Abstract
Prenatal alcohol exposure (PAE) is associated with extremely high rates of psychopathologies, which may be mediated by the hypothalamic-pituitary-adrenal (HPA) dysregulation observed in exposed individuals. Of relevance, PAE carries an increased risk of exposure to stressful environments throughout life. Importantly, stressful experiences during adolescence increase vulnerability to psychopathologies. However, little is known about how adolescent stressful experiences in the context of PAE-induced HPA dysregulation may further alter the developmental trajectory and potentially contribute to the disproportionally high rate of psychopathologies observed in this population. Here we investigate the short-and long-term effects of adolescent chronic mild stress (CMS) on the emergence of anxiety-/depressive-like behaviors (open-field and forced swim test - FST) and on HPA activity (corticosterone and type 1 CRH receptor – CRHR1) in PAE male and female rats. Under non-CMS conditions, open field results indicate that PAE induced inappropriate behavior (increased time in center) in males and females, with increased activity in female adolescents, but anxiety-like behavior in adult PAE females. Conversely, FST results indicate that PAE induced depressive-like behavior in adolescent males. Exposure to CMS resulted in increased activity in adolescent males and anxiety-like behaviors in adult females. Moreover, PAE and/or CMS altered corticosterone and CRHR1 expression in the mPFC and amygdala. Together, these results suggest that PAE and adolescent CMS induce dynamic neurobehavioral alterations that manifest differently depending on the age and sex of the animal. These results highlight the importance of using both sexes as well as an ontogenetic approach when investigating the effects of environmental adversity.
Keywords: Prenatal alcohol exposure, Adolescence, Chronic mild stress, Anxiety-like behavior, Depressive-like behavior, CRHR1
1. Introduction
Prenatal alcohol exposure (PAE) has consistently been shown to induce a wide range of cognitive, neurobehavioral, and physiological deficits, including dysregulation of the stress system. In both human and rodent studies, offspring exposed to alcohol in utero show increased hypothalamic-pituitary-adrenal (HPA) activation and/or a delayed return to basal levels compared to controls when faced with a wide range of stressful stimuli (Jacobson et al., 1999; Lee and Rivier, 1996; Nelson et al., 1986; Taylor et al., 1982; Weinberg et al., 2008). Moreover, the rates of psychopathologies such as anxiety and depression are extremely high in individuals exposed to alcohol during gestation (Famy et al., 1998; O’Connor et al., 2002; Pei et al., 2011). These psychopathologies can manifest early in childhood and persist into adulthood, with increasingly aggravated symptoms (Steinhausen and Sphor, 1998). Altered HPA regulation and stress hyperresponsivity following PAE may be important factors mediating the increased vulnerability of these individuals to develop psychopathologies. Indeed, depression is often described as a stress-related disorder; episodes of depression often occur in the context of some form of stress, and people with major depression typically exhibit HPA dysregulation (Nestler et al., 2002). Using a rat model of PAE, we have demonstrated that PAE rats exposed to chronic, unpredictable but mild stressful experiences in adulthood show increased anxiety- and depressive-like behaviors, and do so in a sexually dimorphic manner (Hellemans et al., 2008, 2010a,b). These behavioral alterations are associated with functional and structural changes in brain areas underlying stress and emotional regulation, including the medial prefrontal cortex (mPFC) and amygdala (Lan et al., 2015; Raineki et al., 2014; Uban et al., 2013). However, little is known about how exposure to stressful environments during adolescence may further alter the developmental trajectory of key brain areas such as the mPFC and amygdala, and potentially contribute to the disproportionally high rate of psychopathologies following PAE.
Adolescence is a transitional period between childhood and adulthood, during which a variety of sexually dimorphic developmental changes in behavioral, cognitive, and physiological parameters occurs (McCormick and Mathews, 2010; Spear, 2000). Additionally, adolescence can encompass a time of significant vulnerability to mental health problems. The clinical literature indicates that the onset of many psychopathologies such as anxiety, depression, substance abuse, and eating disorders, occurs generally around adolescence (Andersen and Teicher, 2008; Costello et al., 2002; Paus et al., 2008). The underlying mechanisms supporting this increased vulnerability are still not fully understood. However, it has been proposed that exposure to stressors during adolescence may change the developmental trajectory of brain areas involved in stress and emotional regulation, which in turn could result in increased vulnerability to psychopathologies (Eiland and Romeo, 2013; Hollis et al., 2013; McCormick and Green, 2013). A contributing factor for this stress-related vulnerability is that adolescence is characterized by higher HPA activity in response to both acute and chronic stressors than is found in adulthood (Doremus-Fitzwater et al., 2009; Hollis et al., 2013; McCormick and Mathews, 2010; Romeo et al., 2004, 2006). Additionally, adolescence is a time of extensive functional and structural reorganization in brain areas involved in stress and emotional regulation, including the mPFC and amygdala - areas that are also among the most sensitive to the effects of corticosterone (Eiland and Romeo, 2013; McCormick and Mathews, 2010; Spear, 2000).
Besides being the key neuropeptide regulating the HPA axis, corticotropin-releasing hormone (CRH) and its receptors are broadly distributed in the central nervous system (Van Pett et al., 2000; Sawchenko and Swanson, 1989) and dysregulation of the central CRH system has been implicated in the pathogenesis of many psychopathologies, including depression and anxiety (Binder and Nemeroff, 2010; Holsboer and Ising, 2008). Preclinical studies indicate that many of the behavioral abnormalities associated with depression and anxiety are mediated by the type 1 CRH receptor (CRHR1). Specifically, decreased activity of the CRHR1 system is associated with reductions in anxiety-like behavior, as shown in studies using CRHR1-deficient mice (Smith et al., 1998; Timpl et al., 1998), conditional inactivation of the CRHR1 in limbic areas (Müller et al., 2003), and CRHR1 antagonism in rats (Keck et al., 2001; Sandi et al., 2008; Veenit et al., 2014). Additionally, results from clinical trials suggest that CRHR1 antagonists may be a promising pharmacological treatment for depression and anxiety, especially for patients with high central CRH levels (Holsboer and Ising, 2008). Stress during adolescence has been shown to alter the expression of CRHR1 in the amygdala and mPFC (Iredale et al., 1996; Li et al., 2015; Veenit et al., 2014). These stress-induced changes in CRHR1 receptor expression may underlie the anxietylike, social and stress-coping behavior dysfunctions observed in animals exposed to adolescent stressors (Li et al., 2015; Márquez et al., 2013; Toledo-Rodriguez and Sandi, 2011; Veenit et al., 2014).
As adolescence is a period during which the individual shows unique hyperresponsivity to stressors (Eiland and Romeo, 2013; Hollis et al., 2013; McCormick and Mathews, 2010), and as PAE results in HPA dysregulation, here we investigate if exposure to chronic mild stress (CMS) during adolescence has a greater impact on PAE than on control animals. Accumulating evidence suggests that the effects of chronic stress during adolescence may manifest differently depending upon when observations are made: some effects are only observed shortly after stress, some are only manifest long after the termination of stress, and some are enduring and can be observed any time following stress exposure (Bourke and Neigh, 2011; McCormick and Green, 2013; Tsoory et al., 2008). Accordingly, we examine both short- and long-term effects of adolescent CMS on anxiety- and depressive-like behavior and HPA activity. Additionally, as the central CRH system plays a critical role in emotional and stress regulation, we also evaluate the impact of adolescent CMS on CRHR1 expression in the amygdala and mPFC of PAE animals.
