Abstract
After lesions of the somatosensory dorsal column (DC) pathway, the cortical hand representation can become unresponsive to tactile stimuli, but considerable responsiveness returns over weeks of post-lesion recovery. The reactivation suggests that preserved subthreshold sensory inputs become potentiated and axon sprouting occurs over time to mediate recovery. Here, we studied the recovery process in 3 squirrel monkeys, using high-resolution fMRI CBV-fMRI mapping of contralateral somatosensory cortex responsiveness to stimulation of distal finger pads with low and high level electrocutaneous stimulation (ES) before and 2, 4, and 6 weeks after a high cervical level contralateral DC lesion. Both low and high intensity ES of digits revealed the expected somatotopy of the area 3b hand representation in pre-lesion monkeys, while in areas 1 and 3a, high intensity stimulation was more effective in activating somatotopic patterns. Six weeks post-lesion, and irrespective of the severity of loss of direct DC inputs (98%, 79%, 40%), somatosensory cortical area 3b of all three animals showed near complete recovery in terms of somatotopy and responsiveness to low and high intensity ES. However there was significant variability in the patterns and amplitudes of reactivation of individual digit territories within and between animals, reflecting differences in the degree of permanent and/or transient silencing of primary DC and secondary inputs 2 weeks post-lesion, and their spatio-temporal trajectories of recovery between 2 and 6 weeks. Similar variations in the silencing and recovery of somatotopy and responsiveness to high intensity ES in areas 3a and 1 are consistent with inter-individual differences in collateral damage to and recovery of secondary (e.g. spinothalamic) pathways. Thus, cortical deactivation and subsequent reactivation depends not only on the degree of DC lesion, but also on the severity and duration of loss of secondary as well as primary inputs revealed by low and high intensity ES.
Keywords: CBV, MION, somatosensory cortex, ascending spinal pathways, spinal cord injury
INTRODUCTION
The dorsal column of the spinal cord that terminates in the cuneate nucleus is the major activation pathway of the hand representations in primary somatosensory cortex (area 3b), the adjoining representation area 1, and the tactile component of areas 3a and 2. Thus, when the dorsal columns (DC) are cut at high cervical levels (above the level where afferents from the hand enter the spinal cord), the hand region in area 3b and other somatosensory cortical areas become unresponsive to touch on the hand. However, over weeks to months of recovery in monkeys with spinal cord lesions, much of the hand representation in area 3b, and other cortical areas, becomes responsive to touch on the hand, and the pattern of reactivation in cortex preserves aspects of the normal somatotopic order (Bowes et al., 2013; Chen et al., 2012a; Jain et al., 1997; Qi et al., 2011). Some of this reactivation may originate from those few primary afferents that often survive such spinal cord lesions. However the spared afferents of the second order spinal cord neurons that normally provide sub-threshold or modulatory inputs to the cuneate nucleus may also become an effective source of cortical reactivation (Liao et al., 2015). The importance of these secondary inputs may increase with increasing severity of primary deafferentation. In addition, the relative contribution of these two components of cortical reactivation, and their relation to functional recovery, remain less well understood, in large part because of the challenges and constraints imposed on longitudinal studies within individual animals by the invasive nature of electrophysiological mapping studies (Jain et al., 1997; Merzenich et al., 1983a; Merzenich et al., 1983b). Progress in high resolution functional MRI mapping now permits non-invasive longitudinal studies of cortical activation at high spatial resolution, with submillimeter coregistration of functional maps across sessions (Chen et al., 2012a; Corbetta et al., 2002; Frey et al., 2008; Lecoeur et al., 2011b; Lecoeur et al., 2011a; Makin et al., 2015a; Makin et al., 2015b; Moore et al., 2000; Yang et al., 2014; Zhang et al., 2010). Furthermore, by comparing cortical activation by low with high threshold pathways via low and high levels of tactile or electrocutaneous stimulation (ES), the relative contributions of dorsal and other pathways to local cortical innervation can be characterized (Dutta et al., 2014; Wang et al., 2012; Zhang et al., 2007). By combining these approaches, the spatiotemporal patterns of cortical reactivation as well as the relative contributions of reactivation of primary (low threshold dorsal column) and expansion of secondary (high threshold spinothalamic) inputs to cortical reactivation can be studied longitudinally in the same animal, thus controlling for inter-animal variations in somatotopy, and lesion severity and location (Chen et al., 2012a; Yang et al., 2014).
Here we exploit differences in the fMRI response properties of cortical areas to dorsal column vs. spinothalamic inputs to track and characterize the afferents that drive cortical reactivation following varying degrees of loss of DC afferents. Thus, while tactile stimulation of the hand effectively activates low-threshold cutaneous afferents from the hand, contributions of higher threshold afferents from the hand and those of subsequent second and perhaps higher level afferents in the spinal cord and brainstem may not be revealed. Previous studies of blood-oxygen-level dependent (BOLD) contrast imaging have shown that nociceptive stimulation in humans and non-human primates can activate area 3b, as well as higher order areas of somatosensory cortex (Zhang et al., 2007), most likely via the spinothalamic pathway (Bingel et al., 2004; Chen et al., 2011; Chen et al., 2012b; DaSilva et al., 2002). Thus, pathways dependent on more intensive and even nociceptive stimuli could contribute to cortical reactivations after DC lesions. ES levels can be adjusted to largely activate low threshold tactile afferents or higher threshold afferents as well (Wang et al., 2012). Co-activation of both sets of afferents may also more effectively activate higher order spinal cord and other sensory pathways.
To monitor the spatiotemporal evolution of cortical responses resulting from sensory loss and the origins of subsequent cortical reactivation, we collected high-resolution cerebral blood volume (CBV)-based functional maps of somatosensory cortex from three squirrel monkeys before and three times after a lesion of the contralateral dorsal columns at a mid-cervical level. To examine the potential contributions of low and high threshold sensory pathways in cortical activations and reactivation, we applied low and high ES to the distal finger pads of digits 1–3 during imaging sessions in anesthetized monkeys. We reasoned that the low intensity innocuous ES would selectively activate pre-lesion and spared post-lesion low threshold primary Aβ afferents, and their secondary spinal cord neuronal targets, while high intensity nociceptive ES would recruit higher threshold Aδ and C-fibers and would more strongly activate lower threshold afferents and subsequent second order spinal cord relays. To gain insights into the roles of low and high threshold inputs to cortical reactivation, our study had three goals: first, to determine the spatial trajectories of recovery of cortical responsiveness to low and high intensity ES of digits 1–3 before, and 2, 4, and 6 weeks after a DC lesion; second, to compare temporal trajectories of activation response magnitudes in cortical areas 3b, 3a, and 1 at low and high levels of stimulation; third, to compare the somatotopic organization revealed by ES fMRI after 6 weeks of recovery to that obtained from microelectrode mapping using tactile stimulation.
As expected, high level ES to the digits effectively activated contralateral somatosensory cortex before and after a large unilateral lesion of the dorsal column at a high cervical level. Consistent with previous results, the larger, nearly complete lesion most effectively deactivated the contralateral hand region in area 3b and adjoining areas shortly after the lesion, while less complete lesions were less effective. Subsequent microelectrode mapping clearly matched those of the final imaging session. The results suggest that preserved DC afferents from the hand are important in maintenance and recovery of activation of cortex, and that second order spinal cord pathways likely play a role in reactivation after nearly complete lesions. In addition, the results reveal the usefulness of fMRI and electrocutaneous stimulation of the skin in evaluating the recovery process in primates with spinal cord injury.
MATERIALS AND METHODS
Animal preparation and DC lesion surgery
Three adult (4 yr old) male New World squirrel monkeys (Saimiri boliviensis) were used in this study. The somatosensory cortex in these three subjects was scanned via high-resolution functional magnetic resonance imaging (fMRI) before, and 2, 4, and 6 weeks after a DC lesion. During the MRI scans, each monkey was anesthetized and mechanically ventilated, with isoflurane (0.5–1.1%) delivered by a mixture of N2O/O2 in a 70:30 ratio. The head and body were stabilized in an MRI compatible frame. Vital signs were monitored and maintained throughout the imaging sessions.
After pre-lesion imaging data were collected, all animals underwent a unilateral dorsal column lesion at cervical level C4-C5 in the spinal cord, that possibly spares some of the primary afferent inputs from digit 1 and even digit 2 (Florence et al., 1991; Qi et al., 2011). A DC lesion was made under aseptic conditions and general anesthesia. The animal was intubated for ventilation, skin incision was made in the back of the neck, and muscles were separated to expose the dorsal vertebrae near cervical level C4-C5. A small opening was made on the dorsal arch of the vertebrae by first slicing the superficial connective tissue between the two vertebrae outside of the spinal cord, then carefully removing small pieces of bone on the dorsal arch of 1 segment using rongeurs. The dura and pia covering the cervical spinal cord were resected. Unilateral dorsal columns were sectioned on the right side of the spinal cord for all three monkeys with a pair of surgical iris scissors. To close, the dura was replaced by Gelfilm, and muscles were re-closed and sutured using absorbable suture. The skin was closed using surgical staples. The monkeys were carefully monitored until they fully recovered from anesthesia before they were returned to their home cage. The monkeys received antibiotics and analgesics for 3 days after surgery. All experimental procedures were approved by the Vanderbilt University Animal Care and Use Committee and followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
FMRI acquisition
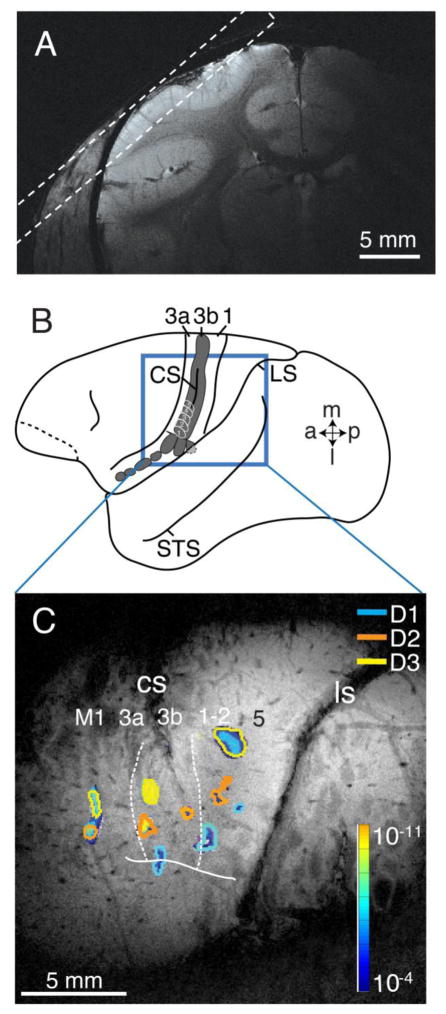
MRI scans were acquired at 9.4T (Agilent) using a 3 cm surface transmit-receive coil positioned over primary somatosensory cortex (Fig. 1).
Figure 1.
Methods used for imaging somatosensory cortex of squirrel monkeys. A. A high-resolution coronal T2**-weighted image of frontal cortex collected to guide the placement of oblique slices over anterior parietal somatosensory cortex. The dashed lines indicate the imaging plane relative to the cortical surface. B. The area of interest in the cortex of a squirrel monkey brain showing the locations of somatosensory areas 3a, 3b (shaded), and 1. The locations of the representations of digits 1–5 are outlined in white in area 3b just below the short, shallow central sulcus. The lateral sulcus and superior temporal sulcus are shown for reference. C. Cerebral blood volume (CBV) signals overlaid on a high-resolution coronal T2**-weighted image with somatosensory areal borders. The most superficial MRI slice with the orientation shown in A for squirrel monkey SM-Ge. The cerebral blood volume (CBV) signals resulted from electrocutaneous stimulation of digits 1 (blue), 2 (orange), and 3 (yellow). The strength of the evoked CBV responses is indicated from dark (weak) to light color (strong, see spectrum scale bar). The most anterior (left) activation foci are in area 3a, the middle foci are in area 3b, while the caudal (right) foci are in area 1, possibly involving area 2. The solid line indicates anatomically and electrophysiologically defined hand-face border with the face lateral and the hand medial. Abbreviations: 3a, area 3a; 3b, area 3b; 1, area 1; 2, area 2; a, anterior; CS, central sulcus; D1, D2, D3, digits 1, 2 and 3; l, lateral; LS, lateral sulcus; m, medial; M1, primary motor cortex; p, posterior; STS, superior temporal sulcus
Structural Imaging
3D whole brain isotropic images were collected (TR/TE=5/2.39 ms, 128 × 128 × 128 matrix; 0.5×0.5×0.5 mm3) and used for planning of coregistered high resolution structural and functional images. These images were also used for coregistration of imaging studies between sessions (Lecoeur et al., 2011a, 2011c). High resolution T2*-weighted gradient echo structural images (TR/TE 400/16 ms, 16 slices, 512×512 matrix; 68 × 68 × 500 μm3 resolution) were acquired as a multi-slice oblique stack positioned for maximal in-plane coverage of somato-sensory areas 3a, 3b and 1 (Fig. 1A). These images were used to identify cortical venous structures used to locate somatosensory cortex and provide structural landmarks for co-registration of fMRI maps across imaging sessions.
CBV-fMRI
Cerebral blood volume-based functional mapping used a multi-slice multi-shot gradient echo-echo planar imaging (GE-EPI) sequence (2-shot, TE/TR 10/750 ms, 128 × 128 matrix; 273×273 μm2 in-plane resolution, 2 mm thick, no gap, 4 slices). Images were collected using the same plan coordinates, and were thus intrinsically coregistered with the T2* weighted high resolution structural images. CBV-fMRI mapping studies began 10 minutes following a slow i.v. bolus of monocrystalline iron oxide nanocolloid contrast agent (MION, 12 mg/kg). Each functional imaging run consisted of 300 image frames.
Activation Protocol
During a single imaging run, seven alternating 30 s blocks of baseline and single digit electrocutaneous stimulation (ES) were delivered to digits 1, 2, or 3. ES was generated by a Grass Technologies S88 stimulator (Natus Neurology, West Warwick, RI), stimulus isolation unit (SIU5) and constant current unit (CCU1). The stimulation was delivered through gold leads and electrode gel (Spectra360, Parker Laboratories, Inc, Fairfield NJ) placed on the lateral aspects of a distal finger pad. The ES consisted of an 8 Hz train of square wave pulses each with duration 2 ms and pulse amplitude at 2 mA (low intensity) or 4 mA (high intensity). Parallel studies confirmed that low intensity stimulation engaged area 3b in a somatotopically organized manner consistent with activation of low threshold primary afferents, while the high intensity, which was close to human pain thresholds (Inui et al., 2003; Ruben et al., 2006), maximally activated areas 3a, 3b, 1 and 2 consistent with activation of both low and high threshold (primary, secondary and possibly higher order) afferents. Specifically, the response properties of areas 3a, 3b and 1 to increasing ES intensity were measured in the same animals pre-lesion. These measurements confirmed that 2 mA ES engaged only area 3b, whose CBV-fMRI response increased monotonically with increasing current, saturating at ~4 mA, while areas 3a and 1 were unresponsive to ES currents below 3 mA. Beginning at 3 mA ES, area 3a and 1 responses increased monotonically with increasing current until plateauing at 6–8 mA (Wang et al., 2012). These stimulation parameters are similar to those used in other studies in squirrel monkeys (Schroeder et al., 1995). No movement (digit twitch) was observed when digits were stimulated at either intensity under anesthesia.
