Abstract
Edited MRS allows the detection of low-concentration metabolites, whose signals are not resolved in the MR spectrum. Tailored acquisitions can be designed to detect, for example, the inhibitory neurotransmitter γ-aminobutyric acid (GABA), or the reduction-oxidation (redox) compound glutathione (GSH), and single-voxel edited experiments are generally acquired at a rate of one metabolite-per-experiment. We demonstrate that simultaneous detection of the overlapping signals of GABA and GSH is possible using Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES). HERMES applies orthogonal editing encoding (following a Hadamard scheme), such that GSH- and GABA-edited difference spectra can be reconstructed from a single multiplexed experiment. At a TE of 80 ms, 20-ms editing pulses are applied at 4.56 ppm (on GSH),1.9 ppm (on GABA), both offsets (using a dual-lobe cosine-modulated pulse) or neither. Hadamard combinations of the four sub-experiments yield GABA and GSH difference spectra.
It is shown that HERMES gives excellent separation of the edited GABA and GSH signals in phantoms, and resulting edited lineshapes agree well with separate Mescher-Garwood Point-resolved Spectroscopy (MEGA-PRESS) acquisitions. In vivo, the quality and signal-to-noise ratio (SNR) of HERMES spectra are similar to those of sequentially acquired MEGA-PRESS spectra, with the benefit of a saving half the acquisition time.
Keywords: GABA, glutathione, editing, HERMES, Hadamard, simultaneous
Graphical abstract
1. Introduction
GABA (γ-aminobutyric acid) is the principal inhibitory neurotransmitter in the human brain. GABAergic inhibition shapes and regulates patterns of neuronal activity, serving a key role in cortical information processing and plasticity. Glutathione (GSH) is the most abundant redox (reduction-oxidation) compound in the brain, serving an important role in minimizing the damage caused by reactive oxygen species. GABAergic dysfunction and oxidative stress are two commonly postulated mechanisms in neurological and psychiatric disease, being implicated in, for example, Amyotrophic lateral sclerosis (ALS) (1–3), Parkinson’s disease (4–6) and Schizophrenia (4,7,8). Both metabolites are present in the brain at millimolar (mM) concentrations (9,10) and are, in principle, detectable by 1H (proton) magnetic resonance spectroscopy (MRS). In vivo 1H-MRS suffers from incomplete resolution of metabolite signals, due to limited dispersion along the chemical-shift dimension, broad in vivo linewidths and multiplet splittings due to scalar coupling. Thus, signals of low-concentration metabolites are often overlapped by larger signals and cannot be reliably quantified. Spectral editing techniques, such as MEscher-GArwood Point RESolved Spectroscopy (MEGA-PRESS (11)), simplify the spectrum, selectively revealing signals from metabolites of interest, such as GABA (12) and GSH (13), and removing overlying signals of more concentrated metabolites.
A significant drawback of MEGA-PRESS is that it usually only edits one metabolite at a time, and from a single brain region. Since editing is typically applied to lower-concentration compounds, relatively long acquisition times are required, and studies are almost always severely restricted in terms of both the numbers of brain regions and metabolites that can be studied within the time constraints of an MR examination. Thus, although GABA-edited MRS is increasingly widely used (14), it is rarely combined in studies with the edited detection of other metabolites of interest, such as GSH, because such a study would require multiple long edited acquisitions.
There are occasions when the edited detection of more than one metabolite is possible. A mixed signal of glutamate and glutamine (Glx) is co-edited in GABA-edited acquisitions, giving an edited signal at a different chemical shift (3.75 ppm) to the GABA signal (3.0 ppm). It has also been shown that GSH and ascorbate can be edited in a single acquisition, using Double Editing With (DEW)-MEGA-RESS (15), because both the editing target signals (at 4.01 and 4.56 ppm) and the detected signals (at 2.95 and 3.73 ppm) of GSH and ascorbate, respectively, are resolved in the spectrum. Simultaneous detection of GABA and GSH is not possible with MEGA or DEW-MEGA editing because the detected edited signals are not resolved – that is, the edited GABA signal at 3 ppm and the edited GSH signal at 2.95 ppm would overlap.