2. Methods
2.1. Animals and breeding
Male and female Sprague-Dawley rats were obtained from Charles River Laboratories (St. Constant, Canada). Rats were pair-housed by sex and maintained at a constant temperature (21 ± 1 °C) and on a 12 h light-dark cycle (lights on at 0700 h) with ad libitum access to water and standard lab chow (Harlan, Canada). After a 10-day acclimation period, male and female pairs were placed together for breeding. Vaginal smears were taken each morning, and the presence of sperm indicated gestation day 1 (G1). All experiments were performed in accordance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals, Canadian Council on Animal Care guidelines, and were approved by the University of British Columbia Animal Care Committee.
2.2. Prenatal alcohol exposure
On G1, females were single-housed and randomly assigned to prenatal alcohol exposure (PAE) or control prenatal treatment groups. PAE dams (n = 21) were offered liquid ethanol diet ad libitum, with 36% ethanol-derived calories (Weinberg-Keiver High Protein Experimental Diet # 710324, Dyets Inc., Bethlehem, PA), formulated to provide adequate nutrition to pregnant rats regardless of ethanol intake (Lan et al., 2006). Ethanol diet was introduced gradually over the first 3 days with bottles containing: Day 1 – 66% control diet, 34% ethanol diet; day 2 – 34% control diet, 66% ethanol diet; day 3 – 100% ethanol diet. Fresh diet was provided daily within 1 h of lights off. Blood alcohol levels (BAL) were measured as previously reported (Workman et al., 2015); alcohol-consuming dams had BALs of 118.20 ± 8.11 mg/dl. Control dams (n = 22) were offered ad libitum access to a pelleted form of the liquid control diet (Weinberg-Keiver High Protein Pelleted Control Diet #710109). All animals had ad libitum access to water. Experimental diets were continued through G21. Beginning on G22, PAE and control dams were offered ad libitum access to standard laboratory chow and water, which they received throughout lactation. Pregnant dams were left undisturbed except for weighing (G1, G7, G14, and G21) and cage changing (G1, G7, and G14). On the day of birth (postnatal day 1, PN1) litters were culled to 12 pups (6 males, 6 females when possible). Dams and pups were weighed and cages changed on PN1, PN8, PN15, and PN22. On PN22 pups were weaned and group-housed by litter and sex. See supplementary section for body weight data for dams during gestation and lactation and pups.
2.3. Adolescent chronic mild stress (CMS)
One male and one female from each litter were randomly assigned to either the CMS or the non-CMS condition and were pair-housed with another animal of the same sex and prenatal group (see supplementary section for detailed description of animal numbers per group). Animals in the CMS condition were subjected to 10 consecutive days of chronic, unpredictable, mild stressors. To account for the sexually dimorphic time of pubertal onset (McCormick and Mathews, 2010), males and females were exposed to CMS at ages consistent with puberty onset - PN31–41 for females, PN 37–47 for males. On each CMS day, animals received two different stressors at random times in the morning (between 0800 and 1200 h) and afternoon (between 1300 and 1800 h) with a minimum of 2 h between stressors. On day 1 of CMS and the day immediately following the end of CMS, within 2 h of lights on, basal blood samples were obtained from all animals (including those in the non-CMS group) via tail nick. After tail nick, all animals were weighed and placed in a new home cage. Except for blood sampling and routine husbandry, animals in the non-CMS condition were left undisturbed during this period. CMS and non-CMS animals were housed in different colony rooms so that non-CMS animals were not exposed to the disturbance and stress odors of the CMS animals. The order and type of stressor was randomized, but all animals received the same number of exposures to each stressor over the 10-day period. Stressors included: 1) Platform: exposure to an elevated Plexiglas platform (20 × 20 cm) mounted on 90 cm high post for 10 min; 2) Cage tilt: the home cage was tilted at a 30° angle for 2 h; 3) Novel cage: exposure to a novel cage without food and water, with a small amount of a different kind of bedding for 1 h; 4) Soiled cage: exposure to a soiled cage of another animal of the same sex for 1 h; 5) Restraint: restraint in PVC tubes (tube size varied to ensure proper restraint/immobility of each animal) for 30 min; 6) Social isolation without food and water: overnight isolation in a smaller cage (28 × 17 cm with 12.5 cm high) for 12 h; and 7) Empty water bottle: animals given their empty water bottles for 1 h following the social isolation/food and water deprivation period.
2.4. Open field
All animals were tested on the open field before and after CMS exposure. Prior to CMS, animals were first acclimated to the open field for 15min with littermates (females: PN28; males: PN34) and then tested individually on the following two days (females: PN29-30; males: PN35-36). For the post-CMS test, half the animals were allowed to rest for a day and then were tested in the open field to evaluate the short-term effects of CMS (females: PN42; males: PN48); the remaining animals were tested in adulthood (PN90-PN100) to evaluate the long-term effects of CMS. The open field apparatus was an age-appropriate, clear Plexiglas box, 42 × 42 cm × 38 cm high for animals tested in adolescence, and 84 × 84 cm × 38 cm high for animals tested in adulthood. Both open fields were fitted within two frames of infrared beams, with the top frame positioned to a height that would track rearing behavior, and the bottom frame adjusted to track movement and activity. Data were recorded and automatically analyzed by MotorMonitor™ software. Testing occurred in a dimly lit room between 0800 and 1200 h, and the first 10 min of the tests were evaluated.
2.5. Forced swim test (FST) and brain collection
Following post-CMS open field-testing, all animals were exposed to the FST. Animals were habituated (15 min) and then tested (5 min) the following day in a clear Plexiglas cylinder filled with water (25±1°C; depth prevented escape and tail touching bottom). Test sessions were recorded and time spent immobile (passive floating without struggling, slightly hunched but upright position with minor movements to maintain head above water) was measured using Noldus Observer software. Animals were decapitated 30-min after the end of testing on day 2 and brains collected, quickly frozen on dry ice, and stored at −80 °C. Testing and brain extractions were performed between 0800 and 1200h.
2.6. Corticosterone radioimmunoassay
Pre- and post-CMS blood samples were centrifuged at 32,000 rpm for 10 min at 4°C, and serum stored at −80 °C until assay using ImmunChem™ Double Antibody Corticosterone 125I RIA kits (MP Biomedicals, Orangeburg, NY).
2.7. Neural assessment of CRHR1 by in situ hybridization
2.7.1. Probe
The ribonucleotide probe was prepared using CRHR1 1.3 kb template provided by Dr. Victor Viau (source: Dr. Cyntia Donaldson, Perrin et al., 1993), 35S UTP (Perkin-Elmer, Waltham, MA), and the Promega Riboprobe System (Promega Corp., Madison, WI) with polymerase T7 for antisense probe. The probe was purified using Micro Bio-Spin 30 Columns (Bio-Rad, CA, USA) and 0.1 M DTT was added to prevent oxidation.