FMRI Analysis
All data were analyzed in Matlab (MathWorks, Natick, MA). Functional data were not smoothed in the spatial domain. The initial 5 volumes of each run were discarded from data analysis so that only steady state signals were included. CBV time courses were drift corrected, then temporally smoothed with a low-pass filter (0.25 Hz cutoff). Activation maps were determined using voxel-wise correlation of fMRI signal with stimulus waveform (Chen et al., 2007). The area of single digit activation in different brain areas were then determined using a threshold at p < 10−4 (uncorrected for multiple comparisons) and a cluster size of k ≥ 2. CBV signal time courses from significantly activated voxels in each of the areas 3a, 3b, and 1 were extracted and averaged across runs within a session, and peak amplitudes of CBV responses to stimuli were obtained and compared.
Co-registration
Our study required longitudinal co-registration of fMRI results across sessions, as well as co-registration of the final fMRI session with microelectrode maps, and with histological tissue processed for architecture. High resolution T2*-weighted images provided the detailed vascular architecture and landmarks used for the co-registration of structural images collected across sessions, allowing anatomic co-registration to within ~50 μm between imaging sessions (Lecoeur et al., 2011b). The coordinates of those landmarks were then used in a point-based registration algorithm to superimpose the CBV activation maps (Chen et al., 2007). A point-based registration transformation between these two sets of coordinates was also applied to a picture acquired during electrophysiological mapping, allowing for an overall superposition of the CBV activation maps, electrophysiology map, and a high-resolution T2*-weighted structural image. Once the MRI-map and the blood vessel photograph used for electrophysiological map were aligned, the activation region of CBV responses could be outlined and superimposed onto the electrophysiological map.
Microelectrode mapping
To test if the CBV signals from fMRI faithfully reflect the neuronal reactivation in the cortex, we determined the topographic maps in the deprived hand representations in areas 3b and 1 by single microelectrode mapping methods. Neuronal responsiveness, receptive field properties, and somatotopic maps with a high resolution (around 300 μm) in deprived somatosensory cortex were determined 1, 2 and 3 weeks following the final fMRI mapping (i.e. 7, 8 and 9 weeks after DC lesion) for SM-BB (98% loss), SM-Ge (79% loss) and SM-A (40% loss) respectively. This allowed us to compare the results from fMRI activation and microelectrode mapping. Surgical procedures are fully described elsewhere (Jain et al., 2008; Kaas and Florence, 1997; Merzenich et al., 1978; Qi et al., 2011). In brief, each monkey was given an initial ketamine injection (10–30 mg/kg, i.m.) for sedation, followed by isoflurane anesthesia (1–2%). After reaching a deeply anesthetized level, the monkey was secured in a stereotaxic device for surgery and the duration of the experiment. The monkey was then artificially ventilated. A craniotomy and durotomy were performed to expose the forelimb regions of areas 3a, 3b, 1 and 2, and posterior parietal cortex. The opening in the skull was covered with silicon oil to prevent desiccation. The cortical surface was photographed, and the blood vessel pattern was used to guide the placement of electrode penetrations, which were marked on the photograph, and were further used for alignments of fMRI images and the microelectrode receptive field maps.
To record the responses of cortical neurons, a low impedance tungsten microelectrode (1MΩ, Microprobes, Gaithersburg, MD) was held on a stereotaxic manipulator and inserted perpendicularly into the cortex with a hydraulic micromanipulator (Narishige International USA, Inc, East Meadow, NY). Within the hand representations of areas 3b and 1, the microelectrode penetrations were placed densely in ~300 μm spacing with allowances for blood vessels. An effort was made to cover most or all of the hand representations in areas 3b and 1 during the recording session. More limited recordings were obtained from areas 3a, 2, and 5. Magnitudes of multi-neuronal responses to the tapping and manipulation of the hand, as indicated by the audio signals of the neuron firing, were constantly evaluated as the microelectrode passed through the superficial to the middle layers of cortex (up to 700 μm depth). For each penetration, we characterized neurons’ receptive field location, size, and stimulus preference to light touch, tapping, and joint movement stimuli, at the site where the strongest evoked response occurred. Neurons were classified as cutaneous if they responded to light contact on the skin or hair movement, high threshold if they required taps to the skin, and non-cutaneous or “deep” if they responded only to the manipulation of joints and muscles. The minimum receptive field was determined by the area of skin where light touches with a hand held probe activated the recorded neurons (Merzenich et al., 1978). When a receptive field was defined, it was outlined on a drawing of the hand or other relevant body part. At the end of the recording sessions, electrolytic lesions were made by passing anodal direct current (10 uA) as an electrode was withdrawn from a range of depths (i.e. 3.5 – 5 mm) in cortex to the pial surface.
These marking lesions were later used to relate the recording results to cortical architecture from histological tissue processing.
Co-registration of fMRI with single electrode receptive field mapping
FMRI activation maps evoked with Low and high intensity ES maps were intrinsically coregistered within each imaging session (pre-lesion, 2, 4, 6 weeks post-lesion). Activation maps were co-registered across sessions using automatic longitudinal registration (Lecoeur et al., 2011a, 2011c). fMRI images were then aligned with a high resolution photograph of the sensorimotor cortical areas after a craniotomy and durotomy that was used for more precise electrophysiological mapping. The sulci and blood vessels alignments between these 2 images were done without knowledge of the electrophysiological mapping results; finally, at the conclusion of the electrophysiological mapping experiment, electrolytic lesion marks were made at strategic penetration sites (usually at electrophysiological boundaries). Photomicrographs were taken from a tangentially cut cortical section processed for myelin, cytochrome oxidase (CO), or vesicular glutamate transporter 2 (vGluT2) to confirm architectural boundaries and to locate electrolytic lesion marks. As a result, MRI images were superimposed on the electrophysiological maps based on sulci and blood vessel landmarks. Thus, the foci from MRI responses were transferred onto the reconstructed electrophysiological map.
Estimates of axon sparing in cuneate nucleus
Tracer injection
To determine the levels and extents of the DC lesion, transganglionic transport tracer cholera toxin subunit B (CTB, Sigma-Aldrich Co. LLC, St. Louis, MO) was subcutaneously injected into the tips of digits 1, 3, and 5 of both hands (1% in dH2O, 5 μl at each injection site) while monkeys were anesthetized with 1–3% isoflurane. Procedures followed those described previously (Florence et al., 1991; Qi et al., 2011; Qi and Kaas, 2006). To allow for appropriate transportation time, injections were placed 4 – 6 days before terminal microelectrode mapping procedures.
Measurement of CTB labeled axon terminal fields in cuneate nucleus
In order to quantitatively measure the distribution (areal size) of CTB-labeled terminal fields in the cuneate nuclei of the brainstem, we used NIH Image J v.144 software (available as freeware from http://rsbweb.nih.gov/ij/). We measured CTB-labeled terminal fields to quantitatively estimate the effectiveness of the dorsal column lesion in each monkey. Detailed procedures are described elsewhere (Qi et al., 2011). In brief, we measured the differences in CTB labeled axon arbors in the cuneate nucleus of the brainstem on both lesioned and non-lesioned sides by determining the cross-sectional area of the labeled foci in individual brainstem sections from caudal to rostral through the nuclei. The measured foci were those areas that exceeded a threshold of optical density measured with NIH ImageJ software. All measurements were then exported to Excel software for illustration purposes, and statistical comparisons between measures of the lesion and intact sides of sections were performed in Matlab (Wilcoxon matched-pairs signed-ranks test, α = 0.05).
Tissue Processing
At the conclusion of the terminal microelectrode mapping session, the monkey was given a lethal dose of anesthetic (sodium pentobarbital), and when areflexive, perfused transcardially with phosphate buffered 0.9% saline (PBS, pH 7.4) followed by 2–4 % paraformaldehyde in PB, and then by 2–4% paraformaldehyde with 10% sucrose in PB. The brain and spinal cord were removed. Cortex was separated from subcortical structures, manually flattened, and kept flat between glass slides (see (Gharbawie et al., 2011) for progressive steps of flattening squirrel monkey cortex). The cortex, brainstem, and spinal cord were stored overnight in 30 % sucrose in PB for cryoprotection. The flattened cortex was cut parallel to the surface at 40 μm thickness on a freezing microtome, and the sections were stained for myelin (Gallyas, 1979), cytochrome oxidase (Wong-Riley, 1979), or vesicular glutamate transporter 2 (vGluT2, e.g., (Hackett and de la Mothe, 2009) to reveal the cortical areal boundaries related to area 3b, the locations of the electrolytic lesion markers, and the electrode tracks. The brainstem was sectioned in a coronal plane and the spinal cord in the horizontal plane, both at 40 μm thickness. Every fourth section of the brainstem and every other section of the spinal cord were processed with immunostaining to reveal CTB (Qi et al., 2011). Another series of brainstem sections were processed for cytochrome oxidase to reveal brainstem architecture (Qi and Kaas, 2006).
Reconstructions
Spinal cord lesion
In order to reconstruct spinal cord lesions, images of the spinal cord sections were acquired using a Nikon E800 microscope and a Nikon DXM1200 camera (Nikon Inc., Melville, NY). All horizontally cut spinal cord sections were aligned along the midline and a pinhole in the spinal cord made by a penetration perpendicular to the plane of section. The maximal extent of the lesion, the white matter, and the grey matter of each section were measured, and a coronal view of the lesion site was reconstructed from the series of sections with Adobe Illustrator software (see (Qi et al., 2011) for details).
Microelectrode mapping
Representations of the hand and adjoining parts of the face and arm in somatosensory cortex were reconstructed by relating receptive field locations to cortical recording sites transposed from their marked locations on photographs. The electrode penetrations and recording sites were then superimposed on images of histologically processed flattened cortical sections with the aid of electrolytic lesion markers, blood vessels, and sulci landmarks apparent in both the brain section and surface photograph. The known distances between microlesions were used to correct for the slight shrinkage of brain sections due to tissue processing. The borders of areas 3b and 1 were estimated from the architectonic results and physiological data. Designated locations for cortical areas are based on our best estimates, particularly for areas 1, 2, and 5 which have borders that are difficult to define architectonically.
RESULTS
The primary goal of this study was to investigate how deafferentation of somatosensory cortex from a mid-cervical spinal cord injury affects cerebral blood volume (CBV) responses to the electrocutaneous stimulation of digits of the contralateral hand. The results are presented in 3 sections: (1) the nature of the lesions; (2) CBV responses in somatosensory areas 3a, 3b, 1, 2, and 5 before and after DC lesion; and (3) neuronal receptive fields and topography characterized with microelectrode recordings, as well as the verification of the CBV activation maps with the electrophysiological maps. As the extents and levels of the lesions varied, the results are presented on a case-by-case basis starting with the monkey with the most extensive lesion.
Lesion Characteristics
Behavioral observations after DC lesion
While no quantitative behavioral assessments were made, we observed the monkeys’ general behavior patterns in their home cages for several weeks following the DC lesion. Immediately following DC lesion, monkey SM-BB (98% loss) was more reluctant to use the affected hand (ipsilateral to the DC lesion). Specific changes included: not using the affected hand to reach out and grasp food items; rarely using the affected hand to hold onto the cage wall; less frequent use of the affected hand for walking and climbing. Within a few weeks these alterations in home cage activity had largely resolved and were not obvious. In contrast, the other 2 monkeys (SM-Ge, SM-A) showed only minimal impairments of regular home cage behaviors after a week of recovery from DC lesion.
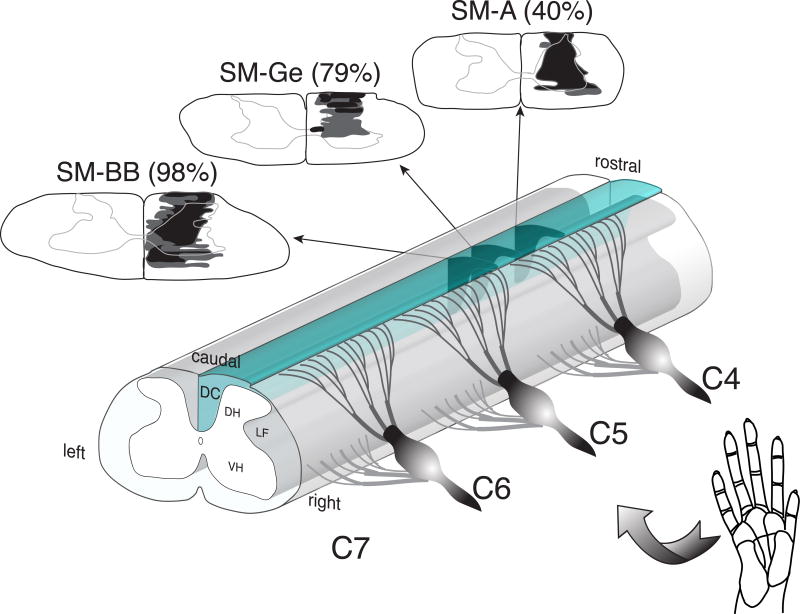
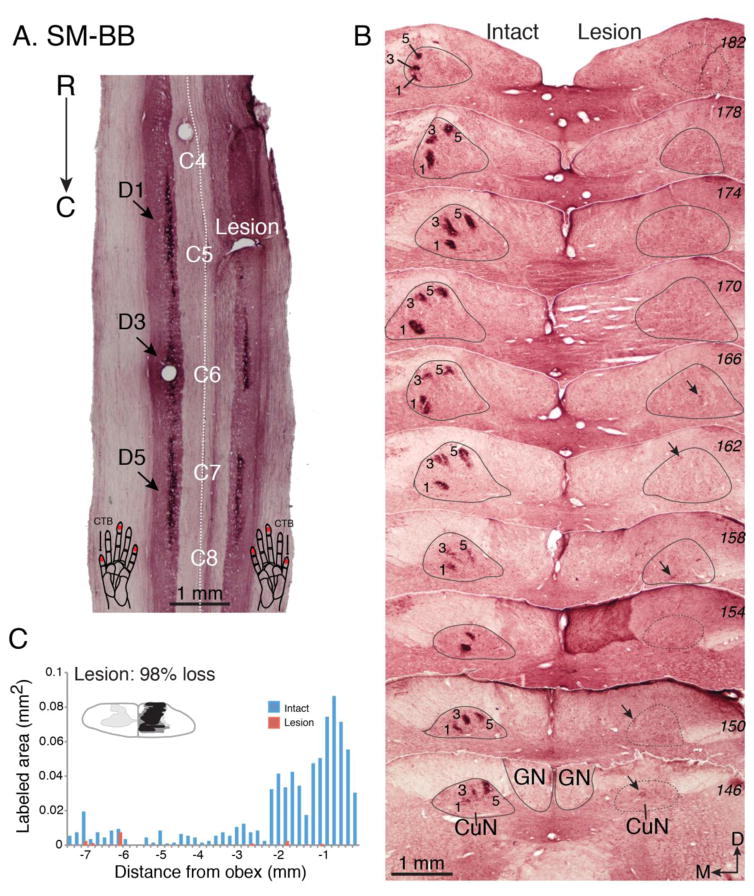
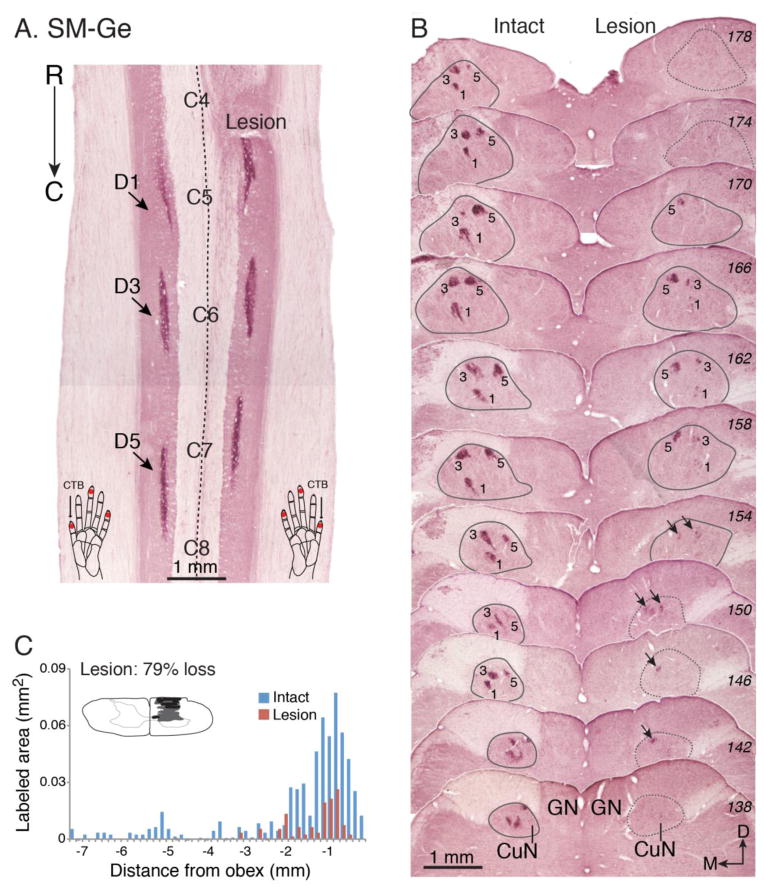
While all three animals received DC lesions at the same C4-C5 cervical spine level (Fig. 2), the final lesion extent, and its impact on the loss of cortical inputs varied between animals. Comprehensive histology and CTB labeling results are summarized in Figs. 3–5. In squirrel monkey SM-BB (98% loss), the DC lesion was at the rostral tip of C5, the level at which afferents from D1 enter (Fig. 2, Fig. 3A). There was almost total absence of CTB staining (2% of contralateral control) in the lesion-side midbrain cuneate nucleus (CuN) - the first synaptic relay station of primary, as well as some secondary, ascending afferents – confirming that the vast majority of inputs from the digits and hand on the (right) lesioned side were interrupted (Fig. 3B, C).
