Abstract
Mounting evidence indicates that adolescents exhibit heightened sensitivity to rewards and reward-related cues compared to adults, and that adolescents are often unable to exert behavioral control in the face of such cues. Moreover, differences in reward processing during adolescence have been linked to heightened risk taking and impulsivity. However, little is known about the processes by which adolescents learn about the appetitive properties of environmental stimuli that signal reward. To address this, Pavlovian conditioning procedures were used to test for differences in excitatory conditioning between adult and adolescent rats using various schedules of reinforcement. Specifically, separate cohorts of adult and adolescent rats were trained under conditions of consistent (continuous) or intermittent (partial) reinforcement. We found that the acquisition of anticipatory responding to a continuously-reinforced cue proceeded similarly in adolescents and adults. In contrast, responding increased at a greater rate in adolescents compared to adults during presentations of a partially-reinforced cue. We subsequently compared the ability of adolescent and adult rats to dynamically adjust the representation of a reward-predictive cue during extinction trials, in which a secondary inhibitory representation is acquired for the previously-reinforced stimulus. We observed significant age differences in the ability to flexibly update cue representations during extinction, in that the appetitive properties of cues with a history of either continuous or partial reinforcement persisted to a greater extent in adolescents relative to adults.
Keywords: Adolescence, Learning, Reinforcement, Appetitive, Pavlovian
1. Introduction
Adolescence is a dynamic period of development that is characterized by unique changes in the brain as well as behavior [1–7]. In particular, adolescent humans and other animals tend to make more impulsive decisions and engage in more risk-taking behavior compared to either adults or pre-adolescents [8–12]. Although this behavioral phenotype is important for gaining skills and experience necessary for becoming an independent adult, it can also lead to negative outcomes. Indeed, adolescence is marked by vulnerability to substance abuse, increased chance of injury and premature death [10, 13].
The behavioral and neurobiological factors that contribute to heightened risk-taking and impulsivity during adolescence remain unclear. However, a growing body of evidence indicates that at least one contributing factor is an inability to control behavior in the face of reward [8–10, 14]. Prior studies have demonstrated an increased sensitivity to primary rewards during adolescence compared to adulthood [2, 9, 15–22, 23]. Similarly, environmental cues that come to predict reward drive behaviors performed in service of obtaining reward to a greater extent in adolescents than in children or adults [1, 2, 18, 24–28].
For example, findings in laboratory animals suggest that motivation for many natural rewards may be enhanced during adolescence. Indeed, during a conditioned place preference paradigm adolescent rats will spend more time than adults in a chamber that has been previously paired with a rewarding stimulus, such as a novel object or social interaction, relative to an alternative chamber [8, 29]. Similarly, adolescent humans exhibit difficulties suppressing responding specifically to appetitive cues (e.g. emotionally salient faces) compared to either children or adults [2, 12, 30]. Along similar lines, substantial research suggests that adolescents are highly sensitive to the rewarding properties of alcohol and other drugs of abuse [31–40], which may contribute to the development of drug use and addiction during adolescence. Thus, an understanding of how appetitive conditioning processes develop across adolescence may be useful to mitigate health issues that present during this period [13].
Most prior studies of reward-related behavior in adolescents have focused on behavioral responding during tasks where performance has reached asymptotic levels [1, 2, 19, 20, 26, 27]. In comparison, surprisingly little is known about whether developmental differences exist in the acquisition of stimulus-reward associations (i.e., excitatory conditioning). Alterations in the rate or magnitude of excitatory conditioning could impact many aspects of behavior, particularly during adolescence when individuals are engaged in increased exploration and novelty-seeking, thus experiencing a range of environmental cues for the first time and actively learning the meaning of those cues. For example, more rapid acquisition of stimulus-reward contingencies could contribute to the emergence of maladaptive behaviors, such as smoking and substance abuse. Alterations in excitatory conditioning could also affect an organism’s ability to inhibit behavior. Indeed, studies in both humans and laboratory animals, using a range of behavioral paradigms, indicate that adolescents often experience difficulty using environmental cues to withhold behavior [2, 5, 41–50]. This could result, for example, from stronger associations among competing excitatory cues.
To address this, the present experiments tested how Pavlovian stimuli acquire reinforcing properties in adolescent and adult rats. In Experiment 1, rats were trained on a continuous reinforcement schedule, in which they learned to discriminate between a cue that was always followed by reinforcement (continuously reinforced cue; CRF+) and a second cue that was never reinforced (CRF−). In Experiment 2, another set of adolescent and adult rats were trained on a partial reinforcement schedule, in which they learned to respond to a cue that was reinforced on only a subset of trials (partially reinforced; PRF+/PRF−), in order to determine if there are age-related differences in associations involving partial versus continual reinforcement. After the conditioning phase, rats in both experiments underwent extinction training to test for age-related differences in the ability to learn a secondary representation of a previously-reinforced stimulus. Indeed, the ability to update the meaning of a stimulus that has previously been paired with reward is critical for adaptive behavior in a changing environment, yet few studies have examined extinction in adolescents.
