory responses to baroreflex activation (BA) in dogs with reduced kidney mass and high salt intake. *P<0.05 versus high salt.
The table summarizes several of the values determined from the blood samples. In parallel with the net retention of sodium, there was ~ a 6% increase in extracellular fluid volume (from 6502±280 to 6884±293 mL) on day 7 of HS, whereas PRA fell to undetectable levels. There were no other hormonal changes. Similarly, during HS there were no significant day 7 changes in plasma concentrations of potassium, protein, sodium (NS control=148±1 mmol/L; data not shown) or hematocrit.
Table.
Hormonal and Humoral Responses to high Salt Intake and Baroreflex Activation
| CONDITION | PRA (ng ANG I/mL/hr | PALDO (ng/dL) | PCORT (μg/dL) | HCT | PProt (g/dL) | PK (mmol/L) | Na Iothal Space (mL) |
|---|---|---|---|---|---|---|---|
| CONTROL | 0.62±0.28 | 2.0±0.2 | 1.7±0.2 | 33±2 | 7.3±0.3 | 4.7±0.2 | 6502±280 |
| HIGH-SALT | 0* | 1.6±0.1 | 1.9±0.2 | 32±1 | 7.0±0.3 | 4.6±0.2 | 6884±293* |
| HIGH SALT+BA | 0* | 1.8±0.3 | 1.8±0.1 | 28±2* | 6.6±0.4* | 4.8±0.3 | 7042±351* |
| HIGH SALT | 0* | 1.7±0.1 | 1.1±0.1 | 30±1 | 6.9±0.3 | 4.6±0.3 | 6764±396 |
| RECOVERY | 0.82±0.53 | 2.2±0.4 | 1.5±0.3 | 33±2 | 7.2±0.4 | 4.3±0.2 | 6232±273 |
Values are mean±SE: n=5. PRA indicates plasma renin activity; PALDO plasma aldosterone concentration; PCORT, plasma cortisol concentration; HCT, hematocrit; PProt, plasma protein concentration, PK, plasma potassium concentration Na Iothal Space, sodium iothalamate space. BA, baroreflex activation.
P<0.05 vs control.
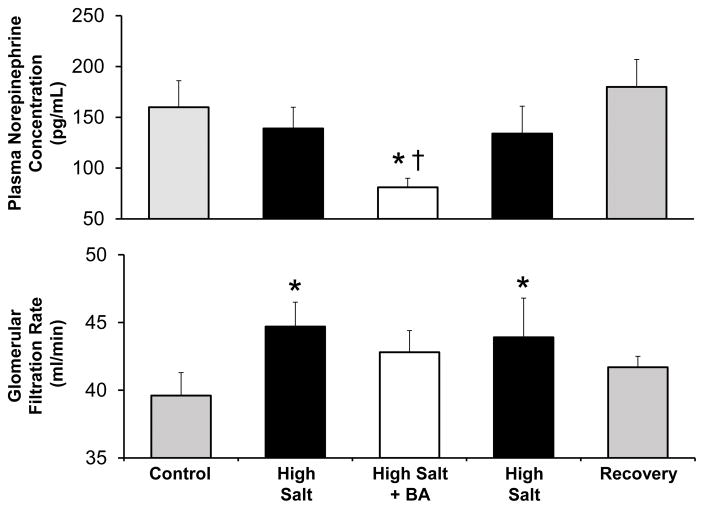
Figure 3 illustrates the changes in GFR and plasma NE concentration in this study. During NS GFR was 39.6±1.7 mL/min or ~ 60% of the value we have reported in dogs with normal kidneys of comparable body weight.33 This indicates considerable adaptive hyperfiltration during the postoperative period in remnant nephrons following the 70% surgical reduction in renal mass. Notwithstanding this compensatory hyperfiltration in remnant nephrons, there was still a further increase (~ 13% ) in GFR during HS. There were no significant changes in plasma NE concentration during HS.
Figure 3.
Changes in plasma norepinephrine concentration and glomerular filtration rate in dogs with reduced kidney mass in response to high salt intake with and without baroreflex activation (BA). *P<0.05 versus control; †.P<0.05 versus high salt.