Figure 2.
The spinal segment and extent of the dorsal column lesions. A drawing of the cervical spinal cord indicates the levels of the lesion of the right dorsal columns for squirrel monkeys SM-BB, SM-Ge, and SM-A. The reconstructed extents of these lesions are shown on drawings of spinal cord transverse views for the three monkeys (arrows). Black shading depicts the damage resulting in tissue loss (a hole); dark grey areas surrounding the black shading depict abnormal morphology of the spinal cord tissue due to DC lesion. Cervical spinal cord segments 4–7 (C4-C7) receive inputs from the digits 1–5.
Figure 3.
The effectiveness of the dorsal column lesion in squirrel monkey SM-BB. The dorsal column lesion was evaluated by comparing the amounts of labeled peripheral nerve terminations in the cuneate nucleus of the lesioned right side with the intact left side. The terminations of peripheral afferents in the dorsal horn of the spinal cord and cuneate nucleus of the brainstem were labeled by injections of cholera toxin subunit B (CTB) into the distal phalange of digits 1, 3, and 5. A. Photomicrograph of a horizontally cut CTB-immunoreacted section of the cervical spinal cord shows the location of the dorsal column lesion (Lesion) and labeled axon terminals in the dorsal horns (indicated by arrows) after tracer injections. Note that the lesion was centered within the terminations from digit 1. Injection sites in the digits are indicated by red dots on the schematic drawings of hands at the bottom of panel A. B. A series of coronally cut CTB-immunoreacted sections (labeled with numbers in italic on right) through dorsal column nuclei of brainstem. The cuneate nucleus is outlined in grey, and numbers 1, 3, 5 mark foci of afferents labeled by injections in digits 1, 3, and 5. Note that there are only a few detectable foci of axon fibers on the lesioned side where indicated by arrows. The numbers on the right are series section numbers. C. The lesion was reconstructed from a series of horizontally cut sections as in A. Bar graph compares the areal sizes of CTB-labeled axon arbor foci in the cuneate nuclei of the brainstem for the lesioned and intact sides. The numbers on the x-axis are the distance (in mm) measured from the beginning of the obex. The negative values indicate the measured distances were caudal to the obex. The y-axis value is the areal size (in mm2) of the combined foci of CTB-label for each section through the cuneate nucleus of the brainstem. The inset is a transverse view of spinal cord through cervical segment C5 indicating the reconstructed extent of the lesion in black. Further damage is marked in grey. Abbreviations, C4-C8, spinal cord cervical segments 4–8; CuN, cuneate nucleus of brainstem; C, caudal; D, dorsal; D1, D3, D5, digits 1, 3, 5; GN, gracile nucleus of brainstem. M, medial; R, rostral.
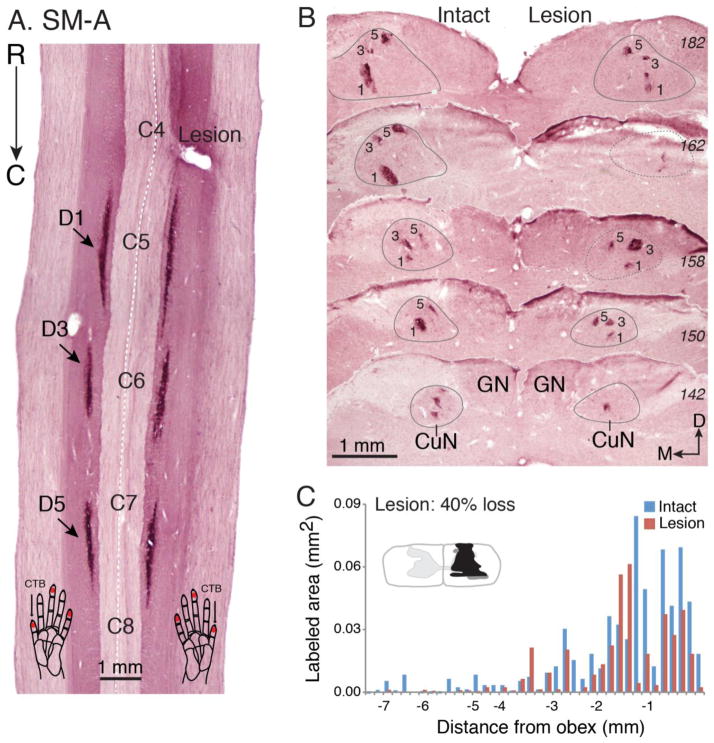
Figure 5.
The effectiveness of the dorsal column lesion in squirrel monkey SM-A. A. Photomicrograph of a horizontally cut CTB-immunoreacted section of the cervical spinal cord shows the location of the dorsal column lesion (Lesion) and labeled axon terminals in the dorsal horns (indicated by arrows) after tracer injections. B. A series of CTB-immunoreacted coronal sections (labeled with numbers in italic on right) through dorsal column nuclei of brainstem. Note that foci of CTB label appeared on both sides indicating the dorsal column lesion was not complete. C. Bar graph compares the areal sizes of CTB-labeled axon arbor foci in the cuneate nuclei of the brainstem for the lesioned and intact sides. Conventions follow Figure 4.
Lesion Histology
The DC lesions in squirrel monkeys SM-Ge (79% loss) and SM-A were less extensive. Although both lesions were placed at the cervical C4 level and the lesion sizes were comparable to that of SM-BB (98% loss), the locations of the cuts were more lateral, allowing more afferents in the cuneate fasciculus to survive, as reflected in denser CTB-label in CuN on the lesion side. Thus, the fraction of axon sparing on the lesion side compared to the intact side was 21% in SM-Ge (Fig. 4B, C), and 60% in SM-A (Fig. 5B, C). The summed areal differences of the CTB patches in CuN between the lesion and non-lesion sides were highly significant in all 3 cases (Wilcoxon matched-pairs signed-ranks test, p ≤ 0.002). The lesions also encroached into the dorsal horn, intermediate zone, and some portions of the ventral horn, to differing extents, in all three monkeys. The lateral and ventral ascending pathways such as dorsolateral funiculus, spinocervical, and spinothalamic tracts were intact, as were the major descending pathways of the corticospinal, rubinospinal, and cerebellospinal tracts
Figure 4.
The effectiveness of dorsal column lesion in squirrel monkey SM-Ge. A. Photomicrograph of a horizontally cut CTB-immunoreacted section of the cervical spinal cord shows the location of the dorsal column lesion (Lesion) and labeled axon terminals in the dorsal horns (indicated by arrows) after tracer injections. B. A series of coronally cut CTB-immunoreacted sections (labeled with italic number on right) through dorsal column nuclei of brainstem. Note that foci of CTB label on the lesion side were less widely distributed and less dense (arrows) compared to those of the intact side. C. Bar graph compares the areal sizes of CTB-labeled axon arbor foci in the cuneate nuclei of the brainstem for the lesioned and intact sides. Conventions follow Figure 3.
In summary, the severity of the DC lesion measured by loss of CuN afferents varied, with SM-BB (98% loss) > SM-Ge (79% loss) > SM-A (40% loss). This allowed us to exploit longitudinal fMRI studies, with each animal serving as its own pre-lesion control, to examine the influence of severity of primary deafferentation on the degree, nature and spatiotemporal trajectory of cortical deactivation and subsequent reactivation, as well as providing direct electrophysiological correlates with the final fMRI time point.
CBV-fMRI maps in cortex before and after dorsal column lesion
Pre-lesion Maps
Before the spinal cord lesion, low level ES of digits 1, 2, and 3 evoked a focus of blood volume increment in contralateral area 3b for each digit (Figs. 6–8, Panel A), but did not evoke any significant CBV responses in areas 3a, 1 or 2. The area 3b foci were arranged in a mediolateral sequence reflecting the normal representational territories of these digits (Sur et al., 1982). They also recapitulated the small but stable inter-subject variations in detailed somoatotopy revealed by repeated fMRI mapping studies (Chen et al., 2007; Zhang et al., 2010). Thus, low level ES of the digits revealed a somatotopic pattern that closely matched those previously reported microelectrode mapping experiments where neurons in area 3b responded to tactile stimulations (e.g., Merzenich et al., 1978; Merzenich et al., 1987; Sur et al., 1982).
Figure 6.
High-resolution cerebral blood volume (CBV) functional mapping of digit responses to electrocutaneous stimulation for squirrel monkey SM-BB. CBV functional images in sensorimotor areas were collected with monocrystalline iron oxide nanoparticles (MION) enhancement in a 9.4 T scanner during electrocutaneous stimulation of digits 1, 2, and 3 at pre- and post-lesion times. The DC lesion for this monkey was the most extensive (98% loss). A–D. Composite maps collected during low intensity 2 mA stimulation at pre-lesion (A) and at post-lesion weeks 2 (B), 4 (C), and 6 (D). The activation foci (P<10−4) from stimulating D1 (blue), D2 (orange), and D3 (yellow) are outlined. E–H. Composite maps collected during high intensity 4 mA stimulation at pre- (E) and post-lesion weeks 2 (F), 4 (G), and 6 (H). Dotted lines indicate the borders of area 3b. The color code within outlines reflects the magnitude of the response. The strength of the evoked CBV responses is indicated from dark (weak) to light color (strong, see spectrum scale bar in Fig. 1C). The solid line indicates anatomically and electrophysiologically defined hand-face border with the face lateral and the hand medial. Conventions follow Figure 1.
Figure 8.
High-resolution cerebral blood volume (CBV) functional images to digit stimulation for squirrel monkey SM-A. The DC lesion for this monkey was the least extensive (40% loss) among the 3 cases. Conventions follow Figures 1 and 6.
High level ES activated rostral to caudal bands of somatotopically organized foci in hand regions of areas 3a, 3b and 1. The foci in these areas were in their expected locations in a mediolateral sequence for digits 3, 2, and 1. The results varied somewhat across the three cases, again reflecting inter-individual variations in fine-scale organization. Less consistent activation of more rostral (presumed M1: SM-BB (98% loss), SM-Ge (79% loss)) and/or caudal (presumed areas 2/5: SM-BB) bands of higher order were also detected (Figs. 6–8, Panel E). Case SM-Ge (Fig. 7) had foci for the 3 digits in expected locations for area 3b, but foci for only digits 2 and 3 in area 3a, and digits 1 and 2 in area 1. Additional foci were detected caudomedially in area 2 or perhaps area 5, where more convergence of inputs from digits is expected (Cooke et al., 2014; Padberg et al., 2009; Padberg et al., 2010; Pons et al., 1985).
Figure 7.
High-resolution cerebral blood volume (CBV) functional images to digit stimulation for squirrel monkey SM-Ge. The DC lesion for this monkey was extensive, but not complete (79% loss). Conventions follow Figures 1 and 6.
The somatotopy and area boundaries defined by fMRI were consistent with the known areal and somatotopic organization, and inter-session co-registration of functional images can be as accurate as ~ 140 um (Lecoeur et al., 2011b). Some residual mis-registration between fMRI-defined, and terminal electrophysiological maps and histologically defined area boundaries was evident. These mis-registrations arise from the imperfect correction for non-linear distortion of area boundaries during flattening of cortical tissue for histology, and by imperfect correspondence between the planar orientation of the functional imaging slice, and the projection of the curved cortical surface onto a flat photographic plane during the terminal physiological mapping procedure. Nonetheless, mis-registration was generally 100 – 200 μm for SM-Ge (79% loss) and SM-A, and was no more than ~500 μm for SM-BB (98% loss).
Overall, the results support the contention that electrocutaneous stimulation at even high intensity reveals normal aspects of somatotopic organization, but some care is needed in reconciling apparent differences between fMRI, electrophysiology and histologically defined maps due to occasional residual mis-registration.
Post-lesion maps
By 6 weeks post-lesion, all animals showed reactivation of digits 1–3 in area 3b by low amplitude ES in a somatotopically organized manner that closely resembled each animal’s pre-lesion map (Figs. 6–8). However as expected, given the variable loss of primary afferents and inter-subject differences in the impact of the c-spine lesion on secondary and higher order inputs, the temporo-spatial trajectories of cortical reactivation revealed by low and high intensity ES CBV mapping at intermediate time points varied, and are described in detail for individual animals
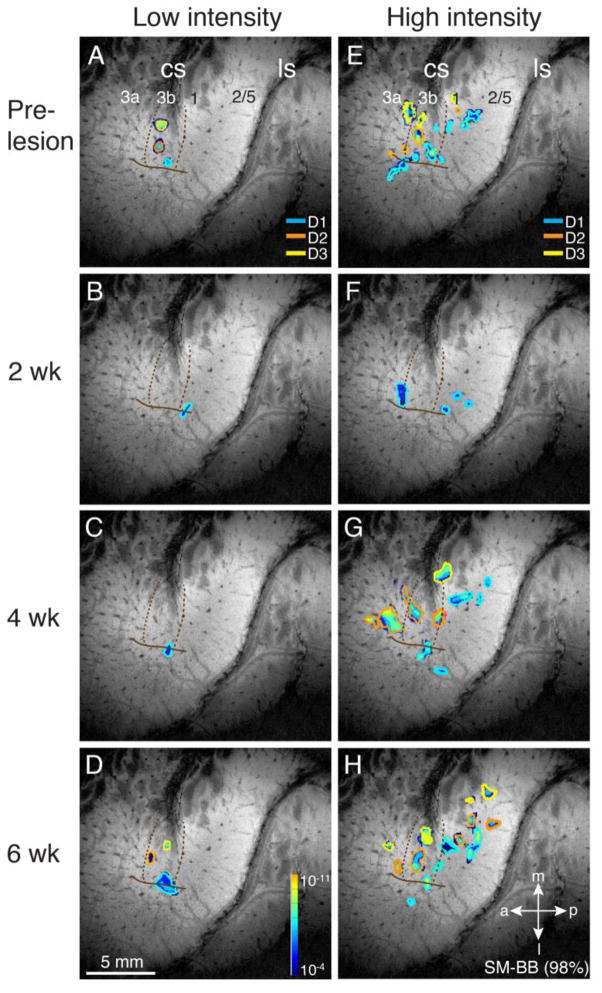
SM-BB (98% loss)
Deactivation of cortex 2 weeks post-lesion was extensive in the case of SM-BB, whose C5 level lesion prevented all but some remnant DC afferents from digit 1 reaching the cuneate nucleus (Figs. 2, 3). Consistent with this, 2 weeks post-lesion, the hand territories in contralateral areas 3b, 3a, 1, and 2 were unresponsive to low and high intensity stimulation of digits 2, and 3 (Fig. 6B, F). Only stimulation of digit 1 elicited any cortical activation. A small focus at the hand/face boundaries of area 3b/1 was activated by low intensity stimulation of digit 1. High intensity stimulation of digit 1 further activated this and two additional foci near the hand-face border of area 1, as well as a large focus in hand cortex of area 3a overlapping the digit 1 high intensity activation in 3a pre-lesion, suggesting the activation of preserved proprioceptive afferents in the spinal cord.