2. Materials and Methods
2.1 Subjects
Naïve male Long Evans rats were obtained from Harlan Laboratories (Indianapolis, IN) at either 21 (n = 32) or 56 (n = 32) days of age. Juvenile rats were weaned from their dam on PND 21, and were shipped and received on the same day. Rats were initially group-housed and allowed at least one week to acclimate to the colony room prior to beginning food restriction and behavioral training. The colony room was maintained at 72°F. Water was available ad libitum throughout the experiment. Food (2014 Teklad Global 14% Protein Rodent Maintenance Diet, Harlan Laboratories) was available ad libitum until one week prior to behavioral training. During the week prior to behavioral training, rats were separated into individual cages, handled and weighed daily. Body weights were gradually reduced over a four day period to 85% of the daily weight of free-feeding age-matched control rats. All groups remained food restricted until completion of behavioral training, with supplemental rat chow provided after each daily session to maintain the target weight. The colony room was maintained on a 14:10 h light–dark cycle and monitored and cared for in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
2.2 Behavioral apparatus
Behavioral procedures were carried out in standard conditioning chambers (Med Associates). The chambers (24 × 30.5 × 29 cm) consisted of aluminum front and back walls, clear acrylic sides and top, and grid floors. Each chamber was outfitted with a dimly illuminated food cup, recessed in the center of the front wall, and a speaker located 15 cm above and to the right of the food cup, used to present the auditory conditioned stimuli, a white noise (78-dB) and a clicker (Experiment 1) or a 1500-Hz, 78-dB tone (Experiment 2). Delivery of two 45-mg food pellets (Bioserv) served as the unconditioned stimulus (US). Each chamber was equipped with a pair of infrared photocells located across the entrance to the food cup to monitor entries into the cup and connected to a PC-clone computer. Each chamber was enclosed in a sound-attenuating cubicle (62 × 56 × 56 cm) with an exhaust fan to provide airflow and background noise (~68 dB) and a red house-light (mounted on the ceiling) to provide background illumination. The cubicles also contained surveillance cameras used to monitor the rats during behavioral training.
2.3 Behavioral procedure
At the start of each training session rats were moved in plastic transporters from the colony room to the conditioning chambers. Rats were first trained to eat from the food cup during a single 61-min session in which one 45-mg food pellet was delivered every minute, with the exception that no more than three food pellets could accumulate in the food cup.
2.3.1 Experiment 1: Continuous reinforcement conditioning
During the Acquisition phase of the experiment, rats underwent eight daily training sessions of Pavlovian excitatory conditioning using a continuous schedule of reinforcement. This procedure consisted of daily 61-min sessions with eight reinforced and eight non-reinforced trials. During reinforced trials the white noise or clicker (counterbalanced; CRF+) was presented for 5 sec and followed immediately by delivery of the US. On non-reinforced trials, the other cue (clicker or white noise, counterbalanced; CRF−) was presented for 5 sec, after which no US was delivered. The two trial types occurred pseudo-randomly during each session, with no more than two consecutive reinforced or non-reinforced trials. The presentation order and the inter-trial intervals (ITI) varied daily (average ITI of 3.6 min, ranging from 1.7 to 5.6 min). Rats in the adolescent group began training on postnatal day (PND) 35 and rats in the adult group started training on PND 70.
Immediately following Acquisition, rats underwent two Extinction Training sessions that were each 66-min in duration and consisted of 30 non-reinforced presentations of each auditory stimulus (a total of 60 trials). The two stimuli occurred pseudo-randomly during each session, with no more than three consecutive presentations of either stimulus. The presentation order and the ITIs varied daily (average ITI of 1 min, ranging from 0.3 to 1.7 min). Finally, rats underwent an Extinction Test session (30 non-reinforced presentations of each stimulus) four days following the end of extinction training. This test session occurred when rats in the adolescent group were age PND 48.
2.3.2 Experiment 2: Partial reinforcement conditioning
Previous research has established that learning is mediated by the information that one stimulus provides about another [51–54]. In particular, the temporal information that a stimulus provides about a reinforcer is critical in establishing the conditioned response patterns to that stimulus [55–58]. Experiment 1 considered how adult and adolescent rats learned about a cue that acquired excitatory and inhibitory properties in sequence. Conversely, Experiment 2 tested for age differences in acquiring similar competing properties simultaneously, i.e., a partially reinforced cue. Traditionally, the more reliably the stimulus is associated with the reinforcer, the more motivated the animal becomes to produce a conditioned response [59, 60]. To date, minimal research has considered how adolescents respond to differences in the predictive validity of a stimulus. However, recent evidence has indicated that adolescents more readily engage in behaviors for which the outcome is uncertain relative to adults [61, 62]. Furthermore, mounting evidence in adults indicates that the reinforcement history of a cue influences both initial and long-term learning about the cue’s meaning, as well as the responses that occur in the presence of the cue [63–69]. Thus, Experiment 2 determined how adolescents learn about a conditioned reinforcer that does not always predict reinforcement (i.e., a partial reinforcement schedule).
During the Acquisition phase, rats underwent 10 daily training sessions in a partial reinforcement procedure consisting of daily 61-min sessions in which a 5-sec tone was presented 16 times. Four of these presentations were immediately followed by delivery of the US, while the other 12 presentations were not reinforced. The two trial types occurred pseudo-randomly during each session, with no more than three consecutive reinforced (PRF+) or non-reinforced (PRF−) trials. The presentation order and the ITIs varied daily (average ITI of 3.6 min, ranging from 1.7 to 5.6 min). Rats in the adolescent group began training on PND 35 and rats in the adult group started training on PND 70.
Immediately following Acquisition, rats underwent two Extinction Training session (68-min each) consisting of 32 presentations of the tone. The ITIs varied daily (average ITI of 1 min, ranging from 0.3 to 1.7 min). Rats underwent a final Extinction Test session (32 non-reinforced presentation of the tone) four days following the end of extinction training. The Test session occurred when rats in the adolescent group were age PND 50.