Recently, the Hadamard Encoding and Reconstruction of MEGA-Edited Spectroscopy (HERMES) approach (16) was demonstrated, that separately edits more than one metabolite with overlapping signals within a single acquisition. In this manuscript, we show that HERMES can be used to simultaneously and separably detect edited signals from GABA and GSH. Through phantom measurements, we demonstrate that a four-step HERMES editing scheme allows the independent manipulation of the GABA and GSH spin systems, and Hadamard transformation of the acquired data yields separate difference-edited spectra for GABA and GSH. We present in vivo measurements showing that simultaneously acquired HERMES spectra are comparable to sequentially acquired GABA- and GSH-edited MEGA-PRESS acquisitions with twice the total scan time. We also demonstrate that HERMES-accelerated editing is compatible with spatial acceleration methods, such as Parallel Reconstruction In Accelerated Multivoxel (PRIAM (17)) spectroscopy, which excites more than one voxel simultaneously and uses a parallel-imaging-like reconstruction to separate the signals from each.
2. Methods
2.1 HERMES editing of GABA and GSH
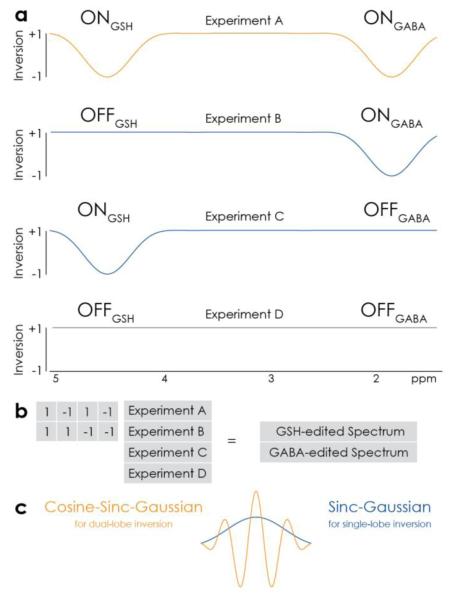
The key principle of HERMES is that editing pulses can be separately applied to GABA spins at 1.9 ppm and GSH spins at 4.56 ppm. Four sub-experiments (labeled A, B, C and D, respectively) can be performed that apply editing to both GABA and GSH (ONGABA, ONGSH), GABA-only (ONGABA, OFFGSH), GSH-only (OFFGABA, ONGSH) or neither (OFFGABA, OFFGSH), in an interleaved fashion. This editing scheme, and the inversion profiles of the editing pulses needed to implement it, are shown in Figure 1a. The GABA-edited difference spectrum is calculated by subtracting the two OFFGABA scans from the sum of the two ONGABA scans. Similarly, the sum of the two ONGSH scans minus the sum of the two OFFGSH scans gives the GSH-edited difference spectrum. Because the encoding is based upon columns of a Hadamard matrix (which are mutually orthogonal), the GABA-edited spectrum does not contain edited signal from GSH and vice versa, as outlined in Figure 1b.
Figure 1.
HERMES editing of GSH and GABA. a) Inversion profiles of editing pulses applied in the four sub-experiments A-D. HERMES acquires all combinations of (ONGSH, OFFGSH) and (ONGABA, OFFGABA). b) Hadamard transformation of the sub-experiments yields the separate GSH- and GABA-edited spectra. c) Dual-frequency inversion in Experiment A is achieved using a cosine-sinc-Gaussian editing pulse. Experiments B and C use a more conventional sinc-Gaussian pulse to invert at a single offset.
HERMES can be thought of as two different MEGA-PRESS experiments being acquired at the same time, with orthogonal ON/OFF editing patterns. Both experiments are PRESS-localized, and J-difference-edited. Many of the limitations of MEGA-PRESS, e.g. loss of edited signal from spatially heterogeneous coupling evolution (18) are shared by HERMES, as are its strengths.
2.2 RF pulse design and simulations
Experiments B and C require the inversion of a single frequency, and a sinc-Gaussian pulse was used for this purpose. For Experiment A (ONGABA, ONGSH), since the two editing frequencies (4.56 ppm for GSH, and 1.9 ppm for GABA) are well separated, a dual-lobe inversion pulse is required. It can be generated by multiplying the same sinc-Gaussian waveform by 2cos(π ΔΩ t), where ΔΩ is the frequency difference between 4.56 ppm and 1.9 ppm (340 Hz at 3T). The factor of 2 accounts for the pulse splitting energy between two different offsets. Both RF pulses are plotted in Figure 1c.
The inversion performance of the pulses as a function of offset (which is an excellent approximation to the offset-dependence of editing, as shown in (19)) is simulated using FID-A ((20), function rf_blochSim), starting with pure z-magnetization and plotting the zmagnetization across a 5 kHz range in steps of 1 Hz.