After 4 weeks of recovery, low intensity stimulation failed to activate additional cortex (Fig. 6C). High intensity stimulation evoked more and larger foci for digits 1 and 2 in area 3b, additional foci for digit 1 in area 3b, and multiple foci for digits 1 and 2 in areas 1 and 2 (Fig. 6G).
Finally after 6 weeks of recovery, low intensity stimulation of digits 3, 2, and 1 evoked small foci of activity in approximately the expected mediolateral locations in area 3b (Fig. 6D). With high intensity stimulation, more activity was apparent in areas 3a, 3b, 1, and 2, with multiple foci and somatotopic reorganization in areas 1 and 2 (Fig. 6H). Thus, over 6 weeks of recovery, considerable reactivation of cortex occurs even after a nearly complete disruption of digit projections to the cuneate nucleus via the dorsal columns.
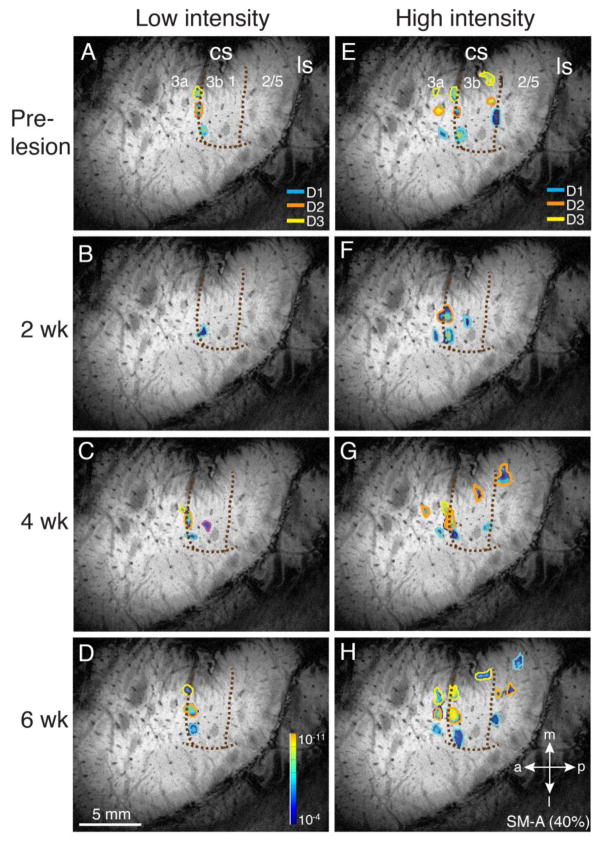
SM-Ge (79% loss)
2 weeks after DC lesion, low and high intensity stimulation elicited spatial patterns of activation for digits 1–3 in areas 3b (low and high intensity) and 3a (high intensity) that matched the pre-lesion maps. Notably, when compared with pre-lesion dimensions (Fig. 7A, E), the areal extent of area 3a and 3b high intensity activations was greater for all digits 2 weeks post-lesion, as it was for low intensity digit 3 stimulation in area 3b (Fig. 7B, F). The three low stimulus intensity area 3b foci matching those evoked before the DC lesion remained in the same locations after 4 and 6 weeks of recovery (Fig. 7C, D), while the strength and area of digit 3 response to low intensity stimulus decreased over time to more closely resemble the pre-lesion values. In contrast to the low intensity maps, which remained somatotopically stable, with no additional recruitment or loss of activation areas from 2 to 6 weeks post lesion, the high intensity map showed significant evolution with time (Fig. 7F, G, H). The expanded, but still somatotopically organized areas of activation observed for all three digits in areas 3a and 3b at 2 weeks decreased in areal extent at 4 weeks (and in the case of area 3a digit 1 disappeared), and additional high intensity activation foci for each digit were present in areas 3b, 1, 2, and/or 5 (Fig. 7G). At 6 weeks (Fig. 7H), area 3b responses in all three digit territories remained somatotopically organized and matched pre-lesion centers. However, they were increased compared with week 4, and again resembled the week 2 map, with greater areal extent, and exaggerated CBV responses compared with pre-lesion. In the case of digit 3, there was greater caudal expansion of area of activation within area 3b into more proximal digit areas, reaching the border with area 1. In areas 1,2 and 5, the patterns of activation continued to evolve, and their areas and strength of CBV response were similarly increased compared with week 4 responses.
For SM-Ge (79% loss), the DC lesion reduced direct inputs from digits 1, 3, and 5 to the cuneate nucleus almost 80%. Cuneate terminations from digit 1 were almost undetectable, those from digit 3 were somewhat spared, and those from digit 5 more so. Furthermore, based on the MRI results, it is likely that the inputs from digit 2 more closely resemble digit 3 than digit 1 (Figs. 5, 10). This is consistent with the observed stability of the low intensity, and the spatio-temporal evolution of the high intensity maps. Taken together, these findings provide evidence for preservation and or early recovery of somatotopic activation of area 3b by primary inputs revealed by low intensity stimulation, and later more extensive activation of these and more extended and somatotopically reorganized areas by recovering and/or sprouting secondary and higher order high-threshold inputs.
Figure 10.
Response magnitudes of CBV signal time courses for voxels significantly activated by low and high intensity ES in each of the areas (3a, 3b, and 1). For each panel, CBV signal changes were extracted for each digit and averaged across runs and voxels within a session. Graphs compare CBV peak response amplitudes in somatosensory regions of interest before and after DC lesion elicited by low (2 mA) and high (4 mA) electrocutaneous stimulation to the distal pad of digits 1 – 3 (same color codes as in Figs. 6 – 8) of 3 monkeys (SM-BB (98% loss), SM-Ge (79% loss), SM-A (40% loss)). X-axis, time of scan before (0), and 2, 4, 6 weeks after unilateral dorsal column lesion of cervical spinal cord; Y-axis, mean percentage signal change; [high - low], intensity response differences.
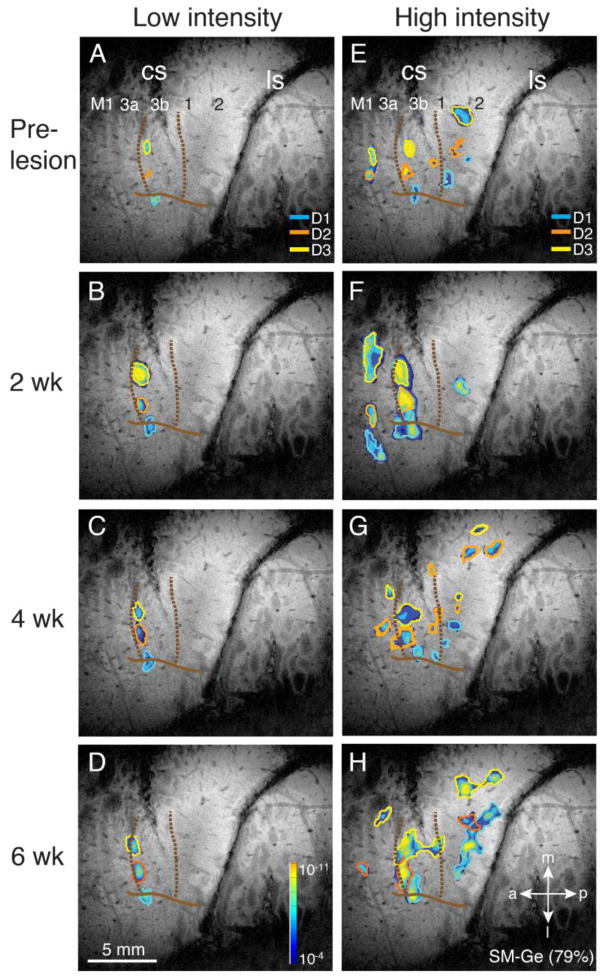
SM-A (40% loss)
Somewhat surprisingly, given the significant sparing of primary DC inputs suggested by significant digit 1 and digit 3 CTB labeling in CuN, low intensity stimulation produced only a small focus of activity in area 3b related to digit 1 after two weeks of recovery (Fig. 8B), with subsequent recovery of digits 2 and 3 at 4 and 6 weeks (very weak digit 3 focus at 4 weeks), all somatotopically organized in their pre-lesion locations with pre-lesion response amplitudes and areas (Fig. 8C, D). There was a similar trajectory of recovery in high intensity stimulus maps (Fig. 8F–H): at week 2, digits 1 and 2 showed somatotopically organized responses in area 3b, as well as a transient more caudal area 3b focus of digit 1 activity. There was only a weak digit 1 focus in area 3a, and area 1 was silent; by week 4, high intensity stimulation evoked activation of digits 1 and 2 across areas 3a, 3b and 1, with some digit 2 activation in area 2/5. At week 6, there was complete recovery of somatotopically organized activations for all three digits in areas 3a, 3b and 1, that closely matched the pre-lesion high intensity map, with small additional digit 1 and digit 2 activation foci in area 2/5. Thus, a lesion that left more than half of the dorsal column afferents intact nonetheless depressed the short term responsiveness of somatosensory cortex to low and high intensity stimuli, but allowed almost complete recovery of responsiveness to both low and high intensity stimuli with near normal somatotopy after 6 weeks of recovery. These patterns of recovery, with preservation of pre-lesion somatotopic organization for both low and high intensity maps, are consistent with the significant sparing of DC inputs to CuN, suggesting that transient non-lethal suppression of both direct and secondary pathways underlies the initial deactivation and subsequent reactivation of somatosensory cortex in this animal.
Overall, the results from the three cases suggest that imaging somatosensory cortex at low and high levels of ES of the digits can provide valuable information about the normal somatotopy of area 3b, but also areas 3a and 1 and posterior parietal cortex. Furthermore, the spatiotemporal trajectories of low and high intensity cortical responses post-lesion suggest that both direct and indirect pathways contribute to the reactivation of cortical areas deprived of inputs by acute deafferentation, with their relative contributions being influenced by the nature and severity of the injury to direct and secondary inputs, and themselves influencing the nature and functional/somatotopic appropriateness of cortical reactivation.
Longitudinal functional mapping at 2, 4, and 6 weeks post-lesion indicates that considerable reactivation of hand cortex can occur over 6 weeks of recovery even after a nearly complete dorsal column lesion. This reactivation was best revealed by the high intensity stimulation, and the results suggest that some somatotopic reorganization occurred following severe loss of inputs. However, recovery of near normal patterns of activation in area 3b and beyond were observed for lesions that left 20% or more of hand related dorsal column inputs intact.
CBV response magnitude after DC lesion and recovery
The effects of a DC lesion and the recovery process on the magnitudes of the CBV responses were evaluated in areas 3a, 3b, and 1. Response magnitudes of CBV signal time courses for voxels significantly activated by low and high intensity ES in each of the areas (3a, 3b, and 1) were extracted for each digit and averaged across runs and voxels within a session. Responses averaged across digit 1, 2 and 3 fields for subject SM-BB (98% loss), where all three digits behaved similarly, illustrate typical CBV response profiles to low and high intensity ES in area 3b before and after a DC lesion (Fig. 9): pre-lesion, low intensity stimuli evoked negative signal responses, consistent with local rCBV increase; high intensity stimuli evoked stronger responses in the same, or slightly larger patches in area 3b. In contrast to area 3b, areas 3a and 1 were relatively unresponsive to low intensity ES, but had similar response profiles to 3b for high intensity stimuli.
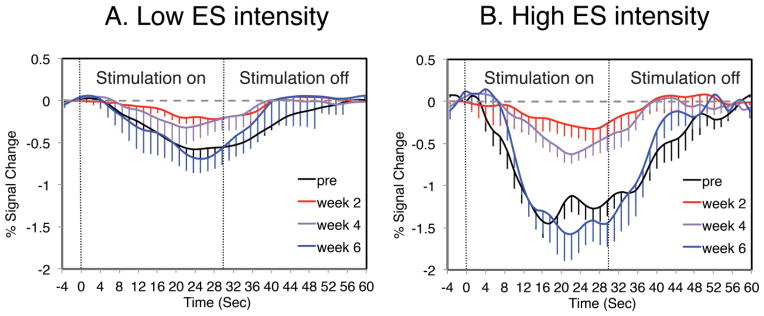
Figure 9.
Representative CBV profiles of percent signal change in area 3b in response to digit 1 stimulation before and after DC lesion in squirrel monkey SM-BB (98% loss). A. CBV response profiles to 2 mA intensity stimulation pre-lesion and 2, 4, and 6 weeks post-lesion. B. CBV response profiles to 4 mA intensity stimulation. Dotted lines indicate the 30 s duration of electrocutaneous stimulation (ES). The error bars show the standard deviation across voxels. Peak percent signal change amplitudes were extracted from temporal profiles and reversed (to positive values) for further comparisons.
Using these CBV response profiles, we tracked area and digit specific patterns of loss and subsequent recovery of CBV responses after DC lesion to gain further insights into the impact of DC lesion on loss of direct (DC) and indirect (e.g. spinothalamic) inputs to somatosensory areas, and the relative contributions of these pathways to subsequent cortical reactivations. We also plotted the time course of the difference between high and low ([high-low]) ES CBV response amplitudes to assess the contribution of secondary pathways to reactivation post-lesion. Figure 10 summarizes these data, and reveals digit-level differences in the patterns of areal reactivation between animals, reflecting differences in the impact of the spinal cord lesion on primary (DC) and secondary digit-specific inputs. Prior to lesioning, only area 3b responded significantly to low intensity ES, while high intensity stimuli elicited similar strong responses in areas 3a, 3b, and 1 for each digit. CBV response amplitudes were similar among the 3 somatosensory areas when the high intensity stimulus was applied, and again, post-lesion responses varied between animals:
SM-BB (98% loss)
2 weeks post-lesion, there was nearly total suppression of low and high stimulus CBV responsiveness for all three digits in all areas. The loss of area 3b responsiveness to low intensity stimuli in all three digits is consistent with the near complete DC lesion. Furthermore, the lack of response of all three digits to high intensity stimuli in areas 3a, 3b and 1, and the reduction in their [high-low] response to near zero, suggest that there was also major loss of secondary inputs to all three areas. By week 4, however, there was some recovery of responsiveness of digits 1 and 2 in area 3b to high intensity stimuli, with the recovery evident in the [high-low] response, consistent with recovery and/or expansion of secondary inputs of digits 1 and 2 to area 3b. At week 6, there was some recovery of low intensity responses in area 3b for all three digits, and further recovery to near pre-lesion high, low and [high-low] amplitudes. High intensity, and [high-low] responses were at (area 1) or near (area 3a) their pre-lesion values for all three digits. In summary, these patterns of decline and recovery of digit-specific areal responses are consistent with major loss of both direct DC as well as secondary pathways for digits 1, 2 and 3. They further suggest an earlier reactivation of area 3b digits 1 and 2 by secondary inputs, followed by later reactivation of all three digits by residual primary and secondary inputs, while reactivation of areas 3a and 1 by indirect inputs occurred later and may still have been incomplete.