2.4 Data analysis
2.4.1 Experiment 1
The primary measure of interest was the number of snout entries into the food cup during presentation of the conditioned stimuli. For each session of the Acquisition phase, the average number of snout entries during each cue (CRF+, CRF−) was calculated and the data were subjected to a repeated measures analysis of variance (ANOVA) with Age as the between subjects factor and Session and Trial type as within subjects factors. During the two Extinction Training sessions, the number of snout entries during each cue was averaged for the first and last two trials in each session. The data were analyzed using a repeated measures ANOVA with Age as the between subjects factor and Block and Trial type as the within subjects factors. During the Extinction Test session, snout entries during each cue was averaged for the first two trials and the data were analyzed using a repeated measures ANOVA with Age as the between subjects factor and Trial type as the within subjects factor. Paired-sample t-tests were used to analyze responding during the CRF+ within each age group between the end of an extinction session and the beginning of the next session (i.e. retention of conditioning during extinction training or spontaneous recovery during the test).
To assess potential group differences in motivation to consume the food pellets, we recorded the number of snout entries into the food cup during the 5-sec period after food was delivered on CRF+ trials during the Acquisition phase. In addition, the number of snout entries into the food cup during the CRF− was used to test for age-related differences in baseline responding (since responding was expected to be very low in the absence of reinforcement). The data were collapsed across sessions and subjected to an independent measures t-test.
All analyses were conducted using SPSS statistical software and used an alpha level of 0.05. Significant ANOVAs were decomposed with appropriate pair-wise comparisons using an alpha level of 0.05.
2.4.2 Experiment 2
For the Acquisition sessions, the average number of snout entries during presentation of the tone were analyzed using a repeated measures ANOVA with Age as the between subjects factor and Session as the within subjects factor. During Extinction Training, snout entries during the tone were averaged for the first and last two trials in each session and the data were analyzed using a repeated measures ANOVA with Age as the between subjects factor and Block as the within subjects factor. During the Extinction Test, snout entries during the tone were averaged for the first two trials and analyzed using an independent samples t-test. Paired-sample t-tests were used to analyze responding during the tone within each age group between the end of an extinction session and the beginning of the next session (i.e. retention of conditioning during extinction or spontaneous recovery during test).
As in Experiment 1, potential group-differences in motivation to consume the food pellets was assessed via the amount of time each rat spent with the snout in the food cup during the 5-sec period after food was delivered during the Acquisition phase. The number of snout entries into the food cup during the 5 sec period immediately before the tone was presented serves as a measure of baseline responding. The data were collapsed across sessions and subjected to an independent measures t-test.
3. Results
3.1 Experiment 1: Continuous reinforcement
One rat in the adult group exhibited abnormally low baseline levels of responding at least one standard deviation below the group average across all three phases of the experiment. The data from this rat was excluded from the analyses, resulting in n = 15 for this group.
3.1.1 Acquisition
As show in Figure 1, the rates and levels of conditioned responding to either the CRF+ or CRF− during the Acquisition phase were comparable between adolescent and adult rats. This was supported by a multi-factor ANOVA, which revealed that responding was greater during CRF+ than CRF−, indicating that rats could discriminate between the reinforced cue and the non-reinforced cue [F(1, 29) = 37.59, p < 0.001]. In addition there was a main effect of Session [F(7, 203) = 6.07, p < 0.001] and a significant Trial type X Session interaction [F(7, 203) = 10.42, p < 0.001], which subsequent pair-wise analysis indicated was due to an increase in responding to CRF+ over the course of training. There was no main effect of age (p > 0.9) nor any other significant interactions (p’s > 0.6), indicating that there were no age differences in conditioning.
Figure 1.
Acquisition of conditioned responding as measured by snout entries into the food cup during presentation of the CRF+ (solid lines) or CRF− (dashed lines) by adult (black) and adolescent (grey) rats. Both adolescents and adults increased responding during the CRF+ compared to low levels of responding during the CRF−. Data are means ± SEM.
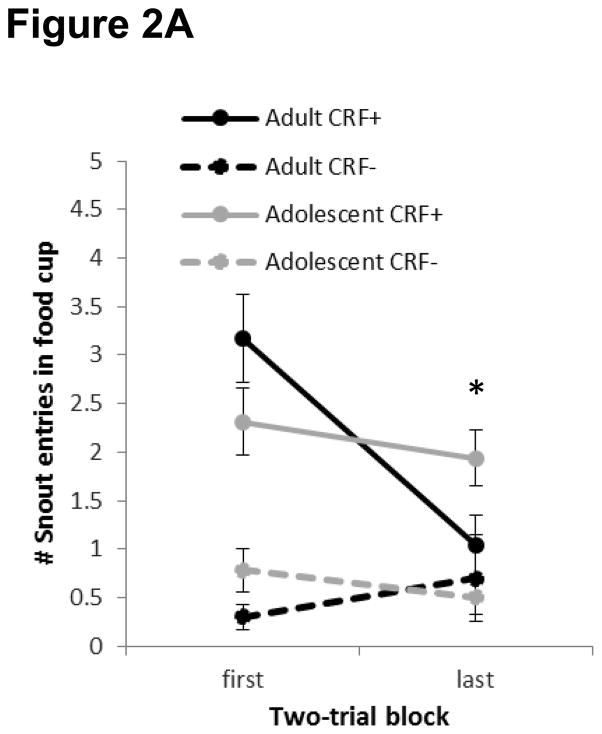
3.1.2 Extinction Training
Conditioned responding during the first and last two-trial blocks of Extinction Session 1 are presented in Figure 2A. A significant interaction between Block and Trial Type [F(1, 29) = 9.94, p < 0.005] and subsequent pair-wise analyses revealed that there was less responding to CRF+ in the second block relative to the first block, indicating that the extinction of conditioned responding was specific to the cue that had previously been paired with food. Importantly, there was also a significant Age X Trial type X Block interaction [F(1, 29) = 8.57, p < 0.01]. To assess the source of this three-way interaction, separate Age x Block ANOVAs were conducted within each level of Trial Type (CRF+ and CRF−). For the CRF−, this analysis revealed no differences across Blocks [F(1, 29) = 0.05, p > 0.8] and no interaction between Block and Age [F(1, 29) = 1.66, > 0.2]. However, for the previously reinforced CRF+, the analysis revealed a main effect of Block [F(1, 29) = 17.88, p < 0.001] and a significant interaction between Age and Block [F(1, 29) = 8.79, p < 0.01], which was further decomposed to reveal significantly higher levels of responding by adolescents than adults specifically at the end of the extinction session [t(29) = 2.10, p < 0.05]. This indicates that there was a greater decrease in responding to CRF+ in adult rats than in adolescents during the first extinction session. In summary, although responding during CRF+ extinguished in both age groups, extinction was greater in adults than adolescents.