Density-matrix simulations of the GSH-cysteine and GABA spin systems were also performed using FID-A (20). Only the voxel-center was simulated for each Experiment. The experimental amplitude-modulated refocusing and editing pulse shapes were included in the simulations; ideal excitation was assumed. Hadamard combinations of the simulated experiments were calculated, and crosstalk between the spectra (i.e. the GABA signal that leaks into the GSH spectrum, and vice versa) was quantified using the root mean square (RMS) of the crosstalk normalized to the RMS of the intended spectra (i.e. the GABA signal in the GABA spectrum, and vice versa).
2.3 MR experimental
Phantom and in vivo data were acquired on a Philips Intera 3T scanner, using the body coil for transmit and a 32-channel phased-array head coil for receive. The bandwidth of the slice-selective excitation and refocusing pulses were 2.2 kHz and 1.3 kHz, respectively. The duration and bandwidth (full-width half-maximum width of each inversion lobe) of both the sinc-Gaussian and cosine-sinc-Gaussian editing pulses were 20 ms and 62 Hz, respectively.
2.4 Phantom Water Saturation Experiments
In order to establish the efficacy of the editing pulses, a saturation-offset series was performed, harnessing the original water-suppression function of MEGA (11). The four editing pulses were played out with additional reference frequency offsets of 0.2 ppm to −0.8 ppm in steps of 0.1 ppm, (to investigate the impact of the GSH-inverting lobes from 4 ppm to 5 ppm), saturating the phantom water signal. One experiment was performed with editing pulses offset by 1.8 ppm to demonstrate whether the editing pulses directly impact the GABA and GSH detected signals. A further set was run to probe the GABA-inverting lobe, with additional reference frequency offsets from −2.4 ppm to −3.4 ppm in steps of 0.1 ppm. A single average of each HERMES experiment (TE 80 ms) was recorded without presaturation of the water signal (2048 datapoints sampled at 2 kHz). Data were line-broadened by 3 Hz, Fourier transformed and a region of the spectrum 0.2 ppm either side of the water signal plotted.
The water signal arising from each experiment was plotted against the editing reference frequency offset (in Hz) applied to acquire each. These data were overlaid on the simulations described in Section 2.2.
2.5 Phantom HERMES
Three phantoms were prepared in phosphate-buffered saline (with 1.5 g/l NaN3) with: 4mM GSH; 4mM GSH and 4 mM GABA; and 10 mM GABA. In each phantom three experiments were performed: HERMES; GABA-edited MEGA-PRESS (editing pulses applied at 1.9 ppm for ON scans); and GSH-edited MEGA-PRESS (editing pulses applied at 4.56 ppm for ON scans). Common acquisition parameters included: chemical shift selective ‘excitation’ water suppression; 3 × 3 × 3 cm3 voxel; TE/TR 80/2000 ms; 20-ms editing pulses; 64 total averages. HERMES was performed as described above, according to the acquisition scheme shown in Figure 1, with a cosine-sinc-Gaussian inversion pulse used for Experiment A, and a single-lobe sinc-Gaussian used for Experiment B and C. Experiment D (OFFGABA, OFFGSH) has no requirement for an editing pulse, so a single-lobe editing pulse was applied at 7.22 ppm. Data were line-broadened by 3 Hz, Fourier transformed and a region of the spectrum from 2.4 to 3.6 ppm was plotted.
2.6 In vivo HERMES of GSH and GABA
Participants
10 healthy adults (4 female, mean age 34.7 ± 8.8 years) were recruited with approval of the Johns Hopkins University Institutional Review Board and gave informed consent to participate.
MRS
HERMES, GABA-MEGA- and GSH-MEGA-edited data were acquired from a single 3.6 × 3.6 × 3.6 cm3 midline parietal region. All in vivo experiments were performed as for the phantom data, except 320 total averages were acquired (i.e. 80 averages each for A, B, C and D in HERMES, and 160 ONs and 160 OFFs for MEGA) and VAPOR water suppression (21) was used. Sixteen non-water-suppressed reference scans were acquired, interleaved throughout the scan, and the transmitter offset was updated based on the water signal offset to reduce magnetic field (B0) drift (19). As proof of principle in a single subject, HERMES was performed with a dualband excitation pulse and PRIAM reconstruction (17), allowing the simultaneous acquisition of HERMES data in two (3.0 cm)3 voxels (left and right insular regions separated by 78 mm laterally). All other acquisition parameters match the single-voxel measurements. The scan duration for each measurement was 11 minutes. By combining two-fold HERMES metabolite acceleration and two-fold PRIAM spatial acceleration, this 11-minute experiment collects data that would take almost 45 minutes to acquire with sequential MEGA-PRESS acquisitions, providing both GABA and GSH measurements from two brain regions in a single scan.