SM-Ge (79% loss)
In contrast to the decreased response to low intensity stimuli in area 3b seen in all three (SM-BB: digits 1, 2, 3) or two (SM-A: digits 2, 3) 2 weeks post-lesion, low intensity responses were intact for all three digits in SM-Ge, and remained so at weeks 4 and 6. Responses to high intensity ES in area 3b were similarly unaffected for digit 3, as were the [high-low] responses. For digits 1 and 2, however, area 3b high and [high-low] intensity responses were elevated at week 2, and remained so 4 and 6 weeks post-lesion. In particular, the time courses of the [high-low] responses suggest an increase in the activation of digit 3 by indirect pathways at week 2, with a similar but smaller increase in digit 2. In areas 1 and 3a high and [high-low] intensity responses were similar to or slightly elevated compared with pre-lesion values for digits 1 and 2. Digit 3 responses in area 1 were depressed 2 and 4 weeks post-lesion but had recovered by week 6, suggesting a transient impact of the lesion on secondary digit 3 inputs to area 1.
The recoveries in digit 3 were consistent with partial sparing of digit 3 inputs to CuN, but the large increases in high and [high-low] responses in area 3b for digits 1 and 2 two weeks post-lesion suggest a significant contribution of secondary inputs to early reactivation in addition to that provided by spared direct (DC) inputs.
SM-A (40% loss)
Despite the sparing of a large fraction of inputs to CuN from all five digits suggested by CTB staining. SM-A showed significant depression of low intensity responses for digits 2 and 3 in area 3b 2 weeks post-lesion (Fig. 8). All three digits showed pre-lesion levels of low intensity responsiveness at weeks 4 and 6. High and [high-low] intensity responses in area 3b were similar to pre-lesion levels for digits 1 and 2 at 2, 4 and 6 weeks post-lesion, while digit 3 responsiveness was profoundly reduced at 2 weeks, but had normalized at 4 and increased further at 6 weeks. In contrast to the sparing of digit 2 (as well as digit 1) high and [high-low] intensity responses in area 3b, there was major compromise of both digit 2 and digit 3 responses in areas 1 and 3a 2 and 4 weeks post-lesion, with partial recovery in area 1 and near complete recovery in area 3a at 6 weeks. Taken together with the post mortem lesion histology, these results suggest that the initial loss but ultimately near complete recovery of low, high and [high-low] intensity responses in area 3b may reflect transient silencing, but not loss, of most DC (digits 2 and 3) and secondary inputs (digit 3) to this area. A similar scenario might explain the delayed trajectories of recovery for digits 2 and 3 in area 3a, while in area 1, it is unclear whether the partial recovery of digits 2 and 3 seen at 6 weeks was continuing, or represents stable axonal loss.
Microelectrode mapping of somatosensory cortex after dorsal column lesion
Multiunit microelectrode mappings were made from a total of 593 microelectrode recordings (SM-BB = 165, SM-Ge = 215, and SM-A = 213) in somatosensory areas 3a, 3b, and 1 in the 3 monkeys 7 – 9 weeks after the DC lesion (i.e., 1–3 weeks following the final fMRI mapping). Consistent with prior mapping studies (Chen et al., 2012b; Jain et al., 1997; Qi et al., 2011), as well as the present fMRI mapping data, the most severely lesioned animal (SM-BB 98% loss) demonstrated evidence of cortical reactivation 7 weeks post-lesion, albeit with significant reorganization, while the two less severely lesioned animals showed less dramatic reorganization, despite the longer recovery periods of 8 and 9 weeks for SM-Ge (79% loss) and SM-A (40% loss) respectively. Given the variation in lesion severity, and period of post lesion recovery amongst the three animals, results from each case are presented individually, and additional extensive mapping information is provided in Supplementary form.
SM-BB (98% loss; 7 weeks post-lesion)
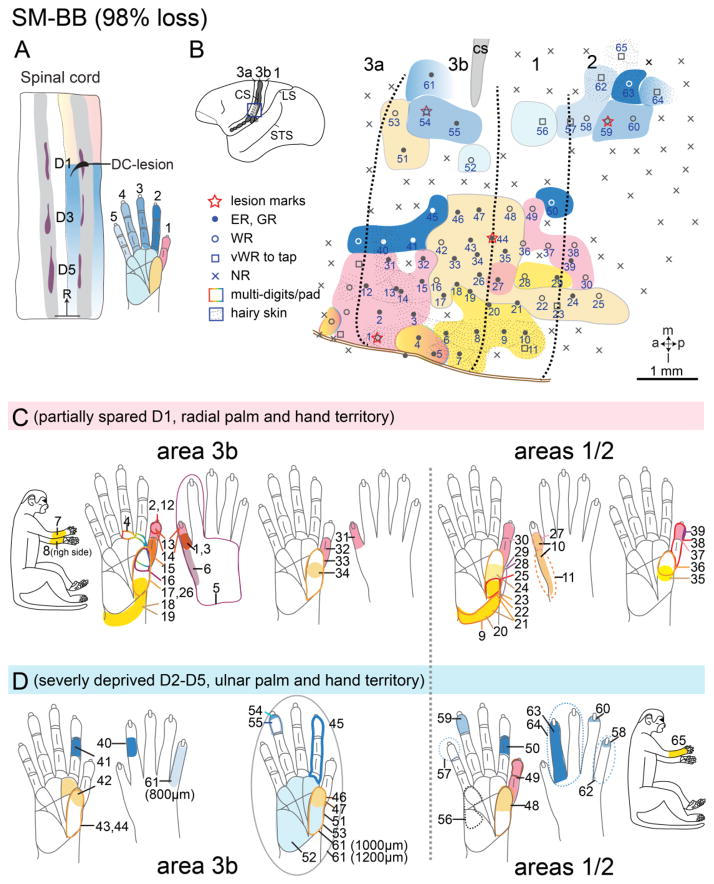
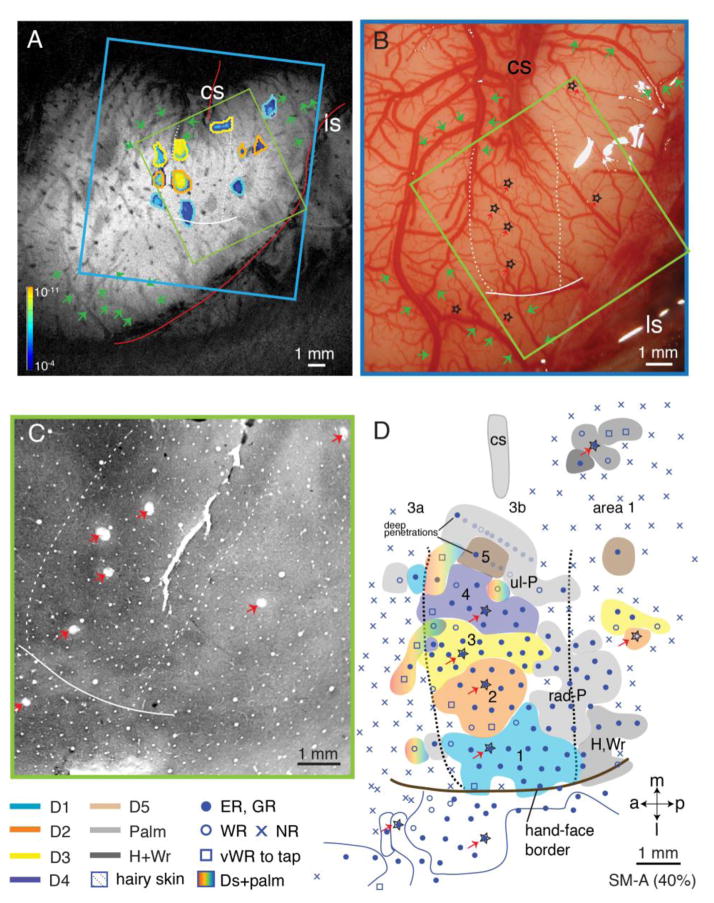
Hand regions in areas 3b and 1 contralateral to the lesion side were mapped 7 weeks after an extensive unilateral DC lesion at the right cervical level 5. Consistent with the severity of the lesion, and the fact that the majority of hand afferents other than some from digit 1 and palm enter the spinal cord below the level of this lesion (Fig. 2), the majority of penetrations in the deprived digits’ territories were unresponsive to tactile stimuli (Figs. 11B, 12D). Thus in deprived area 3b, neurons responded less frequently to tactile stimulation of digits 2 – 5 and pad 1 (P1). Most sites in area 1 responded weakly (Fig. 11B, open circles or squares). Neurons in digit 1 and palm thenar pad (PTH) territories in area 3b were responsive (Fig. 12B, solid dots), but were less responsive in area 1. The neuronal receptive fields on digit 1 and PTH were not unusually large (Fig. 11C), suggesting they probably reflected the spared normal representation in areas 3b and 1. Receptive fields of neurons in the deafferented hand region in area 3b were enlarged when compared with those previously reported for unlesioned animals (Jain et al., 1997; Jain et al., 2000), with the expansion ranging from entire phalange, through a complete digit, to a large portion of palmar pad (Fig. 11D). Furthermore, a large portion of cortex was devoted to representing the dorsal skin (Fig. 11B), suggesting that afferents serving this skin were relatively spared. Thus, the present results are similar to those reported previously after dorsal column lesion in monkeys (Jain et al., 1997; Jain et al., 2000; Jain et al., 2008; Qi et al., 2011; Qi et al., 2014a; Tandon et al., 2009)
Figure 11.
Somatotopic map of areas 3b and 1 of squirrel monkey SM-BB (98% loss). The somatotopic maps of areas 3b and 1 were obtained with microelectrode recordings seven weeks after a nearly complete (98%) section of the contralateral cuneate fasciculus at cervical C5. A. The location of DC lesion in a horizontal section of the cervical spinal cord. Peripheral afferents inputs from digits 2–5 and ulnar hand are below the lesion, with only a portion of inputs from D1 above the lesion (see Fig. 4). Warm colors (pink – orange) indicate spinal cord levels with peripheral nerve inputs above the lesion from digit 1, radial palm and hand; cool colors (shades of blue) indicate spinal cord levels with inputs below the lesion from digits 2 – 5, ulnar palm and hand. Purple patches indicate dorsal horn terminations from digits 1, 3, and 5 labeled by CTB tracer injections. Grey shades the dorsal horn of the spinal cord. B. The reconstructed somatotopic map shows that partially spared D1 and radial palm representations are highly responsive in area 3b and somewhat slightly less responsive in area 1; responses in both areas are organized in topographical manner. Solid circles mark numbered electrode penetrations with good responses; black open circles mark those with weak responses; black diamonds mark those with very weak responses to hard taps; x’s mark microelectrode penetrations with no responses. Dotted patterns mark representations of hairy skin. Thick black dashed lines mark boundaries of area 3b based on myelin architecture and somatotopy defined from microelectrode mapping. C. Receptive fields determined from microelectrode recordings within the partly spared regions in areas 3b and 1 are outlined on drawings of the body and glabrous and dorsal surfaces of the hand. Numbered receptive fields correspond to numbered recording sites in B. D. Receptive fields determined from microelectrode recordings throughout the highly deprived regions in areas 3b and 1. The dashed lines on the boundaries of some RFs indicate that the exact boundaries of the RFs were hard to define due to very weak responses to hard taps. Numbers on the receptive fields on the hands indicate the matching microelectrode penetrations in the map (panel B). Abbreviations: ER, excellent response; GR, good response; NR, no response; WR, weak response; vWR, very weak response to hard taps; R, rostral. Other conventions follow Figure 1.
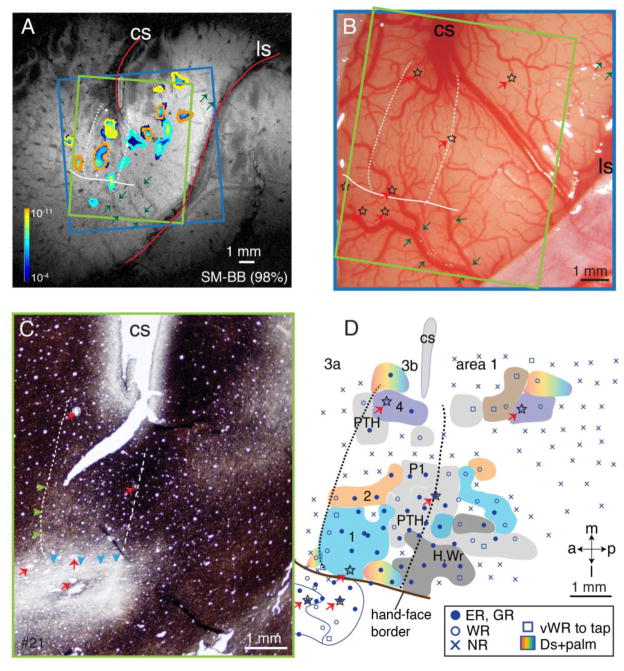
Figure 12.
Alignments of the MR image, microelectrode mapping, and myelin architecture for monkey SM-BB (98% loss). A. Composite CBV activation maps during electrocutaneous stimulation of digits 1–3 at high intensity (4 mA) from post-lesion week 6 scan. Foci outlined in blue were activated by stimulating D1, orange foci from D2, and yellow foci from D3. The strength of the evoked CBV responses is indicated from dark (weak) to light color (strong, see spectrum scale bar in Fig. 1C). The green arrows point to corresponding blood vessels in the MR image and high resolution brain photograph in panel B. B. Photograph of the left somatosensory cortex of monkey SM-BB shows surface blood vessels over microelectrode mapping region. Green arrows mark the blood vessels that can be identified in the MR image (panel A). C. Photomicrograph of a flattened and myelin-stained section through somatosensory cortex showing the central sulcus (CS), the hand-face border (blue arrowheads), septa demarcate digits 1, 2, and 3 territories (green arrow heads), and electrolytic lesion marks (red arrows). D. Reconstructed somatotopic map obtained from dense microelectrode recordings from the same region of interest as in the MR image of panel A. Note that same lesion marks shown in panel B (stars) are also revealed in panels C (red arrows) and indicated in panel D (red arrows and stars).
The blue square outlines indicate the field of view from panel B, the green outlines indicate the field of view from panel C. White dash lines mark rostral and caudal borders of hand region in area 3b; solid brown lines mark hand-face border in area 3b. Abbreviations, H, hand; Wr, wrist. Conventions follow Figures 1 and 11.