Figure 2.
Extinction of conditioned responding as measured by snout entries into the food cup during presentation of the previously reinforced cue (CRF+; solid lines) or CRF− (dashed lines) by adult (black) and adolescent (grey) rats during session 1 (panel A) and session 2 (panel B). Adolescents exhibit difficulties extinguishing responding to a previously reinforced cue compared to adults. Data are means ± SEM. *p < 0.05.
Responding during Extinction Session 2 is shown in Figure 2B. The number of snout entries into the food cup continued to be higher during CRF+ than CRF− [main effect of Trial Type, F(1, 29) = 35.4, p < 0.001]. In addition, the overall level of responding was higher in adolescents compared to adults [main effect of Age, F(1, 29) = 4.6, p < 0.04]. There was no main effect of Block and no significant interactions between any of the variables (p’s > 0.07). In addition, no differences were observed between the levels of conditioned responding during the CRF+ at the end of the first extinction session and the beginning of the second extinction session for either adolescents [t(15) = −1.77, p > 0.09] or adults [t(14) = −1.31, p > 0.2].
3.1.3 Extinction Test Session
Responding during both CRF+ and CRF− were relatively low during the extinction Test session carried out four days after Extinction Session 2. Overall, responding was higher during the previously-reinforced CRF+ than the CRF− [F(1, 29) = 21.42, p < 0.01], but this did not differ by age [F(1, 29) = 2.48, p > 0.1]. There were also no differences in overall levels of responding between age groups [F(1, 29) = 0.01, p > 0.9].
3.1.4 Baseline responding
All rats exhibited similarly low levels of responding to CRF− presentations during Acquisition [mean ± SEM were 1.51 ± 0.13 s for adults and 1.59 ± 0.16 s for adolescents; t(29) = 0.42, p > 0.6]. Thus, differences in baseline behavior could not account for age-related differences in conditioned responding during the CRF+.
3.1.5 Post-CRF+ responding
Analysis of conditioned responding during the 5 sec period after food delivery during the Acquisition session revealed no differences between adolescents and adults [p > 0.07]. The means ± SEMs were 1.16 ± 0.13 s for adolescents and 1.53 ± 0.16 s for adults. Thus, motivation to consume the food pellets appeared to be similar in both age groups.
3.2 Experiment 2: Partial reinforcement
Two rats in the adult group exhibited abnormally low levels of responding at least one standard deviation below the group average across all three phases of the experiment. The data from these rats were eliminated from analyses, resulting in n = 14 for this group.
3.2.1 Acquisition
The average number of snout entries into the food cup during presentation of the tone in the acquisition phase is presented in Figure 4. A 2 (Age: Adolescent, Adult) x 10 (Session) ANOVA indicated that responding to the tone increased across sessions [main effect of Session, F(9, 252) = 19.00, p < 0.001]. Notably, between-subjects analysis revealed that adolescents exhibited higher overall levels of responding relative to adults [main effect of Age, F(1, 28) = 5.48, p < 0.05]. However, the Age X Session interaction was not significant [F(9, 252) = 1.74, p > 0.08].
Figure 4.
Acquisition of conditioned responding as measured by snout pokes into the food cup during presentation of the PRF tone by adult (black) and adolescent (grey) rats. Adolescents increased responding during the CS+ faster than adults and exhibited higher overall levels of responding. Data are means ± SEM. *p < 0.05.
3.2.2 Extinction Training
During the first extinction session (Figure 5A), adolescent rats exhibited higher overall levels of responding relative to adults [main effect of Age, F(1, 28) = 5.69, p < 0.05]. In addition, there was a significant interaction between Age and Block [F(1, 28) = 5.94, p < 0.05]. This was decomposed by subsequent pair-wise comparisons, which revealed that adolescents exhibited significantly higher levels of responding compared to adults during the first block of trials [t(28) = −3.18, p < 0.005] but not the second block [t(28) = 0.14, p > 0.8].
Figure 5.
Extinction of conditioned responding as measured by snout pokes into the food cup during presentation of the previously reinforced tone by adult (black) and adolescent (grey) rats during session 1 (panel A) and session 2 (panel B). Adolescents exhibit initially higher levels of responding than adults and subsequently extinguish at a faster rate than adults. However, responding at the end of the session was similar between age groups. Data are means ± SEM. *p < 0.05, **p < 0.005.
Responding during Extinction Session 2 is shown in Figure 5B. A repeated measures ANOVA revealed a main effect of Block [F(1, 28) = 5.81, p < 0.05], indicating that responding continued to decrease over the course of the session. There were no other significant main effects or interactions. In addition, there were no significant differences in the levels of responding during the last block of Extinction Session 1 and the first block of Extinction Session 2 for either age group (p’s > 0.4].
3.2.3 Extinction Test Session
The number of times the rat poked its snout into the food cup during presentation of the tone was averaged for the first two trials of the test session (Figure 6). Responding did not differ significantly between the age groups [t(28) = −1.90, p > 0.06]. In addition, responding did not significantly differ from the low levels of responding reached at the end of the extinction phase for adolescents [t(15) = −1.90, p > 0.07] or adults [t(13) = −0.59, p > 0.5].