2.7 Post-processing
Data were multiplied by a 3-Hz exponential window function, followed by frequency-and-phase correction using spectral registration in the time domain (22), as incorporated in Gannet (23). This approach alone results in good alignment for the GABA-edited spectrum, but not the GSH-edited spectrum. Therefore, the averaged GSH-ON spectra were further aligned to the GSH-OFF spectra in the frequency domain, varying both frequency and phase to minimize the standard deviation of the choline subtraction artefact. This strategy was unsuccessful in one subject, so their pre-alignment GSH-edited spectrum (which had relatively little subtraction artifact) is presented.
GABA-edited spectra were modeled in Gannet to yield GABA/Cr ratios and signal-to-noise (SNR) ratios. Noise was determined by applying a linear fit to a region of the baseline between −2.3 ppm and −2.8 ppm, and calculating twice the standard deviation of the residuals.
3. Results
3.1 Editing pulse simulation and phantom validation
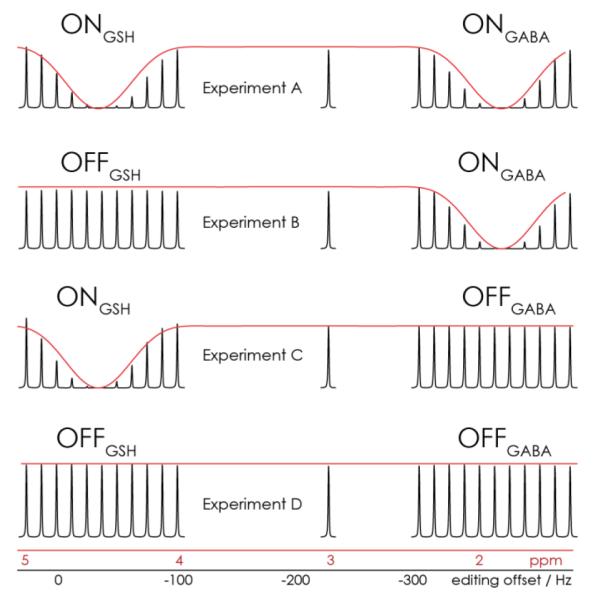
Bloch simulations indicate that the cosine-sinc-Gaussian pulse will effectively invert both the GSH spins at 4.56 ppm and the GABA spins at 1.9 ppm. The results of simulations are shown in red in Figure 2. Also shown in this figure is the water line from the phantom saturation series; the position of each water peak along the x-axis corresponds to the additional reference frequency offset during acquisition (black axis in Hz) and the value it corresponds to when the editing pulses are applied at the correct offset (red axis in ppm). It can be seen that the dual-lobe editing pulse effectively saturates signal at two offsets (as intended for two lobes at 4.56 ppm and 1.9 ppm, Experiment A), and that signal is not saturated at 3 ppm. Experiments B and C demonstrate one saturation lobe as intended.
Figure 2.
Simulations and phantom saturation series. Bloch simulations of the editing pulses in each sub-experiment are shown in red. The water signals resulting from a series of phantom water-saturation experiments (shown in black) demonstrate the effect of editing pulses pulses in each sub-experiment. A secondary x-axis is plotted in black, corresponding to the editing pulse reference frequency offset that was applied to record each signal. As required, editing pulses are applied at 4.56 ppm in Experiments A and C and at 1.9 ppm in Experiments A and B. Signals at 3 ppm are unaffected in all four experiments.
3.2 HERMES density-matrix simulation
Density matrix simulations of GSH and GABA are shown in Figure 3. As intended, the GSH signal at 2.95 ppm is refocused in Experiments A and C (the ONGSH experiments) and inverted in the OFFGSH experiments B and D. Similarly, the GABA signal at 3 ppm shows a refocused triplet in Experiments A and B (the ONGABA experiments) and a “W-triplet” in the OFFGABA experiments C and D. The Hadamard reconstructions of these two experiments, shown in Figure 3b, have edited signals in the intended Hadamard-combined spectra, and very low crosstalk to the other spectra (at a level of 1-5%).
Figure 3.
Simulated HERMES spectra. A range from 2.42 to 3.62 ppm is plotted in all cases. a) HERMES experiments A-D as simulated for GSH-cysteine (left) and GABA (right). Evolution of coupling is refocused in ON experiments and unaffected in OFF experiments, as required. b) Hadamard-reconstructed spectra show GSH-edited signal in one combination and GABA-edited signal in the other.