2.7.2. In situ hybridization
Brains were sectioned coronally (20 µm) using a cryostat (−16°C) and stored at −80°C. Thawed sections were fixed in formalin for 30 min and pre-hybridized as follows: 1× PBS for 10min twice, proteinase K (0.1µg/L; 37°C) for 9min, 0.1 M triethanolamine-hydrochloride (TEA) for 10 min, 0.1 M TEA with 0.25% acetic anhydride for 10 min, 2× SSC for 10 min twice, dehydrated by a graded series of ethanol, chloroform, and 100% ethanol and air-dried. Hybridization buffer (75% formamide, 3× SSC, 1 × Denhardt’s solution, 200 µg/mL yeast tRNA, 50 mM sodium phosphate buffer (pH 7.4), 10% dextran sulphate, 10 mM DTT) was applied (1×106 cpm/slide) and covered with HybriSlips (Sigma-Aldrich, ON). Sections were incubated overnight at 55 °C in chambers humidified with 75% formamide. HybriSlips were removed and slides were rinsed as follows: 2 × SSC twice for 20 min, 2× SSC for 30 min, 50 µg/L RNAse A solution (37 °C) for 60 min, 2× SSC with 0.01 M DTT for 10 min, 1× SSC for 10 min, 0.5× SSC with 0.01 M DTT for 10 min, 0.1 × SSC with 0.01 M DTT (60 °C) for 60 min, 0.1 × SSC for 5 min. Sections were dehydrated by a graded series of ethanol and air dried overnight. The hybridized slides were then exposed to Kodak BioMax MR film, and developed using D-19 developer and Kodak fixer.
2.7.3. Densitometric analysis
The autoradiograph films were scanned and analyzed with ImageJ 1.48v (National Institutes of Health, USA). The left and right subregions of amygdala (basolateral complex and medial) and mPFC (anterior cingulate cortex, prelimbic, and infralimbic) were traced free-hand on two sections per animal to determine mean grey density levels. Corrected grey levels were obtained by subtracting the background level (corpus callosum) from each of the four measurements. Left and right levels in each measured area were averaged together for analysis.
2.8. Statistical analysis
All data are expressed as mean ± SEM. Pre-CMS body weight and basal corticosterone levels were analyzed by Student’s t-test. Pre-CMS open field data were analyzed by repeated-measures analysis of variance (ANOVA; prenatal treatment and test day as factors). All post-CMS data were analyzed by two-way ANOVAs (prenatal treatment and CMS as factors). When significant, ANOVAs were followed by Newman-Keuls post hoc tests. Sex could not be used as a factor in the ANOVAs because females and males were exposed to CMS at different ages (PN31-41 and PN 37–47, respectively). Similarly, age could not be used as factor because open field sizes were age-appropriate and, in situ hybridizations for adolescent and adult brains were run separately, as it was more important to compare groups at each age rather than age within group. Further analyses utilized planned comparisons to test the a priori hypotheses that: 1) PAE will alter behavior, HPA responses, and CRHR1 mRNA expression compared to those in controls; and 2) CMS will differentially alter behavior, HPA responses, and CRHR1 mRNA expression. In all cases, differences were considered significant when p ≤ 0.05. Nonsignificant effects are not reported. Outliers were identified and removed using the Robust regression and Outlier removal (ROUT) method with Q= 0.05.
3. Results
3.1. Body weight and basal corticosterone levels pre- and post-CMS
Pre-CMS body weights of PAE males and females were not different from those of controls (Table 1). Exposure to adolescent CMS resulted in reduced weight gain in both PAE and control males and females [main effects of CMS for males (F(1,62) = 14.74, p = 0.0003) and females (F(1,60) = 6.31, p = 0.015)]. There were no long-term effects of PAE or CMS on body weight in adulthood (data not shown).
Table 1.
Body weight (g) and basal corticosterone (µg/dL) levels pre- and post-CMS.
| Male |
Female |
||||||
|---|---|---|---|---|---|---|---|
| Pre-CMS | Post-CMS |
Pre-CMS | Post-CMS |
||||
| Non-CMS | CMS | Non-CMS | CMS | ||||
| Body weight | Control | 159.4 ±3.9 | 261.3 ±8.3 | 232.4 ±5.8§ | 94.7 ±1.9 | 157.5 ±4.5 | 146.8 ±2.9§ |
| PAE | 163.9 ±2.9 | 257.9 ±5.3 | 238.9 ±5.2§ | 94.1 ± 2.4 | 156.1 ±3.6 | 149.2 ±2.8§ | |
| Corticosterone | Control | 1.05 ±0.16 | 0.81 ±0.22 | 2.22 ± 0.60§ | 1.45 ±0.25 | 1.16±0.57 | 2.71 ±0.76§ |
| PAE | 0.86 ± 0.09 | 0.58 ±0.05† | 1.06 ±0.23†,§ | 1.63 ±0.24 | 0.82 ±0.21 | 4.00 ± 0.93§ | |
PAE animals are different from control animals independent of CMS.
CMS different from non-CMS independent of prenatal treatment.
PAE did not alter pre-CMS basal corticosterone levels in either males or females (Table 1). However, exposure to adolescent CMS increased basal corticosterone levels in males and females of both prenatal treatment groups [main effects of CMS for males (F(1,60) = 8.27, p = 0.0055) and females (F(1,58) = 11.69, p = 0.0011)], with PAE males showing lower basal corticosterone levels than controls, independent of CMS [main effects of prenatal treatment for males (F(1,60) =4.51, p = 0.038)].
3.2. Open field test
3.2.1. Pre-CMS
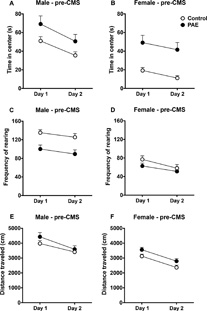
Animals were tested in the open field for two consecutive days prior to CMS exposure (females at PN29-30, males at PN35-36). Overall, PAE males and females spent significantly more time in the center of the open field compared to controls, with male and female rats of both groups spending significantly less time in the center of the open field on day 2 compared to day 1 (Fig. 1A–B) [main effects of prenatal treatment for males (F(1,64) = 4.43, p = 0.039) and females (F(1,55) = 12.79, p = 0.0007); main effects of day for males (F(1,64) = 11.46, p = 0.0012) and females (F(1,55)= 4.58, p = 0.037)]. PAE males, but not females, exhibited less rearing behavior than controls, with all animals displaying significantly less rearing on day 2 compared to day 1 (Fig. 1C–D) [main effect of prenatal treatment for males (F(1,64) = 11.59, p = 0.0011)]; main effects of day for males (F(1,64) = 5.47, p = 0.022) and females (F(1,62) = 21.83, p = 0.0001)]. Moreover, PAE females, but not males, showed higher total distance traveled than controls, with all animals traveling significantly less total distance on day 2 compared to day 1 (Fig. 1E–F) [main effect of prenatal treatment for females (F(1,62) = 5.01, p = 0.029)]; main effects of day for females (F(1,62) = 42.94, p = 0.0001) and males (F(1,64) = 17.95, p = 0.0001)].
Fig. 1.
Effects of PAE on the open field test in rats tested pre-CMS exposure. Circles represent mean ± SEM of the time in the center (A–B), total distance traveled (C–D), or frequency of rearing in the open field (E–F) (males: n=32–34 for all groups; females: n = 25–32 for all groups).