Figure 6.
Test of conditioned responding after a four day interval as measured by snout pokes into the food cup during presentation of the previously reinforced tone by adult (black) and adolescent (grey) rats. Responding did not differ significantly between adolescents and adults. Data are means ± SEM from the first two-trial block of the session.
3.2.4 Baseline responding
All rats exhibited similarly low levels of baseline responding during the 5-sec period before the tone was presented during Acquisition [0.46 ± 0.07 s for adults, 0.35 ± 0.06 s for adolescents; t(28) = 1.24, p > 0.2], Extinction Training [0.40 ± 0.06 s for adults, 0.43 ± 0.06 s for adolescents; t(28) = −0.27, p > 0.7] and Extinction Test [0.36 ± 0.08 s for adults, 0.40 ± 0.07 s for adolescents; t(28) = −0.41, p > 0.6] sessions. Thus, differences in baseline behavior cannot account for age-related differences in conditioned responding during the PRF tone.
3.2.5 Post-PRF responding
Analysis of conditioned responding during the 5 sec period after food delivery during the Acquisition session revealed no differences between adolescents and adults [p > 0.8]. The means ± SEMs were 1.81 ± 0.09s for adolescents and 1.84 ± 0.19s for adults. Thus, as in Experiment 1, motivation to consume the food pellets appeared to be similar in both age groups.
4. Discussion
Learning and updating the meaning of informative environmental cues is critical to adaptive behavior. The present experiments were designed to determine whether learning about the appetitive properties of Pavlovian cues differs between adolescents and adults. In Experiment 1, both adolescents and adults similarly acquired Pavlovian excitatory conditioning to a continuously reinforced auditory cue (CRF+). In addition, both ages exhibited low levels of responding during the CRF−, indicating that adolescents were able to discriminate between the stimuli and differentiate behavioral response patterns directed towards these stimuli like adults. Conversely, in Experiment 2, adolescents acquired excitatory responding to a partially-reinforced cue faster than adults. Thus, age-related differences in excitatory conditioning may be specifically related to the reinforcement schedule of an appetitive cue.
A general feature of learning about a partially reinforced cue is that conditioned responding is initially slow to develop [63–70], because each PRF− trial causes a decrement in associative strength (or an increment in inhibition) that offsets the associative increments that occur on PRF+ trials. However, the occurrence of a reinforcer, even intermittently, may increase the salience of the stimulus in adolescents. Thus, the associative strength may not decrease in the same way as for adults during PRF− trials. In this manner, the increased responding during the acquisition phase by adolescents relative to adults in Experiment 2 may reflect an adolescent proclivity towards the excitatory properties of the partially reinforced cue. An additional factor may be that PRF− trials are less aversive for adolescents. Indeed, adults, but not adolescents favor choices that attach certainty to an outcome over alternatives with identical expected values [71]. Furthermore, adolescents exhibit a higher tolerance for aversive outcomes in general [33, 72–74].
Another major finding of these experiments was that conditioned responding differed between adolescents and adults during extinction training. During the first extinction training session in Experiment 1, both groups reduced responding to the previously reinforced cue once the reinforcer was no longer present. However, extinction leaning was much more robust in adults. Indeed, adult rats initially exhibited higher levels of responding than adolescents, but responding decreased to baseline levels by the end of the session. Conversely, adolescents exhibited a much smaller difference in responding across the session and made significantly more snout entries into the food cup at the end of the session than adults. These findings indicate that adolescents exhibit difficulties extinguishing responding to a previously continuously-reinforced cue compared to adults.
One possible explanation is that adolescents experience difficulties encoding the new meaning of the previously-reinforced (now non-reinforced cue). Indeed, medial prefrontal regions known to be involved in extinction are not fully mature during this time [75–81], whereas subcortical regions such as nucleus accumbens that are involved in the initial reward contingency learning have reached maturity [1]. As a result, enhanced salience of the appetitive properties of a cue could strengthen the initial excitatory representation and subsequently take precedence over the inhibitory representation of the same cue formed during extinction training. The results of Experiment 1 indicate that age-related differences in sensitivity to rewards do not manifest in differences in the acquisition of conditioned responding to a reward-predictive cue. However, the neurobiological processes involved in encoding the meaning of the CRF may have differed. Previously, differences in neuronal activity indicative of reward processing have been shown to differ despite similar learning and performance of operant excitatory conditioning [6, 22]. Thus, heightened activity in brain regions associated with representing the value of a potential reward (e.g. nucleus accumbens [82–85]) during adolescence may have increased the salience of the CRF+.
Notably, the experiments described here were carried out using Pavlovian excitatory conditioning procedures. Although an animal learns to associate a stimulus with a reinforcer, delivery of the reinforcer is not contingent upon a response. Thus, behavior exhibited during the stimulus period (conditioned responding) reflects anticipation of reinforcer delivery. In this way, the experiments tested for age-related differences in how appetitive cues are represented and thus extends previous research that considered differences between adolescents and adults in instrumental responding in the face of reward [86, 87]. For example, the present data are consistent with the performance of adolescents during an operant excitatory conditioning paradigm [87]. Indeed, the authors did not observe age differences in the acquisition a nose poke behavior required to obtain a reinforcer, but did observe perseverative responding by adolescents but not adults when the poke no longer produced a reward. Additional research has also shown that adolescents are slower to extinguish an operant response that previously triggered delivery of a reward [43]. The present results extend these findings to Pavlovian excitatory learning and anticipatory food cup behavior. Moreover, these findings indicate notable similarities between how adolescents represent expectancies about stimuli in the environment and how responding is directed towards predictive stimuli. Moreover, our data are also in line with evidence that adolescents exhibit delayed extinction of a learned fear response to a cue previously paired with a footshock [4, 88].