Responses to BA in Dogs with Reduced Kidney Mass, Salt-Induced Hypertension
Remarkably, during the first day of BA MAP decreased 18±4 mmHg to NS control levels and remained at this level for the duration of the 7days of BA (Figure 2). That is, BA completely abolished the salt-induced hypertension (Figure 1). In addition to this pronounced antihypertensive response, there was appreciable bradycardia during BA (Figure 2). Both MAP and heart rate returned to pre-activation levels during the 4 day recovery period from BA. During the initial fall in MAP on day 1 of BA, there was modest sodium retention (55±13 mmol) before sodium balance was restored on subsequent days (Figure 2). Most of the sodium retained during BA was excreted during the subsequent 4 day recovery period. There was minimal potassium retention on day 1 of BA (~ 6 mmol/day) and an equal net increase in potassium excretion on day 1 of the HS-BA recovery period. Otherwise, there were no other significant changes in potassium excretion during BA.
Concomitant with the modest retention of sodium on day 1, there tended to be a further small increase in extracellular fluid volume during BA, but this did not achieve statistical significance (Table). However, the modest sodium and fluid retention that occurred during BA did lead to statistically significant lower levels of plasma protein concentration and hematocrit when compared to NS but not to HS values. During the 4 day recovery period from BA all values presented in the Table were comparable to HS levels before BA. In addition, there were no further significant changes in hormone levels during BA.
Most notably, the pronounced antihypertensive response to BA occurred in parallel with marked suppression of plasma NE concentration to below control (Figure 3). Furthermore, plasma NE concentration returned to pre-activation levels along with MAP after cessation of BA. Despite blood pressure lowering and abolition of salt-induced hypertension, there was no significant change in GFR from HS levels during BA.
Recovery from High Salt Intake
When salt intake was returned to normal levels, much of the retained sodium (120± 12 mmol) was excreted on day 1 of the NS recovery period before daily sodium balance was achieved on subsequent days. During the 6 day NS recovery period, MAP decreased gradually to normotensive levels. On the 6th and last day of the NS recovery period, MAP and heart rate were 101±3 mmHg and 81 bpm, respectively. Similarly, there were no significant differences in any measured variable when comparing day 6 NS recovery and initial NS values (Table and Figures 2 and 3).
Discussion
The sympathetic nervous system is activated in many patients with RHT and CKD,23–27 and CKD is common in RHT.1,9–14 Despite the likely benefit from suppression of sympathetic activity in patients with RHT and coexistent CKD, these subjects have been largely excluded from clinical trials of device-based therapies using BA and renal nerve ablation to provide global and renal-specific sympathoinhibition. Thus, along with our previous observations in dogs with obesity and aldosterone-induced hypertension,31,33 the current findings provide greater insight into the conditions often present in RHT that likely impact the antihypertensive and renal responses to global sympathoinhibition by BA. Most importantly, the present study in dogs with reduced renal mass and HS shows that BA chronically suppresses sympathetic activity and abolishes the salt-induced hypertension without further impairing GFR. Thus, the BA-mediated responses appear favorable for hypertension therapy in patients with RHT and co-existent kidney disease.
Increased sympathetic activity is commonly associated with both RHT and CKD23–27 and is thought to increase arterial pressure chronically by impairing renal excretory function.38–39 This sympathetically-mediated impairment of renal excretory function is normally achieved by promoting sodium reabsorption directly and indirectly through stimulation of the renin-angiotensin-aldosterone system.38,39 In this regard, our previous studies during suppression of sympathetic activity by BA have emphasized the importance of neurally-mediated inhibition of renin secretion in counteracting pressure-dependent renin release and permitting chronic lowering of arterial pressure.38 However, as the renin-angiotensin system is suppressed during HS, we reasoned that attenuation of the direct effects of neurally-mediated tubular sodium reabsorption may be sufficient to reduce arterial pressure during baroreflex-induced sympathoinhibition.