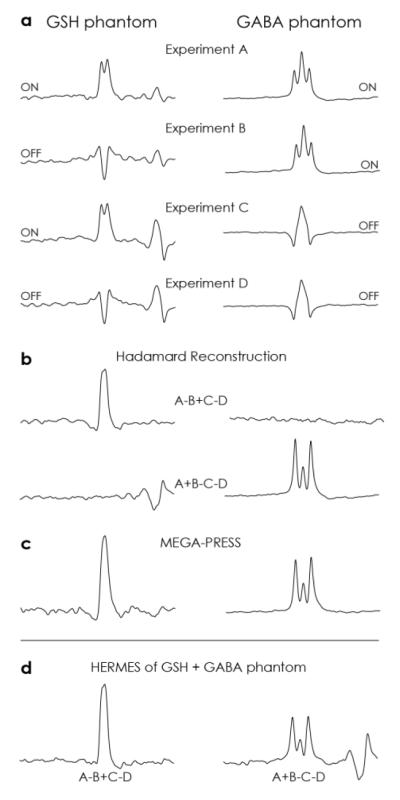
3.2 Phantom HERMES data
The four HERMES subspectra from two phantoms containing GABA-only and GSH-only are shown in Figure 4a. Experiments A-D show the intended multiplet patterns, as described above, and qualitatively resemble simulations. The Hadamard reconstructions of the two experiments (Figure 4b) also strongly resemble the equivalent simulations with crosstalk at or around the noise floor. The lineshapes of these spectra very closely match the lineshapes of traditional MEGA editing shown in Figure 4c. Figure 4d shows the results of HERMES editing in a phantom that contains both GSH and GABA, demonstrating very good separation of the simultaneously acquired signals, which again show strong agreement in terms of lineshape.
Figure 4.
Phantom HERMES spectra. A range from 2.42 to 3.62 ppm is plotted in all cases. a) HERMES experiments A-D as performed on a GSH phantom (left) and GABA phantom (right). Evolution of coupling is refocused in ON experiments and unaffected in OFF experiments, as required. b) Hadamard-reconstructed spectra show GSH-edited signal in one combination and GABA-edited signal in the other. Crosstalk between subspectra (e.g. GABA signal in the GSH-edited combination) is very small. c) MEGA-PRESS spectra of each metabolite show the same edited lineshapes. d) HERMES spectra of a phantom containing both GSH and GABA demonstrate successful simultaneous editing.
3.3 In vivo HERMES data
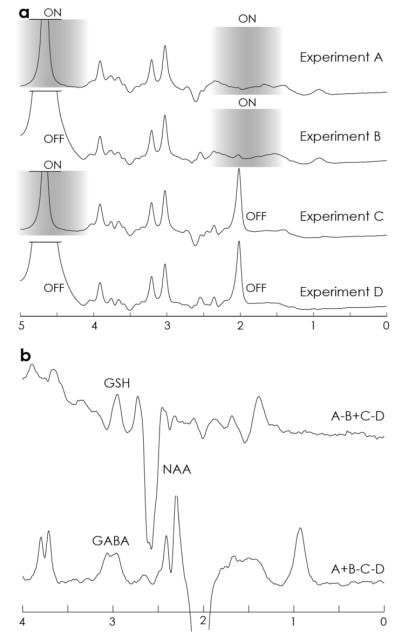
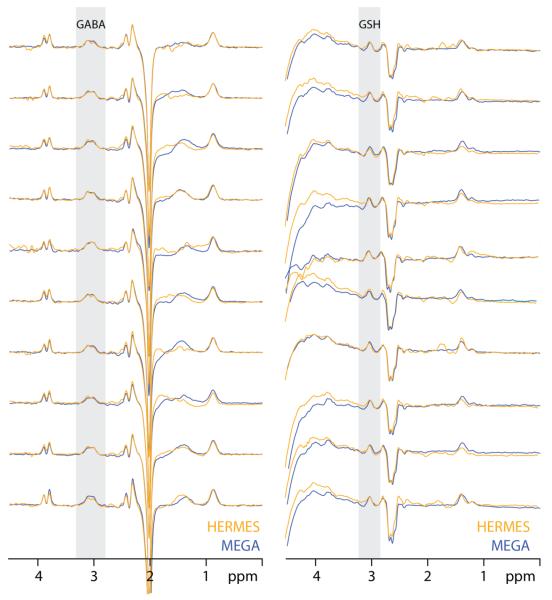
Data from one subject are shown in Figure 5. The four HERMES subspectra are shown separately in Figure 5a to demonstrate that the editing pulses were operating as intended. The GSH-inverting lobe also saturates the residual water signal at 4.68 ppm, whereas the GABA-inverting lobe saturates the N-acetyl aspartate (NAA) signal at 2 ppm. The Hadamard-combined spectra, shown in Figure 5b, have edited GSH and edited GABA signal in the intended Hadamard spectra. Figure 6 shows the HERMES spectra for all ten subjects, overlaid on the separately acquired MEGA-edited spectra for each metabolite, demonstrating consistency of edited signal amplitude and lineshape.