During extinction of the partially reinforced cue in Experiment 2, the greater reduction in responding across the first extinction training session by adolescents compared to adults suggests that adolescents may be able to extinguish responding to a partially reinforced cue more rapidly than adults. However, conditioned responding was very similar between the groups during the second extinction training session. This pattern of results differs from the delayed extinction of conditioned responding exhibited by adolescents in Experiment 1. This result may be particularly noticeable relative to the slow rate of extinction in adult rats. This is common in operant conditioning, where behavior that is reinforced intermittently is more resistant to extinction than behavior that is always reinforced [89–91]. This occurs because the discrepancy, or error, between actual and predicted reward contributes extensively to the extent to which learning occurs [82]. Thus, extinguishing the response to a partially reinforced stimulus may be slow to develop because non-reinforced presentations of the stimulus are expected and thus less prediction error occurs. The present results indicate that adolescents are more able to overcome the low prediction error in order to flexibly update the meaning of the cue. An important avenue for future research will be to determine whether the extinction of a partially reinforced aversive cue during adolescence is also facilitated relative to adulthood. This would provide important insight about the extent to which extinction learning is influenced by reinforcement schedule as well as whether this influence is generalizable to the appetitive or aversive history of a cue.
Developmental differences in associative learning prior to adolescence have also been shown previously. For example, in preweanling rodents, the presence of additional cues, even those unpaired with the outcome, (e.g. CS−) can strengthen learning about a paired cue (CS+) as well as a CS-US relationship [92, 93]. Furthermore, the extent to which a CS− influences learning about a CS+ depends on the amount of information that the CS− provides about the CS-US pairing [94]. Finally, there is evidence that extinction processes in pre-weanlings involves unlearning of the CS-US association, in contrast to the secondary inhibitory association that is believed to be formed during extinction trials in adulthood [95, 96]. Considering these and other developmental findings along with established adult learning and behavioral patterns will aid the interpretation of future research directed at elucidating age-related differences in associative learning.
Taken together, our data suggest that extinction of a Pavlovian excitatory association is subject to age differences. Importantly, the history of reinforcement (i.e., continuous or partial reinforcement) determines whether the rate of extinction is delayed or enhanced in adolescents, respectively. Here, adolescents were delayed relative to adults when extinguishing responding to a cue that had previously been continuously paired with reinforcement, consistent with previous research [4, 43, 87, 88]. Conversely, we show evidence that exposure to non-reinforced presentations of a cue (partial reinforcement) may subsequently facilitate extinction in adolescents. This parallels previous research suggesting that adolescents are more readily able than adults to adjust behavioral patterns directed towards a stimulus that has previously acquired inhibitory properties [86]. Importantly, our findings in this regard may provide insight to unify previous research that has considered age-related differences in flexibly updating cue representations. Indeed, these seemingly disparate results may be attributed to differences in the ratio of reinforced and non-reinforced presentations of a given cue. Moreover, difficulties learning a secondary meaning of a cue during adolescence may be particularly robust when the initially encoded representation of that cue is exclusively excitatory.
The present experiments also extend the current literature by including test sessions following extinction to address the longevity of learning about a Pavlovian appetitive cue. The passage of time after the acquisition and extinction phases can serve as a temporal context shift that increases retrieval interference [97] and may result in spontaneous recovery of the initially conditioned response [98]. In Experiment 1 neither age group exhibited spontaneous recovery of anticipatory responding to the cues previously followed by reinforcement during the test session. In contrast, during the test session in Experiment 2, although responding between the age groups was statistically similar, we observed a trend towards spontaneous recovery of responding by adolescents but not adults, as well as overall higher levels of responding by adolescents than adults. This suggests somewhat less retention of the inhibitory representation of the tone encoded during extinction by adolescent rats. These test sessions were conducted four days after completion of the extinction phase in order to remain within the developmental window of adolescence. However, the interval between extinction and testing is a critical determinant of the magnitude of spontaneous recovery of conditioned responding [98, 99]. Thus, greater differences may be observed with test sessions conducted after longer delays.
In both Experiments 1 and 2, conditioned responding during Extinction Training and Extinction test was consistently higher than baseline responding (CRF− in Experiment 1, Pre-PRF in Experiment 2). This indicates that both groups maintain a representation of the excitatory properties of a cue. This is consistent with the well-established notion that extinction learning does not erase the initial memory, but rather results in a new inhibitory association between the now neutral stimulus and its previously reinforcing outcome [98]. Notably, differential levels of conditioned and baseline responding were observed even after the non-reinforced presentations of the stimulus outnumbered the reinforced presentations. This suggests robust persistence of the initial discrimination learning, consistent with evidence that when time passes from the end of acquisition and extinction training, the representation that was learned first will contribute more to performance [97, 100].
Taken together, the age-related differences in conditioned responding during extinction training (Experiment 1) and test (Experiment 2) phases suggest that adolescents perseverate on the excitatory properties of Pavlovian appetitive cues to a greater extent than adults. Importantly, this appears to be the case for cues with a history of either continuous or partial reinforcement. One explanation for these findings is that the salience of a Pavlovian appetitive cue is increased as a result of the predisposition of adolescents towards positive rewards compared to adults [9, 15–18, 21, 24–28, 41, 101–103]. Indeed, a key theme emerging from research regarding adolescence is that the neurobiological environment during this time provides a basis for behaviors that are biased toward risk, reward, and emotional reactivity [9, 16, 17]. It has been postulated that the differential development of top-down control systems and subcortical areas results in a functional imbalance during adolescence [9, 14, 16, 17, 104]. Ultimately, activity in subcortical regions (e.g. ventral striatum and amygdala) is disproportionately higher than PFC during adolescence [1, 17, 105–109]. As a result subcortical regions exert a stronger influence on behavior, effectively signaling enhanced approach motivation [2].