Acute reductions in arterial pressure during inhibition of sympathetic activity are achieved by decreasing vascular resistance and cardiac output and increasing vascular capacitance. In the present study, the acute fall in arterial pressure in response to these hemodynamic events likely accounted for the modest sodium retention on day 1 of BA, which compared quantitatively to the initial sodium excretory response to BA in normotensive dogs.28–30,32,33 However, the subsequent unabated fall in arterial pressure to control levels throughout the 7 days of BA, with the maintenance of sodium balance, indicates persistent effects of BA to increase renal excretory function. Had BA not had powerful sustained effects to promote sodium excretion, initial reductions in arterial pressure would have been markedly diminished,35,38,39 such as when the natriuretic effects of BA are opposed by inappropriately high plasma levels of either ANG II or aldosterone during chronic infusion of these potent antinatriuretic hormones.29,33,38 Thus, an important finding in this study was that BA abolished the salt-sensitive hypertension associated with a marked reduction in functional nephrons. This response occurred concurrently with a striking fall in plasma NE concentration to below control levels, consistent with previous observations that the chronic antihypertensive response to BA is associated with marked suppression of central sympathetic outflow.30
Given that arterial pressure is usually not adequately controlled in patients with hypertension and CKD or in patients with RHT and the many reports showing that sympathetic hyperactivity is often an associated finding in these conditions,24–27 the intensity of sympathetic suppression during antihypertensive therapy may be of paramount importance in reducing the severity of hypertension and the attendant cardiovascular risk and progression of renal dysfunction in patients with RHT and coexistent CKD. To this point, while lowering of sympathetic activity may occur to some extent with combination drug treatment that includes inhibitors of the renin-angiotensin system, complete elimination of heightened sympathetic activity often does not occur in patients with primary hypertension or CKD.24,25,40,41 In fact, some antihypertensive drugs commonly used in the treatment of RHT, such the dihydropyridine calcium channel blocker amlodipine and the thiazide diuretic hydrochlorothiazide, may stimulate sympathetic activity, a response that would be expected to partially counteract their blood pressure lowering effect.32,40,41 In contrast, experimental studies in dogs show that the lowering of arterial pressure during chronic BA is associated with powerful sustained effects to suppress central sympathetic outflow, regardless of baseline levels of sympathetic activity.28–33 Furthermore, when used in combination with antihypertensive drugs such as amlodipine that increase sympathetic activity, BA lowers arterial pressure further while completely counteracting the drug-induced sympathetic activation.32 Thus, based on its ability to suppress sympathetic activity and chronically lower arterial pressure in several experimental models of hypertension,29,31,33,38 including the reduced renal mass model of salt-induced hypertension reported in the present study, BA may provide a unique approach for therapy in patients with RHT and coexisting CKD.
Another major goal of this study was to determine the changes in GFR in response to BA in dogs with substantial loss of nephrons and salt-induced hypertension. We hypothesized that there may be further, possibly excessive, reductions in GFR associated with BA-induced reductions in arterial pressure. This concern was based on reports that renal autoregulation is impaired in patients with primary hypertension and CKD34 as well as in animals with reduced renal mass17,18 and our previous observations in obese dogs with hyperfiltering nephrons.31 In obese dogs, BA not only abolished sympathetically-driven increases in renin secretion and arterial pressure, but the attendant sympathoinhibition also markedly diminished the elevated rate of sodium reabsorption and the pronounced glomerular hyperfiltration (35% above control) associated with weight gain.31
Since the normal steady-state GFR autoregulatory response to reduced arterial pressure is dependent on both tubuloglomerular feedback (TGF) mediated vasodilation of preglomerular vessels and ANG II mediated constriction of efferent arterioles, we have previously suggested that the diminished hyperfiltration in obese dogs during BA-mediated sympathoinhibition might be attributed to the reduced effects of ANG II on the efferent arterioles as well as TGF-mediated constriction of afferent arterioles evoked by increases in sodium chloride delivery to the macula densa.31 However, we surmised that this mechanism may be less likely to reduce GFR in the hyperfiltering remnant nephrons in the present study because HS decreases the responsiveness of TGF,42,43 thus permitting a compensatory increase in GFR, as reflected by the increase in GFR during HS in the present study. Therefore, inhibition of neurally-mediated sodium reabsorption by BA may not further increase the TGF signal sufficiently to lead to constriction of the afferent arteriole in remnant nephrons exposed to HS. Regardless, given reports that HS blunts TGF and that renal autoregulation is impaired in remnant nephrons, an important finding in this study was that significant reductions in GFR did not occur in remnant nephrons exposed to HS during BA despite pronounced suppression of sympathetic activity and abolition of the salt-induced hypertension.