Figure 5.
In vivo HERMES of one subject. A) The separate subspectra A-D are plotted, showing reduced residual water signal in ONGSH spectra and absent NAA signal in ONGABA spectra. The saturation range of the editing pulses is shown on each spectrum as a grayscale overlay. B) The Hadamard spectra show a GSH-edited signal at 2.95 ppm in the A-B+C-D combination and a GABA-edited spectrum in the A+B-C-D combination.
Figure 6.
In vivo HERMES and MEGA-PRESS spectra from all subjects. Simultaneously acquired HERMES spectra are shown in orange and sequentially acquired MEGA-PRESS spectra are overlaid in each case in blue.
Quantified GABA/water levels in HERMES spectra were 1.25 ± 0.15, compared to 1.36 ± 0.17 for MEGA-edited GABA spectra. GABA/Cr ratios in HERMES spectra were 0.155 ± 0.02, compared to 0.175 ± 0.018 for MEGA-edited GABA spectra. The SNR of GABA-edited signal in HERMES spectra was 15.44 ± 2.7, compared to 15.36 ± 2.7 for MEGA-PRESS.
Figure 7 shows the HERMES-PRIAM spectra from one subject, demonstrating that GSH- and GABA-edited data from two brain regions can be acquired in a single 11 minutes experiment, equivalent to a fourfold net acceleration.
Figure 7.
In vivo HERMES-PRIAM. GSH- and GABA-edited spectra (below) were acquired for two (3 cm)3 voxels (above) in a single 11 minutes acquisition. The orange spectra originate from the orange voxel and the blue spectra from the blue voxel.
4. Discussion
Edited detection of GABA and GSH provides quantitative information on two key brain metabolites, with applications in cognitive and clinical neuroscience. One key limitation of MRS as applied to low-concentration metabolites is the low SNR of measurements, which imposes a lower limit on the duration of measurements for a given acquisition volume (14,24). Sequential measurements of GSH and GABA would generally take 20 minutes per brain region, using the widely used MEGA-PRESS editing technique, and therefore relatively few studies measure both compounds. Simultaneous HERMES editing acquires equivalent information in half the scan time.
Simulations and phantom measurements of HERMES editing show excellent segregation of edited signals into the intended GSH and GABA subspectra, with low levels of crosstalk. Simultaneously acquired data from the GABA+GSH phantom show excellent agreement with separately acquired MEGA-PRESS data from the single-metabolite phantoms. In vivo, the method was successfully applied in ten healthy volunteers, again demonstrating excellent agreement with separately acquired MEGA-PRESS data. In practice, HERMES allows both GABA and GSH to be measured in the same duration as one MEGA-PRESS measurement of GABA (25) (or GSH). We have also demonstrated a fourfold reduction (11 vs ~45 minutes) in scan time using HERMES-PRIAM (Figure 7), allowing both GABA and GSH to be measured in two brain regions simultaneously.
In this study, we implemented HERMES using PRESS for voxel localization. However, HERMES is an editing approach that, like MEGA, is not necessarily tied to a single localization method - HERMES can be applied within other localization sequences, such as LASER (26,27), semi-LASER (28) and SPECIAL (29). HERMES can also be incorporated into 2D (30) and 3D (31) spectroscopic imaging (MRSI) sequences.
These experiments have been performed at an echo time of 80 ms. Generally triplet-like signals such as GABA edit optimally at a TE of 1/2J (or ~70 ms), whereas doublet-like signals such as GSH edit optimally at a TE of 1/J (or 140 ms). For GABA editing in vivo, echo times of 68-80 ms have been applied (12,32), and for GSH echo times of 68-131 ms have been applied (13,33). It has recently been shown that the edited GSH signal in vivo does not vary substantially between 68 and 140 ms (34), so this choice of 80 ms is a reasonable compromise for simultaneous GABA and GSH editing.
J-difference editing requires the subtraction of large signals, such as creatine, to reveal small edited signals such as GABA and GSH. Post-processing frequency-and-phase correction of individual transients can substantially reduce the appearance of subtraction artifacts in difference spectra. Although it is generally agreed that such post-processing is beneficial (14,35), methods of post-processing frequency and phase correction are still being developed (22,33,36) and currently, the optimum strategy depends on the metabolite being edited. For example, GSH-edited data cannot be aligned based on the residual water signal, since it is suppressed in GSH-ON spectra (33). GABA-edited data cannot be aligned based on the creatine signal (36) or the NAA signal which is suppressed in GABA-ON spectra. In the case of HERMES editing, there are now four sub-spectra that must be mutually aligned, and that differ in the appearance of the water, creatine and NAA signals. Correction was achieved here using a two-step approach, which started on the full time-domain data, and then used the choline signal for further alignment. The presence of subtraction artefacts in the edited spectra show that further development of HERMES post-processing is required.