5. Conclusions
The findings described here indicate that differences in excitatory conditioning processes during adolescence may underlie behavioral tendencies that are apparent during this developmental time period. In particular, a bias towards the excitatory properties of cues may outweigh alternative meanings of the same cue and underlie a variety of reward-biased behaviors. These behaviors could in turn contribute to a number of negative outcomes including interpersonal conflicts, increased chance of injury, vulnerability to substance abuse, and even premature death [10, 13, 16, 110–112]. Moreover, we have presented evidence of the robust influence that the reinforcement history of a cue can have on learning, maintaining and updating cue representations during adolescence. The exact conditions that mediate differences in how the schedule of reinforcement contributes to age-related differences in associative learning processes will be an important avenue for further study.
Figure 3.
Test of conditioned responding after a four day interval as measured by snout entries into the food cup during presentation of the previously reinforced cue (CRF+) or CRF− by adult (black) and adolescent (grey) rats. Both adolescents and adults exhibited higher responding during the previously reinforced CRF+ compared to lower levels of responding during the CRF−. Within each trial type, adolescents responded similarly to adults. Data are means ± SEM from the first two-trial block of the session.
Highlights.
Adolescent and adult rats condition similarly to continuously reinforced stimuli
Adolescents exhibit heightened responding to partially-reinforced cues compared to adults
The appetitive properties of cues persist longer in adolescents relative to adults
Flexibly mediating competing cue representations depends on reinforcement history
Acknowledgments
Research supported by NIH Grants F31MH107138 (HCM) and R01DA027688 (DJB). The authors thank Dr. Travis Todd for valuable discussion regarding the experimental design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proceedings of the National Academy of Sciences, USA. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, … Lee FS. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences, USA. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. The Journal of Neuroscience. 2012;32:16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturman DA, Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proceedings of the National Academy of Sciences USA. 2012;109:1719–1724. doi: 10.1073/pnas.1114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somerville LH. The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science. 2013;22:121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Reviews. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Development Perspectives. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey BJ, Caudle K. The teenage brain: Self control. Current Directions in Psychological Science. 2013;22:82–87. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvan A. The Teenage Brain Sensitivity to Rewards. Current Directions in Psychological Science. 2013;22:88–93. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 16.Casey BJ, Getz S, Galvan A. The adolescent brain and risky decisions. Developmental Reviews. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fareri D, Martin L, Delgado M. Reward-related processing in the human brain: Developmental considerations. Development and Psychopathology. 2008;20:1191–1211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- 19.Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- 22.Sturman DA, Moghaddam B. Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. The Journal of Neuroscience. 2011;31:1471–1478. doi: 10.1523/JNEUROSCI.4210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: Taste reactivity and voluntary sucrose consumption. Pharmacology, Biochemistry, and Behavior. 2009;92:566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laviola G, Macrì S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neuroscience & Biobehavioral Reviews. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 25.May JC, Delgado MR, Dahl RE, Stenger A, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, … Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–24. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 29.Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: Effects of social isolation. Physiology and Behavior. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology and Behavior. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 32.Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. European Journal of Pharmacology. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 33.Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 34.Shram MJ, Funk D, Li Z, Lê AD. Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. Neuroscience Letters. 2007;418:286–291. doi: 10.1016/j.neulet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcoholism, Clinical and Experimental Research. 2007;31:2001–2005. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 36.Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary access conditions. Alcoholism Clinical and Experimental Research. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behavioral Neuroscience. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcoholism Clinical and Experimental Research. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology Biochemistry and Behavior. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. International Journal of Developmental Neuroscience. 2009;27:805–815. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: Two behavioral features of adolescence in mice. Behavioral Neuroscience. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- 42.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 43.Andrzejewski ME, Schochet TL, Feit EC, Harris R, Mc Kee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behavioral Neuroscience. 2011;125:93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- 44.Koss WA, Franklin AD, Juraska JM. Delayed alternation in adolescent and adult male and female rats. Developmental Psychobiology. 2011;53:724–731. doi: 10.1002/dev.20543. [DOI] [PubMed] [Google Scholar]
- 45.Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: adolescent-limited and life-persistent patterns of impulsivity. Behavioral Neuroscience. 2011;125:194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behavioural Brain Research. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 47.Lin CC, Chen WJ, Yang HJ, Hsiao CK, Tien AY. Performance on the Wisconsin Card Sorting Test among adolescents in Taiwan: Norms, factorial structure and relation to schizotypy. Journal of Clinical and Experimental Neurospychology. 2000;22:9–79. doi: 10.1076/1380-3395(200002)22:1;1-8;FT069. [DOI] [PubMed] [Google Scholar]
- 48.Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- 49.Newman L, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Developmental Psychobiology. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer HC, Bucci DJ. The ontogeny of learned inhibition. Learning & Memory. 2014;21:143–152. doi: 10.1101/lm.033787.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York, NY: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- 52.Wagner AR. SOP: A model of automatic memory processing in animal behavior. Information processing in animals: Memory mechanisms. 1981;85:5–47. [Google Scholar]