SM-Ge (79% loss)
In squirrel monkey SM-Ge, the extensive but incomplete lesion at the C4-C5 level left ~20% of afferents from the hand in the cuneate fasciculus intact. Despite this significant loss of inputs, mapping of areas 3a, 3b, and 1 (Fig. 13D) revealed that area 3b was quite responsive 8 weeks post-lesion. Area 1 was less responsive, however. Somatotopy was roughly normal, if slightly more disorganized than SM-A: digits 1– 5 were represented lateromedially (Fig. 13D), distal to proximal portions of digits rostrocaudally (Suppl. Fig. 1A). As for SM-BB and SM-A, the dorsal skin was over represented, especially for digit 1 (Suppl. Fig. 1B).
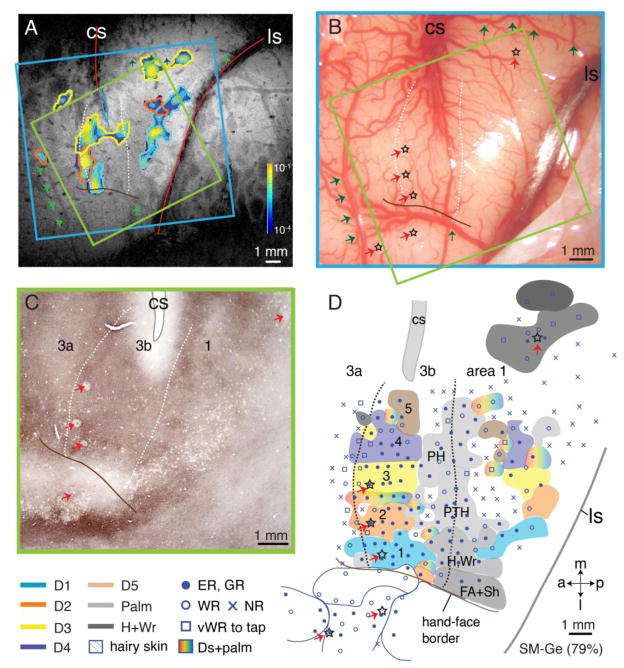
Figure 13.
Alignment of MR image, microelectrode mapping, and brain section stained for vesicular glutamate transporter 2 (vGluT2) antibody in monkey SM-Ge (79% loss). A. Composite CBV activation map collected at post-lesion week 6 with high intensity electrocutaneous stimulation (4 mA) on digits 1–3. The strength of the evoked CBV responses is indicated from dark (weak) to light color (strong, see spectrum scale bar in Fig. 1C). B. Photograph of the left somatosensory cortex of monkey SM-Ge shows surface blood vessels over microelectrode mapping region. C. Photomicrograph of a flattened and vGluT2-immunostained section through somatosensory cortex showing central sulcus (CS), rostral and caudal borders of area 3b, and electrolytic lesion marks (red arrows). D. Reconstructed somatotopic map obtained from dense microelectrode recordings from the same area of interest as in MR image of panel A. Note that same lesion marks shown in panel B (stars) are also revealed in panel C (red arrows) and indicated in panel D (red arrows and stars). White dashed lines mark rostral and caudal borders of hand region in area 3b; solid brown lines mark hand-face border in area 3b. Abbreviations, FA, forearm; H, hand; Sh, shoulder; Wr, wrist. Conventions follow Figures 1 and 11.
SM-A (40% loss)
In squirrel monkey SM-A, mapping 9 weeks following a 40% DC lesion at C4 yielding incomplete loss of afferents from hand revealed a near normal hand representation in area 3b (Fig. 14D). All five digits responded and were represented in a lateromedial order, with distal digits located rostrally and proximal digits and palm caudally (Suppl. Fig. 1C). Some impact of the lesion was noted however. The distal phalanges were over represented in area 3b at the expense of the middle phalange, as neurons responded to stimulation on the middle phalanges in only 2 penetrations (Suppl. Fig. 1C). Again, the representation of dorsal skin was unusually large (Suppl. Fig. 1D), particularly for the digit 2 receptive fields, which were mostly on the dorsal skin. This is not the case in normal New World monkeys (Merzenich et al., 1978; Sur et al., 1982).
Figure 14.
Alignment of the MR image, microelectrode mapping, and cytochrome oxidase (CO) architecture for monkey SM-A (40% loss). A. Composite CBV activation maps during electrocutaneous stimulation of digits 1–3 at high intensity (4 mA) from pre-lesion scans. The strength of the evoked CBV responses is indicated from dark (weak) to light color (strong, see spectrum scale bar in Fig. 1C). B. Photograph of the left somatosensory cortex of monkey SM-A shows surface blood vessels over microelectrode mapping region. C. Photomicrograph of a flattened and CO reacted section through somatosensory cortex showing the central sulcus (CS), hand-face border (double solid lines), and the electrolytic lesion marks (red arrows). D. Reconstructed somatotopic map obtained from dense microelectrode recordings from the same area of interest as in MR image of panel A. Note that same lesion marks shown in panel B (stars) are also revealed in panels C (red arrows) and indicated in panel D (red arrows and stars). White dashed lines mark rostral and caudal borders of hand region in area 3b; solid brown lines mark hand-face border in area 3b. Conventions follow Figures 1 and 11.
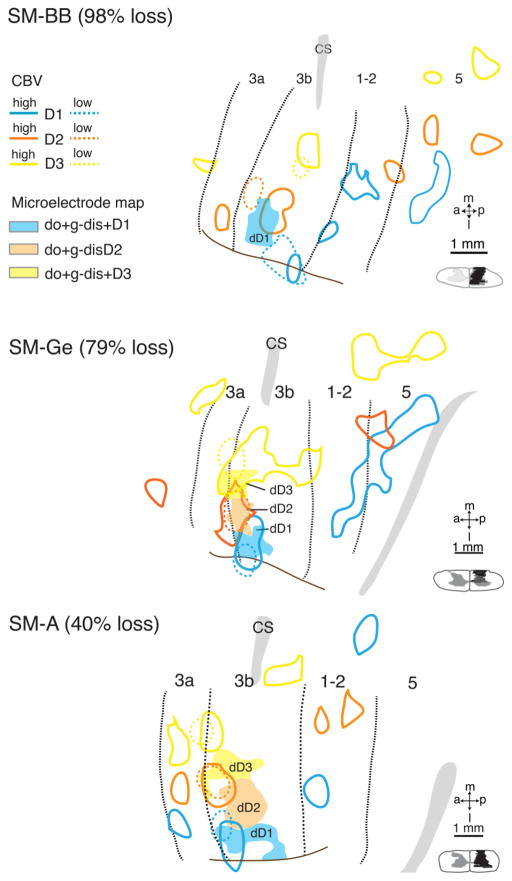
Alignment of CBV-fMRI and microelectrode maps
Finally, alignment of terminal microelectrode maps with histologically defined areal boundaries within somatosensory cortex on the one hand, and with final week 6 fMRI maps and structural images on the other, allowed us to integrate information regarding the dynamics of cortical reorganization available in fMRI maps collected at low and high intensity ES, with the detailed but invasive characterization of cortical reorganization available with electrophysiological mapping. Using intrinsic (sulci, surface and transcortical vasculature), as well as extrinsic (electrolytic lesions) landmarks present in MR, optical, electrophysiological and histologic data, we aligned electrophysiological borders of areas 3a, 3b, and 1 with histologically defined architectonic borders (myelin – Fig. 12C; cytochrome oxidase – Fig. 13C; vGluT2 – Fig. 14C) with the aid of electrolytic lesion markers (red arrows in panels C and D in Figs. 12–14). Structural and vascular features present in brain photographs collected at the time of microelectrode mapping were then coregistered with each animal’s coregistered structural MRIs and fMRI maps (panels A and B in Figs. 12–14). Given the expansion of the receptive fields for dorsal skin in the distal tips of all three animals post-lesion (Suppl. Fig. 1B, D), and the placement of the ES electrodes on the two sides of the distal tip allowing current flow across both dorsal and glabrous surfaces, we combined the receptive fields for dorsal and glabrous skin of the distal phalange for comparison of the terminal electrophysiological and week 6 fMRI maps of digits 1–3 (Fig. 15).
Figure 15.
Co-registrations of high and low intensity CBV maps with the electrophysiological maps for cases SM-BB (98% loss), SM-Ge (79% loss), and SM-A (40% loss). CBV maps collected with low intensity stimulation are in color-coded dashed outlines, CBV maps collected with high intensity stimulation are in color-coded solid outlines, and electrophysiological maps are in solid shades. Black dotted lines indicate areal borders based on architecture and electrophysiological results. Dashed lines mark rostral and caudal borders of hand region in area 3b; solid brown lines mark hand-face border in area 3b. Abbreviations, H, hand; Wr, wrist. Conventions follow Figure 1.
Correspondence between the week 6 fMRI maps and electrophysiology maps was excellent for the two less severely lesioned animals, SM-Ge (79% loss) and SM-A (40% loss). For SM-Ge (Fig. 15B), low intensity ES activations for digits 1 and 2 overlapped the corresponding microelectrode-defined territories mapped 2 weeks later, while the low intensity ES focus for digit 3 overlapped, but had greater medial extent than the corresponding electrophysiological receptive field. For SM-A (Fig. 15C), low intensity activations for digits 1 – 3 were 500 – 700 μm more rostral, and in the case of digit 3 ~900 μm more medial than the corresponding microelectrode maps collected 2 weeks later. For SM-BB (98% loss), the most severely lesioned animal, there was similar relative topography of digits 1–3 within the 6 week fMRI map and the microelectrode map one week later at week 7. The congruence between the two maps, however, was poorer than for SM-Ge and SM-A.
The near perfect congruence of the 6 week and pre-lesion low intensity fMRI maps for SM-Ge (79% loss) and SM-A (40% loss), together with the normalization of the CBV responses to both low and high intensity ES for all three digits to pre-lesion levels by week 4, suggest that any cortical reactivation and/or reorganization in these animals was largely complete by 6 weeks, and the small differences between fMRI and microelectrode maps for these cases can be attributed to residual errors of mis-registration. In the case of SM-BB (98% loss) however, the differences between the week 6 and pre-lesion fMRI somatotopy, the incomplete and ongoing recovery of CBV response amplitudes in all three digits between weeks 4 and 6, and the more extensive reorganization evident in the week 7 microelectrode map all suggest that in addition to any underlying mis-registration errors, continuing processes of cortical reorganization and reactivation may contribute significantly to the discrepancies between the fMRI and microelectrode maps in this animal.
In summary, in the less severely lesioned animals, whose post-lesion recovery and/or reorganization had stabilized by 6 weeks post lesion, there was excellent congruence of the somatotopic maps obtained using CBV-fMRI and dense microelectrode mapping, while in the animal with almost complete loss of DC inputs, continued reorganization and reactivation of somatosensory areas by direct and secondary inputs as late as 6 – 7 weeks post lesion may be a significant contributor to the differences observed between the fMRI and microelectrode maps.
DISCUSSION
In the present study, we characterized the recovery of somatosensory cortex to low and high intensity electrocutaneous stimulation (ES) of the fingers of the squirrel monkey hand with fMRI before and up to 6 weeks after a unilateral section of the contralateral dorsal columns. This recovery pattern has been described previously in studies using tactile stimulation of the hand (Chen et al., 2012a; Jain et al., 1997; Yang et al., 2014). Complete or largely complete lesions at a high cervical level (C4-C5) entirely deactivate the hand portion of somatosensory fields 3a, 3b, 1, and 2. A gradual and incomplete reactivation of the hand cortex follows over a period of weeks. The extent of the reactivation and the restoration of near normal somatotopy depends on the completeness of the DC lesion, and it appears that even a small amount of surviving DC axonal inputs from the hand can promote a near normal somatotopic pattern of reactivation, and the return of skilled hand use (Darian-Smith and Ciferri, 2005; Qi et al., 2013; Qi et al., 2014a). The preserved DC primary afferents are clearly important, but their role is likely facilitated by other pathways whose nature and contribution to reactivation and/or reorganization remain poorly characterized.
In human studies, cortical evoked responses, such as somatosensory evoked potentials (SEP), magnetoencephalography (MEG), electroencephalography (EEG), or fMRI in response to electrocutaneous stimulation of peripheral nerves or a limb are commonly used as clinical tools to detect functional alteration at various levels of the somatosensory pathways (Manganotti et al., 2009). Studies show that electrocutaneous stimulation of the hand by electro-cutaneous, electro-epidermal (that can preferentially activate Aδ fibers), laser (that preferentially activates C-fibers), or mechanical stimulation produce signal changes in multiple cortical areas including the primary and secondary somatosensory cortices (Cauda et al., 2014; Inui et al., 2003; Lui et al., 2008), see (Bushnell et al., 1999; Schnitzler and Ploner, 2000; Treede et al., 1999) for review). Because of the nature of the signal, these methods measure the ensemble activity of a relatively large and heterogeneous neuron population. An accurate interpretation of these human studies, especially after injury, requires knowledge of the activity of the underlying cellular constituents that can more readily be acquired from animal studies.
Here, we show that the expected recovery of cortical activation and somatotopy is apparent when measured by fMRI responses to electrocutaneous stimulation of the digits, with the expected greater reactivation and more normal somatotopy after less extensive DC lesions. In all but the most severely lesioned case, the somatotopy of cortical responses to electrocutaneous stimulation of the digits revealed by fMRI closely resembled that obtained from microelectrode mapping of tactile stimulation of the digits. Discrepancies between fMRI of low intensity ES and subsequent microelectrode mapping of tactile stimuli in the setting of near complete DC loss reflect continuing reorganization and reactivation of cortex in the interval between the two studies.
A second finding of the present study was that greater cortical reactivation was revealed by the high intensity (4 mA) electrocutaneous stimulation. The results suggest that low intensity ES (2 mA) effectively activated low threshold cutaneous afferents of the digits, providing information comparable with the activation of those afferents by light touch, arising from direct inputs in area 3b. fMRI responses to high intensity ES revealed earlier, and more extensive reactivation at several early stages of recovery not only in area 3b, but also in higher order areas including 3a, and 1. Thus, it appears likely that other, higher threshold pathways are also activated, and may potentiate and supplement the activation produced by the low threshold pathway. We suggest that these pathways may involve some of the spinothalamic projections.
These results indicate that fMRI mapping of cortical responses to low and high intensity electrocutaneous stimulation can be exploited to evaluate the reactivation process during recovery. Low ES and tactile stimuli activate the same low threshold pathways, and microelectrode-mapping of tactile stimuli 1 – 3 weeks following week 6 fMRI mapping indicated that after 6 weeks of recovery following a DC lesion, regions that were initially less or unresponsive to low intensity ES, had receptive fields that were relatively large, and somatotopy was rearranged. This reactivation arose not only from spared and/or recovering DC inputs, but also from other inputs driving reactivation of deprived digit territories. In less deprived regions, neuronal receptive fields were not particularly large, and somatotopy was relatively normal.
Congruence of CBV-fMRI responses to electrocutaneous stimulation with microelectrode mapping of tactile responses in somatosensory areas
Digit-specific CBV-fMRI activations in area 3b elicited by low and high intensity ES after 6 weeks of recovery overlapped with the same digit territories obtained from dense microelectrode mapping soon thereafter. In the two animals with less extensive DC lesions, the maps were congruent in area 3b, and both closely resembled their pre-lesion fMRI maps and CBV response amplitudes. However, in the case of severe DC lesion (SM-BB 98% loss), discrepancies between the week 6 fMRI ES maps and the week 7 microelectrode tactile map of cortical reactivation were greater than can be attributed to residual registration errors. Given the extensive loss of both low and high ES fMRI responses 2 weeks post-lesion, and the continued recovery of low, high and [high-low] CBV responses in area 3b between 4 and 6 weeks, the discrepancies may reflect continued cortical reorganization and reactivation during the one week interval between fMRI and microelectrode mapping.