But are these findings relevant to the heterogeneous population of patients with RHT in whom multiple disease processes and target organ damage contribute to treatment resistance? At the present time, there are preliminary clinical findings that do support the clinical relevance of the current findings and suggest that BA may have efficacy for blood pressure reduction and preservation of renal function in patients with RHT.
As noted above, despite the frequent association of CKD with RHT and the likely contribution of progressive renal damage to therapy resistance, few clinical studies have explored the renal responses to BA and the impact of more advanced CKD on blood pressure lowering during BA. In most cases, only patients with either normal or mildly reduced kidney function have been included in clinical trials. In an early feasibility trial in 22 patients with RHT, Scheffers et al. reported a small increase in serum creatinine concentration along with a reduction in systolic blood pressure of 30 mmHg after 1 year of continuous BA.44 In the phase III randomized Rheos Pivotal Trial in 322 subjects with RHT, most with little or no reduction in kidney function (stage 1 and 2 CKD), Alnima et al. reported a mild, nonprogressive decrease in eGFR during 12 months of BA therapy that reduced systolic blood pressure ~ 25 mmHg.8 Of relevance to the current study, there was no significant fall in eGFR during BA in the few subjects (27) with more advanced, moderately reduced kidney function (stage 3 CKD). Consistent with the findings in this small cohort, a recent prospective trial in 23 patients with RHT and more advanced CKD (stage ≥3) found that eGFR was unchanged after 6 months of BA that lowered systolic blood pressure ~ 17 mmHg.45 In contrast, in subjects with CKD refusing BA, eGFR decreased significantly during a 6 months follow up period. Furthermore, in the subjects given BA therapy, there was a significant decline in albuminuria, a response that was correlated with the reduction in arterial pressure. Taken together, these preliminary findings during chronic BA in patients with long standing hypertension and variable comorbidities, along with the more acute observations in the homogenous model of impaired renal function in the present study, suggest that BA may provide long-term renal stability and protection by virtue its ability to chronically suppress central sympathetic outflow and lower intraglomerular pressure by achieving optimal blood pressure control.
Technical Considerations
Despite the solid findings and the potential clinical relevance of this proof-of-concept study, there is a technical issue that may impact future trials using carotid sinus stimulation for hypertension therapy in patients with RHT and co-existent renal disease. The first-generation system used in the present study consisting of tripolar electrodes implanted bilaterally (Rheos system) is no longer available for either experimental or clinical investigation. As cogently discussed,4 patients currently receive only unilateral carotid sinus implants with an electrode of a different design (Barostim Neo). While recent findings using this second-generation system in patients with RHT do show blood pressure reductions with minimal or no side effects,46–48 unlike its predecessor, the Barostim Neo electrode system has only been tested in uncontrolled clinical trials.4–5 Notably, a recent acute study in patients employing 2 min periods of unilateral carotid sinus activation suggested that the new electrode design may diminish efficacy and tolerability.49 However, this has not been our experience when using the Barostim Neo system during chronic bilateral carotid sinus activation in canines.33,50 Randomized controlled trials are needed to thoroughly evaluate whether the Barostim Neo electrode system may have clinical utility in managing RHT.
Perspectives
Treatment of patients with resistant hypertension whose blood pressure is not adequately controlled by the recommended three-drug regimen is challenging. Nonetheless, adding spironolactone to their antihypertensive medication has had favorable results in a significant number of subjects. However, the use of spironolactone is limited in some patients by adverse effects, including worsening renal function and hyperkalemia, particularly in subjects with advanced kidney disease, which is common in resistant hypertension. While device-based therapy may be a treatment option in these patients, the safety and efficacy of BA and renal nerve ablation in patients with RHT and CKD are unclear as these subjects have been largely excluded from clinical trials. To this end, the current study shows that through its ability to chronically suppress sympathetic activity, BA abolishes the salt-sensitive hypertension associated with loss of functioning nephrons, a response critical in reducing the progression of renal function. Furthermore, during BA there are no serious reductions in GFR, despite pronounced blood pressure lowering. Thus, these findings are consistent with the preliminary clinical findings discussed above showing sustained blood pressure lowering and nephroprotection during BA in patients with CKD and RHT exhibiting multiple disease processes and multiple forms of renal parenchymal disease. Nonetheless, controlled prospective trials with stratification of baseline GFR on blood pressure and renal responses to BA are needed to determine whether adding BA to ineffective antihypertensive regimens may be a viable treatment option in patients with RHT and CKD.