- 53.Rescorla RA. Pavlovian conditioning: It’s not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- 54.Pearce JM. Similarity and discrimination: a selective review and a connectionist model. Psychological Review. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- 55.Balsam PD, Gallistel CR. Temporal maps and informativeness in associative learning. Trends in Neurosciences. 2009;32:73–78. doi: 10.1016/j.tins.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balsam PD, Drew MR, Gallistel CR. Time and associative learning. Comparative Cognition & Behavior Reviews. 2010;5:1–22. doi: 10.3819/ccbr.2010.50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen G, Ward RD, Balsam PD. Information: theory, brain, and behavior. Journal of the Experimental Analysis of Behavior. 2013;100:408–431. doi: 10.1002/jeab.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shahan TA, Cunningham P. Conditioned reinforcement and information theory reconsidered. Journal of the Experimental Analysis of Behavior. 2015;103:405–418. doi: 10.1002/jeab.142. [DOI] [PubMed] [Google Scholar]
- 59.Thorndike EL. Animal intelligence: An experimental study of the associative processes in animals. New York, NY: Macmillan; 1911. [Google Scholar]
- 60.Hull C. Principles of behavior. New York: Appleton-Century-Crofts; 1943. [Google Scholar]
- 61.Tymula A, Belmaker LAR, Roy AK, Ruderman L, Manson K, Glimcher PW, Levy I. Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proceedings of the National Academy of Sciences. 2012;109:17135–17140. doi: 10.1073/pnas.1207144109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blankenstein NE, Crone EA, van den Bos W, van Duijvenvoorde AC. Dealing with uncertainty: Testing risk- and ambiguity-attitude across adolescence. Developmental Neuropsychology. 2016;41:77–92. doi: 10.1080/87565641.2016.1158265. [DOI] [PubMed] [Google Scholar]
- 63.Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian interactions. Hillsdale, NJ: Erlbaum; 1977. pp. 67–97. [Google Scholar]
- 64.Gibbon J, Farrell L, Locurto CM, Duncan HJ, Terrace HS. Partial reinforcement in autoshaping with pigeons. Animal Learning & Behavior. 1980;8:45–59. [Google Scholar]
- 65.Davey GC, Cleland GG. The effect of partial reinforcement on the acquisition and extinction of sign-tracking and goal-tracking in the rat. Bulletin of the Psychonomic Society. 1982;19:115–118. [Google Scholar]
- 66.Collins L, Young DB, Davies K, Pearce JM. The influence of partial reinforcement on serial autoshaping with pigeons. The Quarterly Journal of Experimental Psychology B. 1983;35:275–290. doi: 10.1080/14640748308400893. [DOI] [PubMed] [Google Scholar]
- 67.Swan JA, Pearce JM. The orienting response as an index of stimulus associability in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:292–301. [PubMed] [Google Scholar]
- 68.Gottlieb DA. Acquisition with partial and continuous reinforcement in pigeon autoshaping. Animal Learning & Behavior. 2004;32:321–334. doi: 10.3758/bf03196031. [DOI] [PubMed] [Google Scholar]
- 69.Anselme P. Incentive salience attribution under reward uncertainty: A Pavlovian model. Behavioural Processes. 2015;111:6–18. doi: 10.1016/j.beproc.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 70.Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rivers SE, Reyna VF, Mills B. Risk taking under the influence: a fuzzy-trace theory of emotion in adolescence. Developmental Review. 2008;28:107–144. doi: 10.1016/j.dr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacology Biochemistry and Behavior. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- 73.Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Annals of the New York Academy of Sciences. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- 74.Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacology Biochemistry and Behavior. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 76.Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 77.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- 78.Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. The Journal of Neuroscience. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of Neuroscience. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burgos-Robles A, Bravo-Rivera H, Quirk GJ. Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PLoS One. 2013;8:e57575. doi: 10.1371/journal.pone.0057575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pezze M, McGarrity S, Mason R, Fone KC, Bast T. Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. The Journal of Neuroscience. 2014;34:7931–7946. doi: 10.1523/JNEUROSCI.3450-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 83.Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 84.Spicer J, Galvan A, Hare TA, Voss H, Glover G, Casey B. Sensitivity of the nucleus accumbens to violations in expectation of reward. Neuroimage. 2007;34:455–461. doi: 10.1016/j.neuroimage.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response inhibition and behavioral flexibility between adolescent and adult rats. Behavioral Neuroscience. 2013;127:23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behavioral Neuroscience. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: Effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skinner BF. The behavior of organisms: an experimental analysis. New York: D. Appleton-Century; 1938. [Google Scholar]
- 90.Humphreys LG. The effect of random alternation of reinforcement on the acquisition and extinction of conditioned eyelid reactions. Journal of Experimental Psychology. 1939;25:141–158. [Google Scholar]
- 91.Amsel A. Frustration Theory: An Analysis of Dispositional Learning and Memory. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 92.Miller JS, Jagielo JA, Spear NE. Age-related differences in short-term retention of separable elements of an odor aversion. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:194. [PubMed] [Google Scholar]
- 93.Brasser SM, Spear NE. Contextual conditioning in infants, but not older animals, is facilitated by CS conditioning. Neurobiology of learning and memory. 2004;81:46–59. doi: 10.1016/s1074-7427(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 94.Miller JS, Jagielo JA, Spear NE. The influence of the information value provided by prior-cuing treatment on the reactivation of memory in preweanling rats. Animal Learning & Behavior. 1992;20:233–239. [Google Scholar]
- 95.Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biological psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Quirk GJ, Paré D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. The Journal of Neuroscience. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Devenport L. Spontaneous recovery without interference: Why remembering is adaptive. Animal Learning & Behavior. 1998;26:172–181. [Google Scholar]
- 98.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 99.Huff NC, Hernandez JA, Blanding NQ, LaBar KS. Delayed extinction attenuates conditioned fear renewal and spontaneous recovery in humans. Behavioral Neuroscience. 2009;123:834–843. doi: 10.1037/a0016511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Devenport L, Hill T, Wilson M, Ogden E. Tracking and averaging in variable environments: A transition rule. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:450–460. [Google Scholar]
- 101.Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Science. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- 103.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 106.Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinions in Neurobiology. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 107.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neuroscience and Biobehavioral Reviews. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 108.Rosenberg D, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biological Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 109.Rosenberg D, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 110.Arnett JJ. Adolescent storm and stress, reconsidered. The American Psychologist. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- 111.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 112.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]