3.2.2. Post-CMS
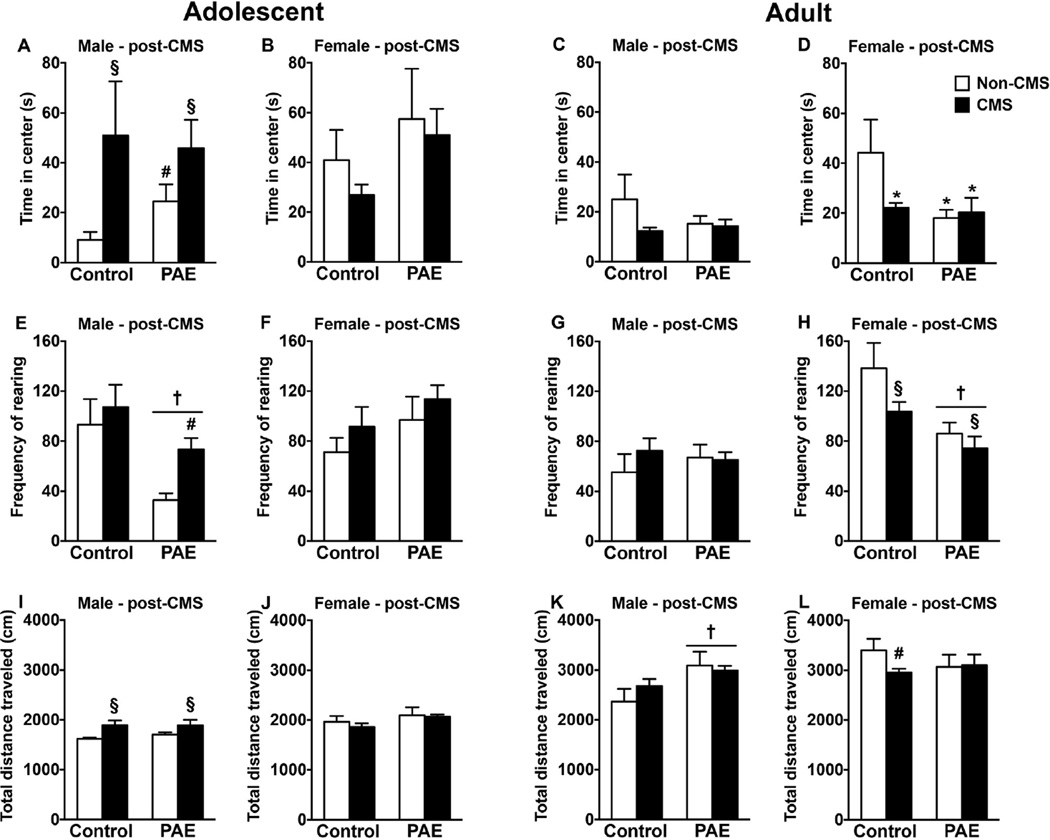
For animals tested in adolescence, immediately following CMS (PN42 for females, PN48 for males; Fig. 2A–B, E–F, I–J), similar to the pre-CMS results, PAE non-CMS males spent significantly more time in the center of the open field compared to control non-CMS males [no significant interaction between prenatal treatment and CMS; a priori analysis comparing control non-CMS to PAE non-CMS (p = 0.047)]. Nevertheless, exposure to CMS significantly increased both time spent in the center and total distance traveled in males, independent of prenatal treatment [main effects of CMS for time in center (F(126) = 5.19, p = 0.031) and total distance traveled (F(1,27) =8.56, p = 0.0068)]. Moreover, PAE males showed reduced rearing behavior independent of CMS exposure [main effect of prenatal treatment (F(1,27) = 9.60, p = 0.0045)]. However, CMS was able to increase rearing behavior in PAE males compared to their non-CMS counterparts [no significant interaction between prenatal treatment and CMS; a priori analysis comparing PAE non-CMS to PAE CMS (p = 0.0016)]. For females, there were no short-term effects of PAE or CMS on open field behavior.
Fig. 2.
Short- and long-term effects of PAE and adolescent CMS in the open field. Bars represent mean ± SEM of the time in the center of the open field (A–D), total distance traveled in the open field (E–H), or, frequency of rearing in the open field (I–L). † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS; for A, # indicates that PAE non-CMS is different from control non-CMS based on a priori comparisons; for E, # indicates that PAE CMS is different from PAE non-CMS based on a priori comparisons (n = 6–10 for all groups).
Results of open field testing in adulthood (PN90-PN100; Fig. 2C–D, G–H, K–L) indicate that PAE males, independent of CMS exposure, showed increased total distance traveled compared to controls [main effects of prenatal treatment (F(1,30) = 6.95, p = 0.013)]. Nevertheless, there were no long-term effects of PAE or adolescent CMS on time in center and rearing for males. However, PAE females, independent of CMS exposure, spent significantly less time in the center of the open field compared to control non-CMS females. Moreover, adolescent CMS decreased time in center and total distance traveled in control females compared to their non-CMS counterparts [interaction between prenatal treatment and CMS (F(1,28) = 3.95, p = 0.05) and main effect of prenatal treatment for time in center (F(1,28) = 5.33, p = 0.028); no significant interaction between prenatal treatment and CMS for distance traveled, a priori analysis comparing control CMS to control non-CMS (p = 0.021)]. Additionally, while exposure to adolescent CMS decreased rearing behavior overall in both PAE and control females, PAE females showed less rearing behavior than control females, independent of CMS exposure [main effects of prenatal treatment (F(1,28) = 13.27, p = 0.001) and CMS (F(1,28) = 4.27, p = 0.048)].
3.3. FST
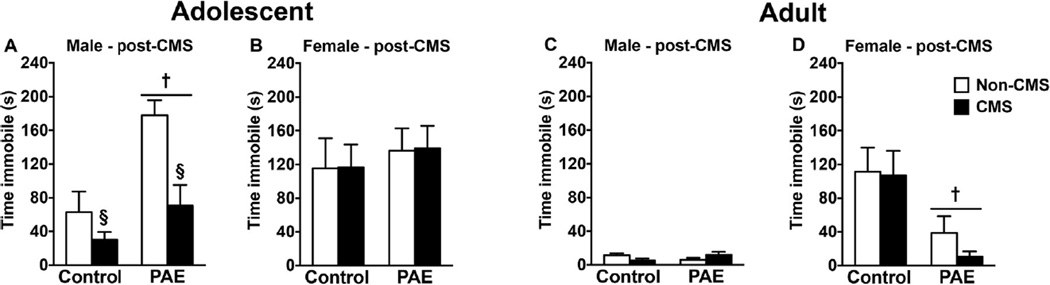
Following the open field test, animals were tested in the FST to evaluate the short- and long-term effects of adolescent CMS on depressive-like behaviors (Fig. 3).
Fig. 3.
Short- and long-term effects of PAE and adolescent CMS in the FST. Bars represent mean ± SEM of time immobile in the FST. † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS (n = 6–10 for all groups).
For animals tested in adolescence immediately following CMS, we observed that adolescent CMS decreased time spent immobile in both PAE and control males. However, PAE males spent significantly more time immobile than controls, independent of CMS exposure [main effects of prenatal treatment (F(1,28) = 14.84, p = 0.0006) and CMS (F(1,28) = 11.97, p = 0.0017)]. For females, there were no short-term effects of PAE or CMS on the FST.
In adulthood, PAE females spent significantly less time immobile than controls, independent of CMS exposure [main effect of prenatal treatment (F(1,26) = 10.76, p = 0.0029)]. However, there were no long-term effects of PAE or CMS on the FST in males.