Supplementary Material
Novelty and Significance.
What is new?
We found in a canine model of CKD produced by surgical reduction of kidney mass that chronic BA has sustained effects to suppresses sympathetic activity and abolish salt-sensitive hypertension.
During BA there is little or no change in GFR despite pronounced lowering of arterial pressure.
What is relevant?
Blood pressure is not adequately controlled in patients with RHT and coexistent CKD, and the hypertension is exacerbated by the prevalence of HS.
Sympathetic activity is commonly increased in both patients with RHT and CKD.
CKD is a present in many patients with RHT, but patients with significant impairment in renal function have been largely excluded from clinical trials using device-based therapies that lower arterial pressure by reducing sympathetic activity.
Summary
By achieving optimal blood pressure control, the findings in this study support the possibility that global suppression of sympathetic activity by BA may have clinical benefit in the treatment of patients with RHT and coexistent CKD.
Acknowledgments
We thank Radu Iliescu for his critical review of our manuscript and John S. Clemmer for construction of the graphs.
Footnotes
Disclosures
Thomas E. Lohmeier: Consultant fees, Scientific Advisory Board, CVRx, Inc.
Eric D. Irwin: Consultant fees, Scientific Advisory Board, CVRx, Inc.
Sources of Funding
National Heart, Lung, and Blood Institute Grant HL-51971.
References
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;117:e510–526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.White WB, Turner JR, Sica D, Bisognano JD, Calhoun DA, Townsend RR, Aronow HD, Bhatt DL, Bakris GL. Detection, evaluation, and treatment of severe and resistant hypertension. Proceedings from an American Society of Hypertension Interactive Forum held in Bethesda, MD, USA, October 10th 2013. J Am Soc Hypertens. 2014;8:743–757. doi: 10.1016/j.jash.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation: mechanisms and potential for hypertension therapy. Hypertension. 2011;57:880–886. doi: 10.1161/HYPERTENSIONAHA.108.119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chobanyan-Jürgens K, Jordan J. Electrical carotid sinus stimulation: changes and challenges in the management of treatment resistant hypertension. Curr Hypertens Rep. 2015;17:71–77. doi: 10.1007/s11906-015-0587-4. [DOI] [PubMed] [Google Scholar]
- 5.Jordan J, Grassi G, Tank J. Device-based treatments in hypertension: think physiology. J Hypertens. 2016;8:00–00. doi: 10.1097/HJH.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 6.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the SYMPLICITY HTN-2 trial): a randomized controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 8.Alnima T, de Leeuw PW, Tan FES, Kroon AA. Renal responses to long-term carotid baroreflex activation therapy in patients with drug-resistant hypertension. Hypertension. 2013;61:1334–1339. doi: 10.1161/HYPERTENSIONAHA.113.01159. [DOI] [PubMed] [Google Scholar]
- 9.Salles GF, Cardoso RL, Pereira VS, Fiszman R, Muxfeld ES. Prognostic significance of a reduced glomerular filtration rate and interaction with microalbuminuria in resistant hypertension: a cohort study. J Hypertens. 2011;29:2014–2023. doi: 10.1097/HJH.0b013e32834adb09. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O’Conner PJ, Selby JV, Ho PM. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutiérrez OM, Irvin MR, Lackland DT, Oparil S, Warnock D, Muntner P. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8:1583–1590. doi: 10.2215/CJN.00550113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muxfeldt ES, de Souza F, Margallo VS, Salles GF. Cardiovascular and renal complications in patients with resistant hypertension. Curr Hypertens Rep. 2014;16:471–482. doi: 10.1007/s11906-014-0471-7. [DOI] [PubMed] [Google Scholar]