Trajectories of CBV-fMRI topography and amplitude following sensory loss
Primary afferents relaying tactile, proprioceptive, nociceptive, and thermal information ascend to the brain through a series of relay stations within the spinal cord dorsal horn, brainstem, and thalamus to finally reach contralateral somatosensory cortex. Damage at any level partially reduces the normal source of activation, depriving all subsequent stages of those inputs. Yet, the central nervous system has capabilities to compensate after injury (Buonomano and Merzenich, 1998; Darian-Smith, 2009; Kaas et al., 2008; Qi et al., 2014b; Xerri, 2012). Most studies of deafferentation of ascending somatosensory pathways in primate models have focused on the tactile modality (Chen et al., 2012a; Florence et al., 2000; Florence et al., 1996; Florence et al., 1998; Jain et al., 1997; Merzenich et al., 1983a; Merzenich et al., 1983b; Pons et al., 1991; Qi et al., 2011; Qi et al., 2014a; Vierck and Cooper, 1998; Vierck et al., 1990). However, little is known about how other somatosensory pathways, such as nociceptive afferents, contribute to cortical reactivation.
Low intensity electrocutaneous stimulation of nerves of the finger (i.e. digital branches of median and ulnar nerves) will activate low threshold cutaneous afferents (via Aβ fibers) associated with touch or hair movement, whereas higher intensities will also activate higher threshold afferents (via Aδ and C fibers) associated with less rapidly conducting inputs related to touch, pain, and temperature (Abraira and Ginty, 2013; Kaas, 2011; McGlone et al., 2014; Todd, 2010). Here, we found that in area 3b prior to the DC lesion, the CBV responses evoked by low intensity ES to (contralateral) single digit tips are focal, topographically organized and congruent with microelectrode mapped tactile representations. Furthermore, the topography and stimulus-response characteristics of CBV responses evoked by low level ES closely resemble those of BOLD responses to tactile stimulation (e.g., (Chen et al., 2012a; Chen et al., 2007; Yang et al., 2014; Zhang et al., 2007). With high intensity stimulation, in addition to area 3b, non-overlapping somatotopically organized lateromedial responses were also detected in areas 3a, 1 and 2, reflecting secondary inputs from high threshold cutaneous afferents via a thalamic relay, and area 3b dependent activation. These findings allowed us to study the dynamic changes in the representation of low and high threshold cutaneous afferents in somatosensory cortex after dorsal column lesions, exploiting the difference in CBV response between high and low intensity ES as a measure of the high threshold component.
This approach revealed significant area- and digit-level variations in recovery of cortical responsiveness to low and high intensity ES between animals. The nature of these variations, and their relation to the nature and severity of the DC lesion provide insights into the nature of the deactivation and subsequent reactivation of somatosensory cortex following spinal cord injury. Unsurprisingly, the nature and degree of activation was highly dependent on the nature and completeness of the DC lesion. As we now review, comparison of the temporal trajectories of topography and amplitude of single digit CBV responses to low and high intensity ES within and between animals, reveals different patterns of loss, recovery and/or reorganization of cortical responsiveness. These patterns appear consistent with lesion-associated acute modulation of primary and/or sensory inputs ranging from temporary (non-lethal) to permanent loss.
SM-BB (98% loss)
A near complete lesion abolished area 3b CBV response to both low and high levels of ES in all three digits (1–3) 2 weeks post-lesion. The reactivation of low intensity responsiveness of digit 1 by week 4, and digits 1–3 by week 6 in area 3b with pre-lesion somatotopy suggests that some of the acute loss of area 3b responsiveness arises from temporary non-lethal silencing of a small number of direct inputs for each digit, and that sparing of even this very small number (~2%), allowed recovery with near normal somatotopy despite the severity of the lesion. The silencing of all three digits’ responses to high intensity stimuli in areas 1 and 3a, with full recovery in area 1 and partial recovery in area 3a, suggests that the severity of the spinal cord lesion in this animal also caused temporary (~4 weeks, area 1) or possibly even permanent (≥6 weeks, area 3a) collateral interruption of indirect pathways to somatosensory cortex. The potential importance of these indirect pathways for reactivation of area 3b is illustrated by the earlier recovery of high, and particularly [high - low] intensity responsiveness for digits 1 and 2 in area 3b. Furthermore, the reorganization observed in higher order somatosensory cortex (areas 1, 2/5) in this animal suggests that even temporary loss of indirect inputs to these areas may drive significant reorganization
SM-Ge (79% loss)
DC lesion had no impact on the amplitudes of CBV responses to low intensity stimuli any digits in area 3b, but there was significant expansion of the size of all three digit representations at week 2 that persisted through to the final week 6 study. Interestingly, this expansion was accompanied by an increase in the high and [high-low] responsiveness of digits 1 and 2 compared with pre-lesion levels, suggesting an increase in indirect inputs to area 3b, possibly as a result of transient (< 2 weeks) suppression of direct inputs, allowing some expansion of indirect inputs in a still somatotopically organized manner.
SM-A (40% loss)
The somatotopy and low, high and [high-low] intensity amplitudes of CBV-fMRI maps 6 weeks post lesion closely matched the equivalent pre-lesion maps in areas 3b, 3a and 1, and were consistent with terminal microelectrode mapping 3 weeks later. All of these findings are consistent with the significant sparing of direct DC inputs. However, despite having the greatest sparing of direct inputs, and essentially complete recovery by week 6, fMRI mapping at weeks 2 and 4 post-lesion revealed significant digit-specific evolution in low, high, and particularly [high-low] intensity cortical responses. The transient loss of low intensity responsiveness of digits 2 and 3, with near complete recovery by week 4 suggests collateral but non-lethal injury of their DC inputs. Digits 2 and 3 showed similar - albeit longer duration - transient suppression of high intensity responsiveness in areas 3a and 1, suggesting there was similar non-lethal collateral damage to indirect pathways. Finally, the divergent high and [high-low] intensity CBV responses of digits 2 and 3 at week 2 suggest that transient interruption of indirect inputs had relatively little impact on cortical reactivation.
Possible mechanisms of cortical reactivation after sensory loss
Several mechanisms may contribute to cortical reorganization after sensory loss, including: (1) Through preexisting, but silent, direct inputs that become effective. As a clear example, Schroeder and colleagues (Schroeder et al., 1995; Schroeder et al., 1997) demonstrated that electrical stimulation of the radial nerve from the skin of the forearm in squirrel monkeys produced a subtle short-latency non-spiking response in layer 4 followed by a clear excitatory multi-unit activity (MUA) 12–15 ms later in the representation of the glabrous hand in area 3b of somatosensory cortex. However, the amplitude of this response was progressively increased and the latency was progressively decreased over weeks of recovery after section of the median nerve to the glabrous hand. Thus, in the absence of a normally effective source of activation, preexisting sources of subthreshold activation can become superthreshold. In the present experiment, the greater effectiveness of the high intensity stimulation may be a result of more effective stimulation of the direct DC pathway. Although peripheral nerve section differs in many ways from dorsal column lesions, similar mechanisms may be involved after deafferentation to the somatosensory system. A large body of evidence indicates that non-dominant dorsal skin (radial nerve territory) is over-represented in the reactivated hand cortex after the dominant inputs from the glabrous skin of the hand are lost due to peripheral nerve injury. This result suggests that preserved dorsal skin afferents are potentiated when dominant glabrous skin afferents are removed (Churchill and Garraghty, 2006; Churchill et al., 1998; Garraghty and Kaas, 1991; Merzenich et al., 1983a; Merzenich et al., 1983b; Merzenich et al., 1984). (2) It is also possible that other, higher threshold pathways are also activated, and may potentiate and supplement the activation produced by the low threshold pathway. Such second order neurons include both those projecting to the cuneate nucleus, which may produce slightly longer response latency of reactivated cortical neurons, as we have observed (Reed et al., 2014); proprioceptive pathways of the lateral cervical spinal cord pathway; or even the spinothalamic pathway, which may amplify the cortical signal relayed from the cuneate nucleus. (3) Through axon sprouting. After DC lesion, surviving direct and indirect inputs to the cuneate nucleus may sprout and synapse onto more neurons as result of the reduction in competition for synaptic space. Thus, more cuneate neurons would be activated by surviving inputs, and in turn activate more cortical territory.
The spinothalamic pathway is likely to provide some of the reactivation of somatosensory cortex since this pathway is uncut with a DC lesion. Although the spinothalamic tract has been associated with pain and temperature sensation, it also includes neurons responsive to even light touch, including wide dynamic range (WDR) neurons that increase activity with stimulus intensity into the range of painful stimuli and eventually relay their activity to somatosensory cortex (Kenshalo et al., 2000). C-tactile afferents have drawn more attention in recent years as studies have found that a slow “second” touch system from hairy skin is mainly involved in the affiliative and rewarding properties of touch (see McGlone et al., 2014 for review). Indeed, current findings, together with early studies from the lab, show that dorsal skin is over represented after DC lesion (Qi et al., 2011; Qi et al., 2014a). The spinothalamic tract projects to several thalamic nuclei with different functional roles, including the ventroposterior inferior (VPI) nucleus and, sparsely, the ventroposterior nucleus (Stepniewska et al., 2003), both of which project to area 3b and other areas (Kaas, 2011). The spinothalamic neurons that project to these targets are found in dorsal horn layers that receive afferents responsive to touch (Craig, 2006; Price and Mayer, 1974).
Our present findings suggest that the spinothalamic tract may mediate some of the reactivation of somatosensory cortex after DC lesion. Low intensity ES evokes restricted CBV responses in area 3b, whereas areas 3a, 1, 2, and 5 require higher intensity stimuli, suggesting that activation of these areas is normally driven in part by spinothalamic tract afferents. Another interesting finding in the present study is that 4 weeks post-lesion, high intensity stimulation evoked CBV responses not only in areas 3a, 3b, and 1, but also in areas 2 and 5. As activation of those areas was rarely detected in pre-lesion fMRI, these results suggest potentiation of remaining sensory inputs well beyond those recorded in monkeys with intact spinal cords. However, the thalamic targets of the spinothalamic projection appear to project mainly to insular cortex, the region of S2 and PV, and cingulate cortex, and only weakly to areas 3b and 1 (Dum et al., 2009). Weak connections may become potentiated during the recovery period after injury.
Conclusions
Functional recovery following spinal cord injury depends in a complex way on the nature and duration of axonal injury, the pathways involved, and the relative contributions of spared and recovering inputs to both salutary and adverse consequences of cortical reorganization and reactivation post-injury. By tracking the spatiotemporal trajectories of cortical reactivation by low and high intensity electrocutaneous stimulation of digits following a dorsal column lesion in monkeys, we were able to distinguish the contributions of permanent axonal loss and temporary silencing of spinal cord pathways to acute cortical deactivation, and the relative contributions of direct and secondary pathways to reactivation and reorganization.
Our results emphasize the heterogeneous nature of the acute injury, and the dynamic and variable trajectories of recovery of cortical responsiveness, even in a relatively well-defined and reproducible SC injury model. They further confirm that significant reactivation of cortical areas can occur despite near total loss of primary cortical inputs: even after an extensive or partial loss of dorsal column inputs from the hand, the cortical hand representation becomes extensively reactivated by the spared afferents originating from the hand. Finally, they provide evidence for the importance of second order pathways for the reactivation and reorganization of cortical areas silenced following loss or even transient silencing of primary inputs.
In concluding, we note the direct translational potential of our approach to the study and treatment of spinal cord injury in patients. High resolution functional mapping of the loss and subsequent recovery of cortical responses to low and high intensity somatosensory stimuli can be used to distinguish between loss and temporary silencing of direct and/or secondary pathways following injury, their respective contributions to cortical reactivation and clinical recovery, and the impact of therapy.
Supplementary Material
Somatotopic maps of areas 3b and 1 of squirrel monkeys SM-Ge (79% loss) and SM-A (40% loss). Somatotopic maps show the locations and receptive field properties of microelectrode penetrations in areas 3b and 1 of squirrel monkeys SM-A (9 weeks) and SM-Ge (8 weeks) after lesion of the contralateral dorsal columns. Both monkeys received incomplete lesions in the DC of cervical spinal cord (40% loss in SM-A, 79% loss in SM-Ge). A. A microelectrode somatotopic map showing the regions of cortex with neurons representing the distal, middle, and proximal parts of digits in squirrel monkey SM-Ge. B. Somatotopic map shows regions where neurons had receptive field on dorsal skin in SM-Ge. C. Somatotopic map of SM-A. The regions of cortex with neuronal receptive fields on distal phalanges of digits D1 – D5 are shaded in dark green, middle phalanges in purple, and proximal phalanges in yellow. Similar to somatotopic maps of normal monkeys reported in earlier studies, distal to proximal parts of the hand are represented in a rostrocaudal orientation in area 3b. In SM-A, the territory representing the distal phalanges of digits is abnormally large. D. Somatotopic map marks the cortical regions with neuronal receptive fields on dorsal skin. Compared to normal monkeys, the dorsal skin representations are abnormally large in SM-A. In AD, solid circles mark penetrations with good responses; black open circles mark those with weak responses; black diamonds mark those with very weak responses to hard taps; x’s mark microelectrode penetrations with no responses. Dotted black lines mark areal boundaries based on myelin architecture. Stars mark electrolytic lesions sites. Conventions follow Figure 1.
HIGHLIGHTS.
High-resolution cerebral blood volume (CBV) functional MRI mapping of cortical responses to low and high intensity electrocutaneous digit stimulation can distinguish and track digit-specific deactivation, reactivation and reorganization of somatosensory cortex by primary and secondary pathways after sensory loss in monkeys.
After a dorsal column lesion, high intensity nociceptive stimulation activates more of the cortical network than innocuous stimuli.
Even after an extensive loss of DC inputs from the hand (98%), the cortical hand representation in areas 3b and 1 becomes extensively reactivated by spared direct and secondary afferents.
The results suggest that the spared afferents and second order spinal cord pathways likely contribute to the cortical reactivation observed after dorsal column lesions.
Acknowledgments
We thank Laura Trice for excellent technical support; Dr. Christina Cerkevich for data collection; Dr. Jamie L. Reed for statistical data analysis and valuable suggestions on the manuscript; Jarrod True, Ken Wilkens, and Daniel Colvin for assistance in MRI. This research was supported by National Institute of Health [NS16446] to JHK, [DA028303] to MJA, [NS067017] to HXQ.