3.4. CRHR1 mRNA expression
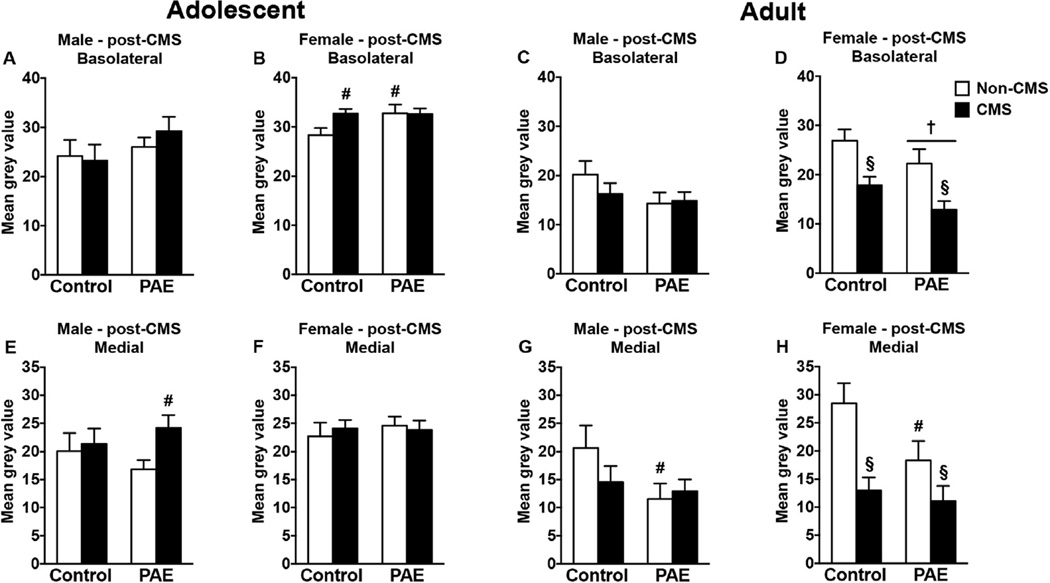
3.4.1. Amygdala
In adolescent males, CMS increased CRHR1 mRNA expression in the medial amygdala in PAE but not control rats; no changes were observed in the basolateral amygdala (Fig. 4A,E) [no significant interaction between prenatal treatment and CMS; a priori analysis for medial amygdala comparing PAE non-CMS to PAE CMS (p = 0.012)]. On the other hand, adolescent PAE non-CMS females showed increased CRHR1 mRNA expression in the basolateral amygdala compared to control non-CMS females (Fig. 4B). Additionally, adolescent CMS increased CRHR1 mRNA expression in the basolateral amygdala of control females compared to their non-CMS counterparts, whereas PAE females showed no significant change in expression with CMS [no significant interaction between prenatal treatment and CMS; a priori analysis for basolateral amygdala comparing PAE non-CMS to control non-CMS (p = 0.033) and control CMS to control non-CMS (p = 0.013)]. Neither PAE nor CMS affected CRHR1 mRNA expression in the medial amygdala of adolescent females (Fig. 4F).
Fig. 4.
Short- and long-term effects of PAE and adolescent CMS on amygdala CRHR1 mRNA expression. Bars represent the mean ± SEM (mean gray value) of CRHR1 mRNA expression in the basolateral (A–D) and medial (E–H) amygdala nuclei. † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS; for B, # indicates that control CMS and PAE non-CMS are different from control non-CMS based on a priori comparisons; for E, # indicates that PAE CMS is different from PAE non-CMS based on a priori comparisons; and for G and H, # indicates that PAE non-CMS is different from control non-CMS based on a priori comparisons (n = 5–10 for all groups).
In adulthood, PAE non-CMS males showed a reduction in CRHR1 mRNA expression in the medial amygdala compared to control non-CMS males (Fig. 4C,G) [no significant interaction between prenatal treatment and CMS; a priori analysis for medial amygdala comparing control non-CMS to PAE non-CMS (p = 0.04)]. However, neither PAE nor CMS affected CRHR1 mRNA expression in the basolateral amygdala of adult males. In females, adolescent CMS reduced CRHR1 mRNA expression in the basolateral and medial amygdala independent of prenatal treatment (Fig. 4D,H) [main effects CMS for basolateral amygdala (F(1,27) = 16.45, p = 0.0004) and medial amygdala (F(1,27) = 14.07, p = 0.0008)]. Moreover, PAE reduced CRHR1 mRNA expression in the basolateral amygdala compared to control females independent of CMS exposure [main effect of prenatal treatment for basolateral amygdala (F(1,27) = 4.51, p = 0.043)]. Additionally, PAE non-CMS females showed a reduction in CRHR1 mRNA expression in the medial amygdala than their control counterparts [no significant main effect of prenatal treatment for medial amygdala; a priori analysis for medial amygdala comparing control non-CMS to PAE non-CMS (p = 0.039)].
3.4.2. mPFC
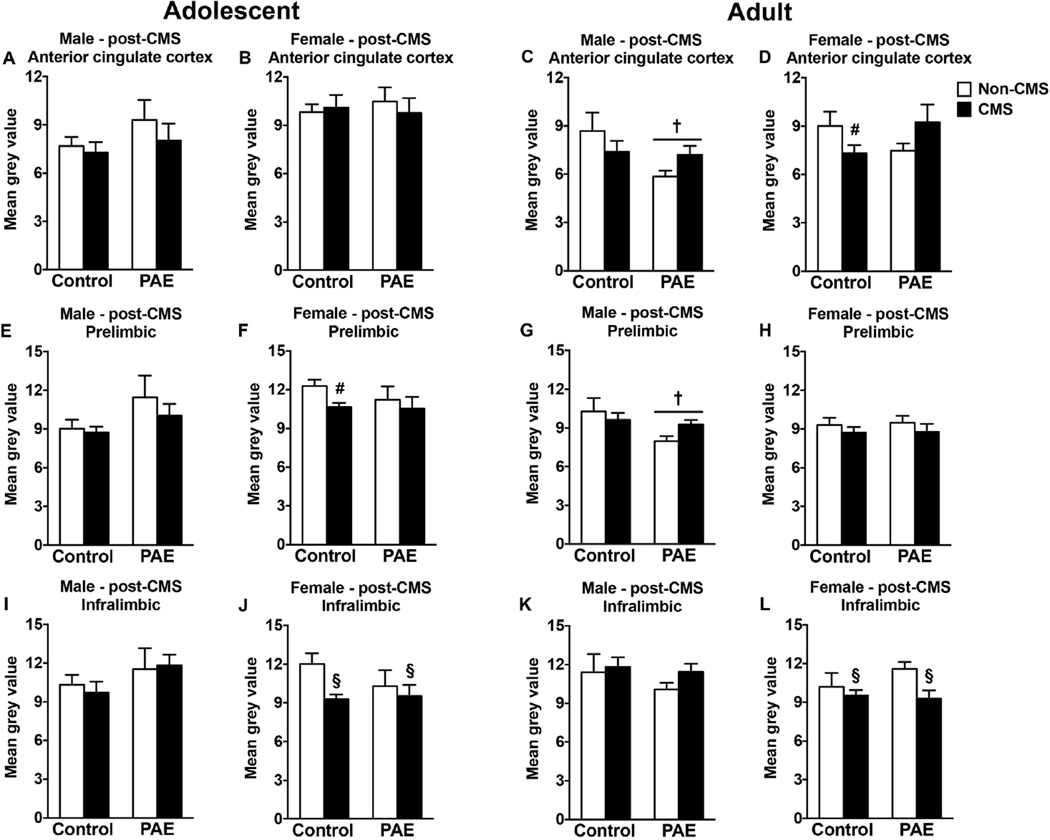
In adolescent males, neither PAE nor CMS affected CRHR1 mRNA expression in any mPFC subregions (Fig. 5A,E,I). In adolescent females, however, CMS exposure decreased CRHR1 mRNA expression in the infralimbic area independent of prenatal treatment (Fig. 5J), and decreased CRHR1 mRNA expression in the prelimbic area of control animals (Fig. 5F). No changes in the anterior cin-gulate cortex were observed in either group (Fig. 5B) [main effect of CMS for infralimbic (F(1,28) = 4.01, p = 0.05); no significant main effect of prenatal treatment for prelimbic; a priori analysis for prelimbic comparing control non-CMS to control CMS (p = 0.0081)].