- 13.Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, Goldsmith D, Heine GH, Jagger KJ, Kanbay M, Mallamaci F, Ortiz A, Vanholder R, Wiecek A, Zoccali C, London GM, Stengel B, Fouque D. The double challenge of resistant hypertension and chronic renal disease. Lancet. 2015;386:1588–1598. doi: 10.1016/S0140-6736(15)00418-3. [DOI] [PubMed] [Google Scholar]
- 14.Thomas G, Xie D, Chen H-Y, Anderson AH, Appel LJ, Bodana S, Brecklin CS, Drawz P, Flack JM, Miller ER, III, Steigerwalt SP, Townsend RR, Weir MR, Wright JT, Jr, Raham M CRIC Study Investigators. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease. Hypertension. 2016;67:387–396. doi: 10.1161/HYPERTENSIONAHA.115.06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner BM. Nephron adaption to renal injury or ablation. Am J Physiol Renal Fluid Electrolyte Physiol. 1985;249:F324–F503337. doi: 10.1152/ajprenal.1985.249.3.F324. [DOI] [PubMed] [Google Scholar]
- 16.Hostetter TH. Hyperfiltration and glomerulosclerosis. Seminars in Nephrol. 2003;23:194–199. doi: 10.1053/anep.2003.50017. [DOI] [PubMed] [Google Scholar]
- 17.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- 18.Brown SA, Finco DR, Navar LG. Impaired renal autoregulatory ability in dogs with reduced renal mass. J Am Soc Nephrol. 1995;5:1768–1774. doi: 10.1681/ASN.V5101768. [DOI] [PubMed] [Google Scholar]
- 19.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage. Implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 20.Coleman TG, Guyton AC. Hypertension caused by salt loading in the dog. III. Onset transients of cardiac output and other circulatory variables. Cir Res. 1969;25:153–160. doi: 10.1161/01.res.25.2.153. [DOI] [PubMed] [Google Scholar]
- 21.Hall JE, Mizelle HL, Brands MW, Hildebrandt DA. Pressure natriuresis and angiotensin II in reduced kidney mass, salt-induced hypertension. Am J Physiol Regulatory Integrative Comp Physiol. 1992;262:R61–R71. doi: 10.1152/ajpregu.1992.262.1.R61. [DOI] [PubMed] [Google Scholar]
- 22.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension. Results from a randomized trial. Hypertension. 2009;54:475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Converse RL, Jacobsen TN, Toto RD, Jost CM, Cosentino CM, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 24.Koomans HA, Blankestijn PJ, Joles JJ. Sympathetic hyperactivity in chronic renal failure: a wake-up call. J Am Soc Nephrol. 2004;15:524–537. doi: 10.1097/01.asn.0000113320.57127.b9. [DOI] [PubMed] [Google Scholar]
- 25.Neumann J, Ligtenberg G, Klein HT, Boer P, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in hypertensive chronic kidney disease is reduced during standard treatment. Hypertension. 2007;49:506–510. doi: 10.1161/01.HYP.0000256530.39695.a3. [DOI] [PubMed] [Google Scholar]
- 26.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell’Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–851. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- 27.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohmeier TE, Irwin ED, Rossing MA, Sedar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 29.Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension. 2005;46:1194–1200. doi: 10.1161/01.HYP.0000187011.44201.2e. [DOI] [PubMed] [Google Scholar]
- 30.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol. 2010;299:H402–H409. doi: 10.1152/ajpheart.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. doi: 10.1161/HYPERTENSIONAHA.111.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iliescu R, Irwin ED, Georgakopoulos D, Lohmeier TE. Renal responses to chronic suppression of central sympathetic outflow. Hypertension. 2012;60:749–756. doi: 10.1161/HYPERTENSIONAHA.112.193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohmeier TE, Liu B, Hildebrandt DA, Cates AW, Georgakopoulos D, Irwin ED. Global- and renal-specific sympathoinhibition in aldosterone hypertension. Hypertension. 2015;65:1223–1230. doi: 10.1161/HYPERTENSIONAHA.115.05155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med. 2002;347:1256–1261. doi: 10.1056/NEJMra020676. [DOI] [PubMed] [Google Scholar]
- 35.Guyton AC. Circulatory Physiology III. Philadelphia, PA: W.B. Saunders; 1980. Arterial pressure and hypertension. [Google Scholar]