Quantification of GABA is relatively similar between HERMES- and MEGA-edited experiments. GABA levels are (non-significantly) lower for HERMES, reflecting the greater remaining subtraction artefacts in the HERMES spectra.
One limitation of simultaneously acquiring GSH and GABA data is that the acquisition durations and volumes are the same for both metabolites. The edited GABA signal is generally larger than the GSH signal and separate acquisitions might choose a shorter scan duration or smaller volume for GABA measurements. An additional limitation to the editing scheme demonstrated is that ONGABA and OFFGABA scans are less rapidly interleaved (every two scans) than ONGSH and OFFGSH scans (interleaved every scan). The HERMES editing scheme now spans four TRs, rather than 2 TRs with MEGA editing. Thus, HERMES might be more susceptible than MEGA-PRESS to subject motion and/or B0 field drift (37), and within HERMES, GABA editing might be more susceptible than GSH editing. Finally, the protocol implemented co-edits a substantial fraction of macromolecular (MM) signal (12). However, by incorporating editing lobes at 1.5 ppm into the OFFGABA scans, MM-suppressed GABA (32,38) could be simultaneously edited with GSH.
5. Conclusion
Hadamard Editing and Reconstruction of MEGA-edited Spectroscopy (HERMES) allow the simultaneous edited detection of multiple metabolites with overlapping signals in the MR spectrum. HERMES has been demonstrated for GABA and GSH editing, allowing a two-fold reduction in scan times compared to sequentially acquired measurements using conventional editing methods. HERMES editing encodes the different editing target signals orthogonally, so that difference-edited spectra for each target can be separately reconstructed using a Hadamard transformation.
Supplementary Material
Highlights.
GABA and GSH can be edited simultaneously and separably using HERMES.
Acquisition times halved compared to sequential acquisitions.
HERMES-PRIAM edits multiple metabolites in multiple brain locations.
Acknowledgements
We are grateful to Vincent Boer for assistance in implementing PRIAM and for sharing reconstruction code. This work was supported by NIH grants R01 EB EB016089, R01 MH106564 and P41 EB015909.
Grant Support: This work was supported by NIH grants R01 EB016089 and P41 015909.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forester BR, Callaghan BC, Petrou M, Edden RA, Chenevert TL, Feldman EL. Decreased motor cortex γ-aminobutyric acid in amyotrophic lateral sclerosis. Neurology. 2012;78(20):1596–600. doi: 10.1212/WNL.0b013e3182563b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiduschat N, Mao X, Hupf J, Armstrong N, Kang G, Lange DJ, et al. Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett. 2014 Jun;570:102–7. doi: 10.1016/j.neulet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Chi L, Ke Y, Luo C, Gozal D, Liu R. Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo. Neuroscience. 2007 Feb;144(3):991–1003. doi: 10.1016/j.neuroscience.2006.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shetty AK, Bates A. Potential of GABA-ergic cell therapy for schizophrenia, neuropathic pain, and Alzheimer’s and Parkinson’s diseases. Brain Res. 2016 May 1;1638(Pt A):74–87. doi: 10.1016/j.brainres.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oz G, Terpstra M, Tkác I, Aia P, Lowary J, Tuite PJ, et al. Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: detection of high GABA concentrations. Magn Reson Med. 2006 Feb;55(2):296–301. doi: 10.1002/mrm.20761. [DOI] [PubMed] [Google Scholar]
- 6.Olanow CW. An introduction to the free radical hypothesis in Parkinson’s disease. Ann Neurol. 1992;32(Suppl):S2–9. doi: 10.1002/ana.410320703. [DOI] [PubMed] [Google Scholar]
- 7.Do KQ, Cuenod M, Hensch TK. Targeting Oxidative Stress and Aberrant Critical Period Plasticity in the Developmental Trajectory to Schizophrenia. Schizophr Bull. 2015 Jul;41(4):835–46. doi: 10.1093/schbul/sbv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shungu DC. N-acetylcysteine for the treatment of glutathione deficiency and oxidative stress in schizophrenia. Biol Psychiatry. 2012 Jun 1;71(11):937–8. doi: 10.1016/j.biopsych.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014 Jan;39(1):1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- 10.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000 May;13(3):129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998 Oct;11(6):266–72. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5662–6. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terpstra M, Henry P-G, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn Reson Med. 2003 Jul;50(1):19–23. doi: 10.1002/mrm.10499. [DOI] [PubMed] [Google Scholar]