Fig. 5.
Short- and long-term effects of PAE and adolescent CMS on mPFC CRHR1 mRNA expression. Bars represent the mean±SEM (mean gray value) of CRHR1 mRNA expression in the anterior cingulate (A–D), prelimbic (E–H), and infralimbic (I–L) cortices. † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS; for D and F, # indicates that control CMS is different from control non-CMS based on a priori comparisons (n = 6–10 for all groups).
In adulthood, PAE males showed a reduction in CRHR1 mRNA expression in the anterior cingulate cortex and prelimbic area compared to control males independent of adolescent CMS, but no changes were observed in the infralimbic area (Fig. 5C,G,K) [main effect of prenatal treatment for anterior cingulate cortex (F(1,30) = 4.35, p = 0.045) and prelimbic (F(1,30) = 4.47, p = 0.043)]. In adult females, adolescent CMS exposure reduced CRHR1 mRNA expression in the infralimbic area independent of prenatal treatment (Fig. 5L) [main effect of CMS for infralimbic (F(1,27) = 5.23, p = 0.03)], and reduced CRHR1 mRNA expression only in control females in the anterior cingulate cortex (Fig. 5D) [interaction between prenatal treatment and CMS for anterior cingulate cortex (F(1 27) = 5.55, p = 0.026); a priori analysis for anterior cingulate cortex comparing control non-CMS to control CMS (p = 0.048)]. However, neither PAE nor adolescent CMS induced long-term effects on CRHR1 mRNA expression in the prelimbic area of females (Fig. 5H).
4. Discussion
Our results demonstrate that PAE and adolescent CMS induce dynamic neurobehavioral alterations that manifest differently depending on the age of testing and sex of the animal. PAE males exhibited depressive-like behavior in the FST (increased immobility) during adolescence, but this effect was transient and disappeared in adulthood. Nevertheless, adult PAE males showed increased activity levels (increased distance traveled) in the open field. Conversely, PAE females displayed anxiety-like behavior in the open field (reduced time in center) in adulthood. However, during early adolescence (pre-CMS test), PAE male and female rats showed an inappropriate behavioral response to the open field test as they spent more time in the center. Exposure to CMS during adolescence resulted in increased total distance traveled and time in center of the open field and reduced time immobile in the FST in males - an effect that was independent of prenatal treatment -suggesting that adolescent CMS resulted in increased behavioral activity in males. By contrast, the long-term effects of adolescent CMS on behavior were evident only in females, as all females exposed to CMS during adolescence showed anxiety-like behaviors in the open field in adulthood. Interestingly, this long-term effect of adolescent CMS was associated with reduced CRHR1 mRNA expression in the amygdala. Together, our results demonstrate differential short- and long-term effects of PAE and adolescent CMS, highlighting the importance of ontogenetic studies when investigating the effects of pre- and postnatal adversity. Furthermore, these data demonstrate that males and females are differentially affected by PAE and/or adolescent CMS, emphasizing the importance of using both sexes. A potential limitation of the present data is the possibility of carry-over effects from repeated testing; however, this experimental design allowed for the comprehensive assessment of multiple neurobehavioral domains within the same animal. Moreover, our findings are generally consistent with previous studies from our laboratory demonstrating differential effects of PAE and CMS exposure in adulthood on anxiety-/depressive-like behavior in males and females, where different cohorts of animals were used for each behavioral test (Hellemans et al., 2010a).
4.1. PAE and adolescent CMS effects on body weight and basal corticosterone levels
Exposure to CMS during adolescence impaired growth as demonstrated by the lower body weight gain observed in both PAE and control males and females at the end of CMS exposure. These results are in line with previous reports showing that exposure to chronic stress during adolescence or in adulthood leads to a reduction in body weight (Eiland et al., 2012; Hellemans et al., 2008; Jankord et al., 2011; Uban et al., 2013; Wulsin et al., 2016). The reduced body weight in CMS animals was temporary, as body weight differences were not present in adulthood. Moreover, PAE rats were not differentially affected by adolescent CMS as they had body weights similar to controls at the end of CMS exposure.
Adolescent CMS also altered basal HPA function as demonstrated by an increase in basal corticosterone levels in both PAE and control males and females exposed to CMS. Similar to the CMS effect on body weight, the CMS effect on basal corticosterone levels corroborates the literature showing that chronic stress during adolescence results in increased basal corticosterone levels (Jankord et al., 2011; Toth et al., 2008; Wulsin et al., 2016).Together, the reduced weight gain and increased basal corticosterone levels in CMS animals validate the effectiveness of our CMS procedure despite the relatively short duration of stress exposure. Interestingly, PAE male rats showed reduced basal corticosterone levels, independent of CMS exposure. This effect was likely driven by the smaller CMS-induced increase in basal corticosterone levels in PAE males, suggesting that HPA function of PAE males was differentially affected by adolescent CMS.
4.2. PAE and adolescent CMS effects on emotionality
Here we demonstrate that the manifestation of the effects of PAE on emotion-related behaviors during adolescence are different from those in adulthood. Indeed, in early adolescence (pre-CMS test), male and female PAE animals spent more time in the center of the open field; in late adolescence (post-CMS test), this effect persisted in males, but disappeared in females. However, when tested in adulthood, PAE females demonstrated increased anxiety-like behavior (less time in the center of the open field; less rearing), whereas PAE males showed increased activity (distance traveled). The increased time spent in the center of the open field observed during early adolescence (pre-CMS test) in PAE male and female rats can be interpreted as an inappropriate behavioral response—a typical response for a rodent is to stick to the periphery and avoid the center, and for this reason, time in center is typically considered an index of anxiety-like behavior (Gould et al., 2009). This inappropriate behavior might reflect a pathological increase in the natural novelty-seeking and risk-taking behaviors observed during adolescence (Spear, 2000). Conversely, it may reflect an inability of PAE animals to read environmental cues or to inhibit inappropriate behavior. Finally, our finding that adult PAE males showed increased activity in the open field replicates previous preclinical data (Randall et al., 1986; Riley, 1990; Rockman et al., 1989) and reproduces one of the most common behavioral symptoms (i.e., hyperactivity) of individuals exposed to alcohol during gestation (Mattson and Riley, 1998; Mattson et al., 2001).