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Glauche V, Knab R, Glascher J, Weiller C, Buchel C. Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. Neuroimage. 2004;23:224–232. doi: 10.1016/j.neuroimage.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Bowes C, Burish M, Cerkevich C, Kaas J. Patterns of cortical reorganization in the adult marmoset after a cervical spinal cord injury. J Comp Neurol. 2013;521:3451–3463. doi: 10.1002/cne.23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Costa T, Diano M, Sacco K, Duca S, Geminiani G, Torta DM. Massive modulation of brain areas after mechanical pain stimulation: a time-resolved FMRI study. Cereb Cortex. 2014;24:2991–3005. doi: 10.1093/cercor/bht153. [DOI] [PubMed] [Google Scholar]
- Chen LM, Dillenburger BC, Wang F, Friedman RM, Avison MJ. High-resolution functional magnetic resonance imaging mapping of noxious heat and tactile activations along the central sulcus in New World monkeys. Pain. 2011;152:522–532. doi: 10.1016/j.pain.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Dillenburger BC, Wang F, Tang CH. Differential fMRI activation to noxious heat and tactile stimuli in parasylvian areas of new world monkeys. Pain. 2012b;153:158–169. doi: 10.1016/j.pain.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Qi HX, Kaas JH. Dynamic reorganization of digit representations in somatosensory cortex of nonhuman primates after spinal cord injury. J Neurosci. 2012a;32:14649–14663. doi: 10.1523/JNEUROSCI.1841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Turner GH, Friedman RM, Zhang N, Gore JC, Roe AW, Avison MJ. High-resolution maps of real and illusory tactile activation in primary somatosensory cortex in individual monkeys with functional magnetic resonance imaging and optical imaging. J Neurosci. 2007;27:9181–9191. doi: 10.1523/JNEUROSCI.1588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill JD, Garraghty PE. The influence of post-nerve injury survival duration on receptive field size: location, location, location. Neurosci Lett. 2006;405:10–13. doi: 10.1016/j.neulet.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Muja N, Myers WA, Besheer J, Garraghty PE. Somatotopic consolidation: a third phase of reorganization after peripheral nerve injury in adult squirrel monkeys. Exp Brain Res. 1998;118:189–196. doi: 10.1007/s002210050271. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Goldring AB, Baldwin MK, Recanzone GH, Chen A, Pan T, Simon SI, Krubitzer L. Reversible deactivation of higher-order posterior parietal areas. I. Alterations of receptive field characteristics in early stages of neocortical processing. J Neurophysiol. 2014;112:2529–2544. doi: 10.1152/jn.00140.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Burton H, Sinclair RJ, Conturo TE, Akbudak E, McDonald JW. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc Natl Acad Sci U S A. 2002;99:17066–17071. doi: 10.1073/pnas.262669099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Retrograde analyses of spinothalamic projections in the macaque monkey: input to ventral posterior nuclei. J Comp Neurol. 2006;499:965–978. doi: 10.1002/cne.21154. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15:149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Ciferri MM. Loss and recovery of voluntary hand movements in the macaque following a cervical dorsal rhizotomy. J Comp Neurol. 2005;491:27–45. doi: 10.1002/cne.20686. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D. Somatotopic activation in the human trigeminal pain pathway. J Neurosci. 2002;22:8183–8192. doi: 10.1523/JNEUROSCI.22-18-08183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci. 2009;29:14223–14235. doi: 10.1523/JNEUROSCI.3398-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Kambi N, Raghunathan P, Khushu S, Jain N. Large-scale reorganization of the somatosensory cortex of adult macaque monkeys revealed by fMRI. Brain Struct Funct. 2014;219:1305–1320. doi: 10.1007/s00429-013-0569-8. [DOI] [PubMed] [Google Scholar]
- Florence SL, Hackett TA, Strata F. Thalamic and cortical contributions to neural plasticity after limb amputation. J Neurophysiol. 2000;83:3154–3159. doi: 10.1152/jn.2000.83.5.3154. [DOI] [PubMed] [Google Scholar]
- Florence SL, Jain N, Pospichal MW, Beck PD, Sly DL, Kaas JH. Central reorganization of sensory pathways following peripheral nerve regeneration in fetal monkeys. Nature. 1996;381:69–71. doi: 10.1038/381069a0. [DOI] [PubMed] [Google Scholar]
- Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. Central projections from the skin of the hand in squirrel monkeys. J Comp Neurol. 1991;311:563–578. doi: 10.1002/cne.903110410. [DOI] [PubMed] [Google Scholar]
- Frey SH, Bogdanov S, Smith JC, Watrous S, Breidenbach WC. Chronically deafferented sensory cortex recovers a grossly typical organization after allogenic hand transplantation. Curr Biol. 2008;18:1530–1534. doi: 10.1016/j.cub.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurological Research. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Kaas JH. Large-scale functional reorganization in adult monkey cortex after peripheral nerve injury. Proc Natl Acad Sci U S A. 1991;88:6976–6980. doi: 10.1073/pnas.88.16.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Kaas JH. Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in new world monkeys. Cereb Cortex. 2011;21:1981–2002. doi: 10.1093/cercor/bhq260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, de la Mothe LA. Regional and laminar distribution of the vesicular glutamate transporter, VGluT2, in the macaque monkey auditory cortex. J Chem Neuroanat. 2009;38:106–116. doi: 10.1016/j.jchemneu.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K, Wang X, Qiu Y, Nguyen BT, Ojima S, Tamura Y, Nakata H, Wasaka T, Tran TD, Kakigi R. Pain processing within the primary somatosensory cortex in humans. Eur J Neurosci. 2003;18:2859–2866. doi: 10.1111/j.1460-9568.2003.02995.x. [DOI] [PubMed] [Google Scholar]
- Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- Jain N, Florence SL, Qi HX, Kaas JH. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci U S A. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Qi HX, Collins CE, Kaas JH. Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. J Neurosci. 2008;28:11042–11060. doi: 10.1523/JNEUROSCI.2334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Somatosensory system. In: Mai JK, Paxinos G, editors. The human nervous system. ElsevierAcademic Press; London: 2011. pp. 1064–1099. [Google Scholar]
- Kaas JH, Florence SL. Mechanisms of reorganization in sensory systems of primates after peripheral nerve injury. Adv Neurol. 1997;73:147–158. [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenshalo DR, Iwata K, Sholas M, Thomas DA. Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol. 2000;84:719–729. doi: 10.1152/jn.2000.84.2.719. [DOI] [PubMed] [Google Scholar]
- Lecoeur J, Wang F, Chen LM, Dawant BM, Avison MJ. Sub-millimeter coregistration of functional maps across imaging sessions. Proc Int Soc Mag Reson Med. 2011b;19:1625. [Google Scholar]
- Lecoeur J, Wang F, Chen LM, Li R, Avison MJ, Dawant BM. Automated longitudinal registration of high resolution structural MRI brain sub-volumes in non-human primates. J Neurosci Methods. 2011a;202:99–108. doi: 10.1016/j.jneumeth.2011.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoeur J, Wang F, Chen LM, Li R, Avison MJ, Dawant BM. Co-registration of high resolution MRI scans with partial brain coverage in non-human primates. Proc SPIE Int Soc Opt Eng. 2011c:7962. doi: 10.1117/12.877024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CC, Reed JL, Kaas JH, Qi HX. Intracortical connections are altered after long-standing deprivation of dorsal column inputs in the hand region of area 3b in squirrel monkeys. J Comp Neurol. 2015 doi: 10.1002/cne.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain. 2008;138:362–374. doi: 10.1016/j.pain.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Makin TR, Filippini N, Duff EP, Henderson Slater D, Tracey I, Johansen-Berg H. Network-level reorganisation of functional connectivity following arm amputation. Neuroimage. 2015a;114:217–225. doi: 10.1016/j.neuroimage.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin TR, Scholz J, Henderson Slater D, Johansen-Berg H, Tracey I. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain. 2015b;138:2140–2146. doi: 10.1093/brain/awv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Formaggio E, Storti SF, Avesani M, Acler M, Sala F, Magon S, Zoccatelli G, Pizzini F, Alessandrini F, Fiaschi A, Beltramello A. Steady-state activation in somatosensory cortex after changes in stimulus rate during median nerve stimulation. Magn Reson Imaging. 2009;27:1175–1186. doi: 10.1016/j.mri.2009.05.009. [DOI] [PubMed] [Google Scholar]
- McGlone F, Wessberg J, Olausson H. Discriminative and affective touch: sensing and feeling. Neuron. 2014;82:737–755. doi: 10.1016/j.neuron.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Sur M, Lin CS. Double representation of the body surface within cytoarchitectonic areas 3b and 1 in “SI” in the owl monkey (Aotus trivirgatus) J Comp Neurol. 1978;181:41–73. doi: 10.1002/cne.901810104. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983a;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983b;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Kaas JH, Stryker MP, Jenkins WM, Zook JM, Cynader MS, Schoppmann A. Variability in hand surface representations in areas 3b and 1 in adult owl and squirrel monkeys. J Comp Neurol. 1987;258:281–296. doi: 10.1002/cne.902580208. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Moore CI, Stern CE, Dunbar C, Kostyk SK, Gehi A, Corkin S. Referred phantom sensations and cortical reorganization after spinal cord injury in humans. Proc Natl Acad Sci U S A. 2000;97:14703–14708. doi: 10.1073/pnas.250348997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Cerkevich C, Engle J, Rajan AT, Recanzone G, Kaas J, Krubitzer L. Thalamocortical connections of parietal somatosensory cortical fields in macaque monkeys are highly divergent and convergent. Cereb Cortex. 2009;19:2038–2064. doi: 10.1093/cercor/bhn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Recanzone G, Engle J, Cooke D, Goldring A, Krubitzer L. Lesions in posterior parietal area 5 in monkeys result in rapid behavioral and cortical plasticity. J Neurosci. 2010;30:12918–12935. doi: 10.1523/JNEUROSCI.1806-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Cusick CG, Kaas JH. A sequential representation of the occiput, arm, forearm and hand across the rostrocaudal dimension of areas 1, 2 and 5 in macaque monkeys. Brain Research. 1985;335:350–353. doi: 10.1016/0006-8993(85)90492-5. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Price DD, Mayer DJ. Physiological laminar organization of the dorsal horn of M. mulatta. Brain Research. 1974;79:321–325. doi: 10.1016/0006-8993(74)90425-9. [DOI] [PubMed] [Google Scholar]
- Qi HX, Chen LM, Kaas JH. Reorganization of somatosensory cortical areas 3b and 1 after unilateral section of dorsal columns of the spinal cord in squirrel monkeys. J Neurosci. 2011;31:13662–13675. doi: 10.1523/JNEUROSCI.2366-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi HX, Gharbawie OA, Wynne KW, Kaas JH. Impairment and recovery of hand use after unilateral section of the dorsal columns of the spinal cord in squirrel monkeys. Behav Brain Res. 2013;252:363–376. doi: 10.1016/j.bbr.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi HX, Kaas JH. Organization of primary afferent projections to the gracile nucleus of the dorsal column system of primates. Journal of Comparative Neurology. 2006;499:183–217. doi: 10.1002/cne.21061. [DOI] [PubMed] [Google Scholar]
- Qi HX, Kaas JH, Reed JL. The reactivation of somatosensory cortex and behavioral recovery after sensory loss in mature primates. Front Syst Neurosci. 2014b;8:84. doi: 10.3389/fnsys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi HX, Reed JL, Gharbawie OA, Burish MJ, Kaas JH. Cortical neuron response properties are related to lesion extent and behavioral recovery after sensory loss from spinal cord injury in monkeys. J Neurosci. 2014a;34:4345–4363. doi: 10.1523/JNEUROSCI.4954-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Qi H-X, Kaas JH. Dorsal column lesions in monkeys differentially affect widespread activity and response properties of putative inhibitory and excitatory neurons in area 3b. Society for Neuroscience Abstract Conference: 44nd Annual Meeting of the Society-for-Neuroscience.2014. [Google Scholar]
- Ruben J, Krause T, Taskin B, Blankenburg F, Moosmann M, Villringer A. Sub-area-specific Suppressive Interaction in the BOLD responses to simultaneous finger stimulation in human primary somatosensory cortex: evidence for increasing rostral-to-caudal convergence. Cereb Cortex. 2006;16:819–826. doi: 10.1093/cercor/bhj025. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Ploner M. Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol. 2000;17:592–603. doi: 10.1097/00004691-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Seto S, Arezzo JC, Garraghty PE. Electrophysiological evidence for overlapping dominant and latent inputs to somatosensory cortex in squirrel monkeys. J Neurophysiol. 1995;74:722–732. doi: 10.1152/jn.1995.74.2.722. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Seto S, Garraghty PE. Emergence of radial nerve dominance in median nerve cortex after median nerve transection in an adult squirrel monkey. J Neurophysiol. 1997;77:522–526. doi: 10.1152/jn.1997.77.1.522. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Sakai ST, Qi HX, Kaas JH. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: potential substrate for parkinsonian tremor. J Comp Neurol. 2003;455:378–395. doi: 10.1002/cne.10499. [DOI] [PubMed] [Google Scholar]
- Sur M, Nelson RJ, Kaas JH. Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: comparisons with other primates. J Comp Neurol. 1982;211:177–192. doi: 10.1002/cne.902110207. [DOI] [PubMed] [Google Scholar]
- Tandon S, Kambi N, Lazar L, Mohammed H, Jain N. Large-scale expansion of the face representation in somatosensory areas of the lateral sulcus after spinal cord injuries in monkeys. J Neurosci. 2009;29:12009–12019. doi: 10.1523/JNEUROSCI.2118-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Jr, Cooper BY. Cutaneous texture discrimination following transection of the dorsal spinal column in monkeys. Somatosens Mot Res. 1998;15:309–315. doi: 10.1080/08990229870718. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Jr, Greenspan JD, Ritz LA. Long-term changes in purposive and reflexive responses to nociceptive stimulation following anterolateral chordotomy. J Neurosci. 1990;10:2077–2095. doi: 10.1523/JNEUROSCI.10-07-02077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Friedman RM, Tang CH, Avison MJ. Vibrotactile and electrocutaneous stimuli activate distinct but overlapping areas in primary somatosensory cortex that differ in their stimulus-response properties. Proc Int Soc Mag Reson Med. 2012;20:2176. [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Research. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Xerri C. Plasticity of cortical maps: multiple triggers for adaptive reorganization following brain damage and spinal cord injury. Neuroscientist. 2012;18:133–148. doi: 10.1177/1073858410397894. [DOI] [PubMed] [Google Scholar]
- Yang PF, Qi HX, Kaas JH, Chen LM. Parallel functional reorganizations of somatosensory areas 3b and 1, and S2 following spinal cord injury in squirrel monkeys. J Neurosci. 2014;34:9351–9363. doi: 10.1523/JNEUROSCI.0537-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Gore JC, Chen LM, Avison MJ. Dependence of BOLD signal change on tactile stimulus intensity in SI of primates. Magn Reson Imaging. 2007;25:784–794. doi: 10.1016/j.mri.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wang F, Turner GH, Gore JC, Avison MJ, Chen LM. Intra- and inter-subject variability of high field fMRI digit maps in somatosensory area 3b of new world monkeys. Neuroscience. 2010;165:252–264. doi: 10.1016/j.neuroscience.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Somatotopic maps of areas 3b and 1 of squirrel monkeys SM-Ge (79% loss) and SM-A (40% loss). Somatotopic maps show the locations and receptive field properties of microelectrode penetrations in areas 3b and 1 of squirrel monkeys SM-A (9 weeks) and SM-Ge (8 weeks) after lesion of the contralateral dorsal columns. Both monkeys received incomplete lesions in the DC of cervical spinal cord (40% loss in SM-A, 79% loss in SM-Ge). A. A microelectrode somatotopic map showing the regions of cortex with neurons representing the distal, middle, and proximal parts of digits in squirrel monkey SM-Ge. B. Somatotopic map shows regions where neurons had receptive field on dorsal skin in SM-Ge. C. Somatotopic map of SM-A. The regions of cortex with neuronal receptive fields on distal phalanges of digits D1 – D5 are shaded in dark green, middle phalanges in purple, and proximal phalanges in yellow. Similar to somatotopic maps of normal monkeys reported in earlier studies, distal to proximal parts of the hand are represented in a rostrocaudal orientation in area 3b. In SM-A, the territory representing the distal phalanges of digits is abnormally large. D. Somatotopic map marks the cortical regions with neuronal receptive fields on dorsal skin. Compared to normal monkeys, the dorsal skin representations are abnormally large in SM-A. In AD, solid circles mark penetrations with good responses; black open circles mark those with weak responses; black diamonds mark those with very weak responses to hard taps; x’s mark microelectrode penetrations with no responses. Dotted black lines mark areal boundaries based on myelin architecture. Stars mark electrolytic lesions sites. Conventions follow Figure 1.