- 14.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014 Feb 1;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terpstra M, Marjanska M, Henry P-G, Tkác I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn Reson Med. 2006 Dec;56(6):1192–9. doi: 10.1002/mrm.21086. [DOI] [PubMed] [Google Scholar]
- 16.Chan KL, Puts NAJ, Schär M, Barker PB, Edden RAE. HERMES: Hadamard encoding and reconstruction of MEGA-edited spectroscopy. Magn Reson Med. 2016 Apr 19; doi: 10.1002/mrm.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boer VO, Klomp DWJ, Laterra J, Barker PB. Parallel reconstruction in accelerated multivoxel MR spectroscopy. Magn Reson Med. 2015 Sep;74(3):599–606. doi: 10.1002/mrm.25718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edden RAE, Barker PB. Spatial effects in the detection of γ-aminobutyric acid: Improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007 Dec;58(6):1276–82. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 19.Edden Richard, Oeltzschner Georg, Harris Ashley D., Puts Nicolaas A. J., Chan Kimberley L., Boer Vincent O., et al. Prospective Frequency Correction for Macromolecule-Suppressed GABA Editing Experiments at 3T. J Magn Reson Imaging. doi: 10.1002/jmri.25304. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med. 2015 Dec 30; doi: 10.1002/mrm.26091. [DOI] [PubMed] [Google Scholar]
- 21.Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999 Apr;41(4):649–56. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015 Jan;73(1):44–50. doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid– edited MR spectroscopy spectra. J Magn Reson Imaging JMRI. 2014 Dec;40(6):1445–52. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puts NAJ, Edden RAE. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012 Jan;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puts NAJ, Edden RAE. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog Nucl Magn Reson Spectrosc. 2012 Jan;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson San Diego Calif 1997. 2001 Dec;153(2):155–77. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- 27.Slotboom J, Bovée WMMJ. Adiabatic slice-selective rf pulses and a single-shot adiabatic localization pulse sequence. Concepts Magn Reson. 1995;7(3):193–217. [Google Scholar]
- 28.Scheenen TWJ, Klomp DWJ, Wijnen JP, Heerschap A. Short echo time1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008 Jan;59(1):1–6. doi: 10.1002/mrm.21302. [DOI] [PubMed] [Google Scholar]
- 29.Mlynárik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006 Nov;56(5):965–70. doi: 10.1002/mrm.21043. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Edden RAE, Ouwerkerk R, Barker PB. High resolution spectroscopic imaging of GABA at 3 Tesla. Magn Reson Med. 2011 Mar;65(3):603–9. doi: 10.1002/mrm.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogner W, Gagoski B, Hess AT, Bhat H, Tisdall MD, van der Kouwe AJW, et al. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. NeuroImage. 2014 Dec;103:290–302. doi: 10.1016/j.neuroimage.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edden RAE, Puts NAJ, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn Reson Med. 2012 Sep;68(3):657–61. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An L, Zhang Y, Thomasson DM, Latour LL, Baker EH, Shen J, et al. Measurement of glutathione in normal volunteers and stroke patients at 3T using J-difference spectroscopy with minimized subtraction errors. J Magn Reson Imaging JMRI. 2009 Aug;30(2):263–70. doi: 10.1002/jmri.21832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan KL, Puts NAJ, Snoussi K, Harris AD, Barker PB, Edden RAE. Echo time optimization for J-difference editing of glutathione at 3T. Magn Reson Med. 2016 Feb 25; doi: 10.1002/mrm.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waddell KW, Avison MJ, Joers JM, Gore JC. A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn Reson Imaging. 2007 Sep;25(7):1032–8. doi: 10.1016/j.mri.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans CJ, Puts NAJ, Robson SE, Boy F, McGonigle DJ, Sumner P, et al. Subtraction artifacts and frequency (mis-)alignment in J-difference GABA editing. J Magn Reson Imaging JMRI. 2013 Oct;38(4):970–5. doi: 10.1002/jmri.23923. [DOI] [PubMed] [Google Scholar]
- 37.Harris AD, Glaubitz B, Near J, John Evans C, Puts NAJ, Schmidt-Wilcke T, et al. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn Reson Med. 2014 Oct;72(4):941–8. doi: 10.1002/mrm.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001 Mar;45(3):517–20. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.