Similar to the differential effects of PAE on the open field in adolescence and adulthood, PAE also resulted in differential and age-dependent behavioral manifestations in the FST. Adolescent PAE male rats showed increased time immobile in the FST – indicating depressive-like behaviors – but this effect was not present in adulthood. Conversely, PAE females were not different from controls in adolescence but showed altered behavior in adulthood, spending less time immobile than controls. Together, our FST and open field data suggest that the effects of PAE on emotion-related behaviors are dynamic and complex, as their manifestation changes throughout the lifespan of the animal. Such dynamic and complex effects of PAE are also reflected in the clinical data, where PAE-related effects change over time, especially those related to emotional regulation (Steinhausen and Sphor, 1998). Importantly, our results add more evidence to the growing body of pre-clinical and clinical literature demonstrating that exposure to alcohol in utero increases the predisposition to mental health problems (Famy et al., 1998; Hellemans et al., 2008, 2010a,b; O’Connor et al., 2002; Pei et al., 2011).
Besides evaluating the unique effects of PAE on emotionality, we also investigated the short- and long-term effects of adolescent CMS on emotional behaviors of PAE animals. Results from several laboratories have demonstrated that chronic adolescent stress results in differential short- and long-term effects on anxiety-like behaviors (Jacobson-Pick and Richter-Levin, 2010, 2012; Mathews et al., 2008; McCormick et al., 2008; Toledo-Rodriguez and Sandi, 2011). For example, while adolescent stress may increase anxietylike behaviors in adulthood, if animals are tested in adolescence immediately after the termination of the stress, the overall outcome is increased activity levels that may reflect a decrease in the expression of anxiety-like behaviors (Eiland et al., 2012; Jacobson-Pick and Richter-Levin, 2010; McCormick et al., 2008; Toledo-Rodriguez and Sandi, 2011). In general, our results are in agreement with the adolescent stress literature, as we observed increased activity levels in adolescent males tested immediately after adolescent CMS exposure as well as increased anxiety-like behaviors in adult females exposed to adolescent CMS, independent of prenatal group. It is possible that the lack of differential response in PAE females may represent a floor effect, as adult PAE females in the non-CMS condition already showed significant anxiety-like behavior.
The literature on the short-term effects of chronic adolescent stress on depressive-like behaviors is somewhat mixed, as some studies report no effects (Jankord et al., 2011; Mathews et al., 2008; Toth et al., 2008; Wulsin et al., 2016) and others report decreases in depressive-like behaviors (Eiland et al., 2012). These discrepancies may be related to the specific stress paradigms utilized, the age of stress exposure, and/or the FST protocols used. Importantly, the current data highlight sex as another possible factor influencing the multifaceted and dynamic short-term effects of adolescent stress. Indeed, in the present study we found decreased depressive-like behaviors (reduced time immobile in the FST) in adolescent males and no short-term effects of CMS on FST behavior in adolescent females. Nevertheless, the reduced time immobile in the FST and the increased total distance traveled and time in the center of the open field observed in adolescent males immediately following CMS exposure corroborate the literature indicating that chronic exposure to stress during adolescence may lead to increased behavioral activity in the short-term (Eiland et al., 2012; Jacobson-Pick and Richter-Levin, 2010; McCormick et al., 2008; Toledo-Rodriguez and Sandi, 2011). By contrast, although in the short-term, adolescent females appear to be less vulnerable to CMS—at least as assessed by the paradigms employed here.
Of note, both the open field and the FST are known to elicit sexually dimorphic behaviors (Gould et al., 2009, Hill et al., 2003; Kokras et al., 2012). However, the different sensitivity of males and females in these tasks does not preclude their utility in elucidating differential effects of our treatments on emotional regulation of males and females, and can enhance our ability to interpret the data. While we could not statistically compare males and females, we did find differential patterns of response to our pre- and postnatal treatments on emotional regulation in males and females.
4.3. PAE and adolescent CMS effects on CRHR1 expression
Clinical and pre-clinical studies indicate that dysregulation of the central CRH system may underlie vulnerability to mental health problems - including depression and anxiety - with CRHR1 playing a critical role (Binder and Nemeroff, 2010; Holsboer and Ising, 2008; Keck et al., 2001; Müller et al., 2003; Smith et al., 1998; Timpl et al., 1998). Importantly, exposure to stress, especially during adolescence, has been shown to result in dysregulation of the central CRH system (Iredale et al., 1996; Li et al., 2015; Veenit et al., 2014), an effect that was observed selectively in females in the current experiment. Our data indicate that in the short-term, adolescent control females exposed to CMS show decreased CRHR1 mRNA expression in the prelimbic and infralimbic cortices and increased CRHR1 mRNA expression in the basolateral amygdala. This CMS-induced reduction in CRHR1 in the prelimbic and infralimbic cortices is in agreement with a previous report indicating CMS-induced decreases in CRHR1 in the frontal cortex of males (Iredale et al., 1996). Moreover, our results indicate that in adulthood, females exposed to adolescent CMS showed a reduction in CRHR1 mRNA expression in the basolateral and medial amygdala. These findings contrast with previous studies showing a long-term increase in amygdala CRHR1 expression in males (Li et al., 2015; Veenit et al., 2014). These differential results may reflect differences in the stress paradigms, the age of stress exposure, sex of the subjects tested, and protocols used to measure CRHR1.
PAE effects on HPA regulation have been extensively investigated over the years in children and animal models (Jacobson et al., 1999; Lee and Rivier, 1996; Nelson et al., 1986; Taylor et al., 1982; Weinberg et al., 2008). The present findings support and extend our previous work (Glavas et al., 2007) indicating that PAE reduces CRHR1 mRNA expression in the pituitary, now demonstrating that PAE alters the central CRH system. Indeed, PAE reduced CRHR1 expression in the medial amygdala of adult males and females, reduced CRHR1 expression in the basolateral amygdala of adult females, and reduced CRHR1 expression in the anterior cingulate and prelimbic cortices of adult males. PAE-induced reduction of CRHR1 expression in the basolateral and medial amygdala in adulthood may be a compensatory response to the increase in CRH mRNA expression in the central amygdala observed in adult PAE animals (Lan et al., 2015). Indeed, while CRH is produced in the central amygdala, the central amygdala contains very few CRHR1 receptors, which are present in abundance in the basolateral and medial amygdala (Van Pett et al., 2000). CRH produced in the central amygdala may be released locally and bind to CRHR1 in the basolateral and medial amygdala, and in the case of PAE, could potentially result in receptor downregulation. Moreover, PAE animals also exhibited a differential response to adolescent CMS, as only adolescent PAE males showed increased CRHR1 in medial amygdala following CMS. These PAE-related effects as well as the unique responses to adolescent CMS in PAE animals indicate that PAE alters the development of the central CRH system, which may, at least in part, underlie the hyperresponsivity to stress observed following PAE (Jacobson et al., 1999; Weinberg et al., 2008).
4.4. Conclusions and implications
Elucidating the mechanisms underlying the increased vulnerability to mental health problems observed in individuals exposed to alcohol during gestation is a critical step towards establishing targeted interventions and/or treatments for affected individuals. The use of animal models has been critical for helping to delineate the specific contributions of PAE and chronic stress exposure to the development of mental health problems; however, the focus has primarily been on adult stress exposure. Here we expand this investigation by examining if exposure to stress during adolescence affects the trajectory of brain development during the sensitive adolescent period, contributing to the increased vulnerability to mental health problems observed within the context of PAE. Overall, our data reveal that PAE and adolescent stress induce unique and dynamic neurobehavioral alterations that are manifested differently in the short- and long-term, depending on the age and sex of the animal. Moreover, this is the first study to investigate the interactive effects of PAE and adolescent stress on emotionality and central HPA activity. That very few interactive effects were observed suggests that adolescent PAE rats may not be more vulnerable than controls to the effects of stress, at least in the paradigm used in the current study. However, adolescence is a dynamic neurodevelopmental period where different types of stress at different stages of adolescent development can result in unique outcomes. As such, more research is needed to evaluate further the specific vulnerability of PAE rats to the effects of stress during adolescence
Supplementary Material
Acknowledgments
This research was supported by NIH/NIAAA grants R37 AA00789 and R01 AA022460, NeuroDevNet (Canadian Networks of Centers of Excellence) grant 20R64153 to JW. We thank all members of the Weinberg laboratory for their assistance, especially Wayne Yu for his help with the radioimmunoassay. We also thank Dr. Wendy Comeau for her assistance with the animal work and for slicing a subset of brains.