- 36.Guyton AC. Renal function curve—a key to understanding the pathogenesis of hypertension. Hypertension. 1987;10:1–6. doi: 10.1161/01.hyp.10.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Hildebrandt DA, Irwin ED, Cates AW, Lohmeier TE. Regulation of renin secretion and arterial pressure during prolonged baroreflex activation. Influence of salt intake. Hypertension. 2014;64:604–609. doi: 10.1161/HYPERTENSIONAHA.114.03788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohmeier TE, Iliescu R. Lowering of blood pressure by chronic suppression of cental sympathetic outflow. J Appl Physiol. 2012;113:1652–1658. doi: 10.1152/japplphysiol.00552.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iliescu R, Lohmeier TE, Tudorancea I, Laffin L, Bakris GL. Renal denervation for the treatment of resistant hypertension: review and clinical perspective. Am J Physiol Renal Fluid Electrolyte Physiol. 2015;309:F583–F594. doi: 10.1152/ajprenal.00246.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grassi G. Sympathomodulatory effects of antihypertensive drug treatment. Am J Hypertens. 2016;29:665–675. doi: 10.1093/ajh/hpw012. [DOI] [PubMed] [Google Scholar]
- 41.Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy. A potential mechanism for long-term morbidity? Hypertension. 2005;45:513–521. doi: 10.1161/01.HYP.0000158312.63381.c1. [DOI] [PubMed] [Google Scholar]
- 42.Welch WJ, Wilcox CS. Role of nitric oxide in tubuloglomerular feedback: effects of dietary salt. Clin Exp Pharmacol Physiol. 1997;24:582–586. doi: 10.1111/j.1440-1681.1997.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PBL, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R. Macula densa nitric oxide synthase 1β protects against salt-sensitive hypertension. J Am Soc Nephrol. 2015;27:00–00. doi: 10.1681/ASN.2015050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheffers IJM, Kroon AA, Schmidli J, Jordan J, Tordoir JJM, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Philipp T, de Leeuw PW. Novel baroreflex activation therapy in resistant hypertension. J Am Coll Cardiol. 2010;56:1254–1258. doi: 10.1016/j.jacc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 45.Wallbach M, Lehnig L-Y, Schroer C, Hasenfuss G, Muller GA, Wachter R, Koziolek MJ. Impact of baroreflex activation therapy on renal function—a pilot study. Nephrology. 2014;40:371–380. doi: 10.1159/000368723. [DOI] [PubMed] [Google Scholar]
- 46.Hoppe UC, Brandt M-C, Wachter R, Beige J, Rump LC, Kroon AA, Cates AW, Lovett EG, Haller H. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens. 2012;6:270–276. doi: 10.1016/j.jash.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Halbach M, Hickethier T, Madershahenian N, Reuter H, Brandt MC, Hoppe UC, Müller-Ehmsen J. Acute on/off effects and chronic blood pressure reduction after long-term baroreflex activation therapy in resistant hypertension. J Hypertens. 2015;33:1696–1703. doi: 10.1097/HJH.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 48.Wallbach M, Lehnig L-Y, Schroer C, Lüders S, Böhning E, Müller GA, Wachter R, Koziolek MJ. Effects of baroreflex activation therapy on ambulatory blood pressure in paptients with resistant hypertension. Hypertension. 2016;67:701–709. doi: 10.1161/HYPERTENSIONAHA.115.06717. [DOI] [PubMed] [Google Scholar]
- 49.Heusser K, Tank J, Brinkmann J, Menne J, Kaufeld J, Linnenweber-Held S, Beige J, Wilhelmi M, Diedrich A, Haller H, Jordan J. Acute response to unilateral unipolar electrical carotid sinus stimulation in patients with resistant arterial hypertension. Hypertension. 2016;67:585–591. doi: 10.1161/HYPERTENSIONAHA.115.06486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lohmeier TE, Iliescu R, Tudorancea I, Cazan R, Cates AW, Georgakopoulos D, Irwin ED. Chronic interactions between carotid baroreceptors and chemoreceptors in obesity hypertension. Hypertension. 2016;68:227–235. doi: 10.1161/HYPERTENSIONAHA.116.07232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.