Role of the funding source
Funding sources did not contribute to experimental design, collection, analysis or interpretation of the data, or decisions regarding submission.
Footnotes
Conflict of interest
All authors report no conflict of interest.
Contributions
All authors participated in experimental design. CR, LC, PM, and LE collected the data. CR analyzed the data. CR and JW wrote the manuscript. All authors edited the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2016.08.015
References
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescence depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety -Insights from human genetic studies. Mol. Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Beiderman J, Goldsmith HH, Kaufman J, Lewinsohn PM, Hellander M, Hoagwood K, Koretz DS, Nelson CA, Leckman JF. Development and natural history of mood disorders. Biol. Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EL, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol. Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produced corticolimbic dendritic architectural remodelling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental health illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am. J. Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu W, Weinberg J. Effects of prenatal alcohol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol. Clin. Exp. Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE. The open field test. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. New York, NY: Humana Press; 2009. pp. 1–20. [Google Scholar]
- Hellemans KGC, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann. N. Y. Acad. Sci. U. S. A. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KGC, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: foetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci. Biobehav. Rev. 2010a;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KGC, Verma P, Yoon E, Yu W, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like disorders in male and female offspring. Alcohol. Clin. Exp. Res. 2010b;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Brotto LA, Lee TTY, Gorzalka BB. Corticosterone attenuates the antidepressant-like effects elicited by melatonin in the forced swim test in both males and female rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:905–911. doi: 10.1016/S0278-5846(03)00149-0. [DOI] [PubMed] [Google Scholar]
- Hollis F, Isgor C, Kabbaj M. The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience. 2013;249:232–241. doi: 10.1016/j.neuroscience.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety—evidence from clinical studies with CRH1 receptor antagonist. Eur. J. Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Iredale PA, Terwilliger R, Widnell KL, Nestler EJ, Duman R. Differential regulation of corticotropin-releasing factor receptor expression by stress and agonist treatments in brain and cultured cells. Mol. Pharmacol. 1996;50:1103–1110. [PubMed] [Google Scholar]
- Jacobson SW, Bihum JT, Chido LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev. Psychopathol. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Richter-Levin G. Differential impact of juvenile stress and corticosterone in juvenile and in adulthood, in male and female rats. Behav. Brain Res. 2010;214:268–276. doi: 10.1016/j.bbr.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Richter-Levin G. Short- and long-term effects of juvenile stressor exposure on the expression of GABAA receptor subunits in rats. Stress. 2012;15:416–424. doi: 10.3109/10253890.2011.634036. [DOI] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F, Lendgraf R. The anxiolytic effect of the CRH1 receptor antagonist R121919 depends on innate emotionality in rats. Eur. J. Neurosci. 2001;13:373–380. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dalla C, Sideris AC, Dendi A, Mikail HG, Antoniou K, Papadopoulou-Daofiti Z. Behavioral sexual dimorphism in models of anxiety and depression due to changes in the HPA axis activity. Neuropharmacology. 2012;62:436–445. doi: 10.1016/j.neuropharm.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. J. Neuroendocrinol. 2006;18:672–784. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Lan N, Hellemans KGC, Ellis L, Weinberg J. Exposure to chronic mild stress differentially alters corticotropin-releasing hormone and arginine vasopressin mRNA expression in stress-responsive neurocircuitry of male and female rats prenatally exposed to alcohol. Alcohol. Clin. Exp. Res. 2015;39:2414–2421. doi: 10.1111/acer.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. Gender differences in the effects prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis responses to immune signals. Psychoneuroendocrinology. 1996;21:145–155. doi: 10.1016/0306-4530(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Li C, Liu Y, Yin S, Lu C, Liu D, Jiang H, Pan F. Long-term effects of early adolescent stress: dysregulation of hypothalamic-pituitary-adrenal axis and central corticotropin releasing hormone receptor 1 expression in adult male rats. Behav. Brain Res. 2015;288:39–49. doi: 10.1016/j.bbr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Márquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, Magistretti PJ, Trono D, Sandi C. Peripuberty stress leads to abnorma aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MB, Zimmermann S, Sillaber I, Hagemeyer T, Deussing JM, Timpl P, Kromann MSD, Droste SK, Kühn R, Reul JMHM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediated anxiety-related behaviors and hormonal adaptations to stress. Nat. Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Wilton A, Styles A, McCormick CM. Heightened neuroendocrine function in males to a heterotypic stressor and increased depressive behaviour in females after adolescent social stress in rats. Behav. Brain Res. 2008;190:33–40. doi: 10.1016/j.bbr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol. Clin. Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld A, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res. Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: investigation in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav. Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 1986;10:397–402. doi: 10.1111/j.1530-0277.1986.tb05112.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Shah B, Whaley S, Cronin P, Gunderson B, Graham J. Psychiatric illness in clinical sample of children with prenatal alcohol exposure. Am. J. Drug Alcohol Abuse. 2002;28:743–754. doi: 10.1081/ada-120015880. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Denys K, Hughes J, Rasmussen C. Mental health issues in fetal alcohol spectrum disorders. J. Ment. Health. 2011;220:473–483. doi: 10.3109/09638237.2011.577113. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning a functional expression of a rat brain corticotropin factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- Raineki C, Hellemans KGC, Bodnar T, Lavigne KM, Ellis L, Woodward TS, Weinberg J. Neurocircuitry underlying stress and emotional regulation in animals prenatally exposed to alcohol and subjected to chronic mild stress in adulthood. Front. Endocrinol. 2014;5:5. doi: 10.3389/fendo.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall C, Becker H, Middaugh L. Effects of prenatal ethanol exposure on activity and shuttle avoidance behavior in adult C57 mice. Alcohol Drug Res. 1986;6:351–360. [PubMed] [Google Scholar]
- Riley EP. The long-term behavioral effects of prenatal alcohol exposure in rats. Alcohol. Clin. Exp. Res. 1990;14:670–673. doi: 10.1111/j.1530-0277.1990.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Markert LE, Delrizzo M. Effects of prenatal ethanol exposure on ethanol-induced activity in rats. Alcohol. 1989;6:353–356. doi: 10.1016/0741-8329(89)90003-7. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Stress and the adolescent brain. Ann. N. Y. Acad. Sci. 2004;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsores IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Sandi C, Cordero MI, Ugolini A, Varea E, Caberlotto L, Large CH. Chronic stress-induced alterations in amygdala responsiveness and behavior—modulation by trait anxiety and corticotropin-releasing factor system. Eur. J. Neurosci. 2008;28:1836–1848. doi: 10.1111/j.1460-9568.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Organization of CRF immunoreactive cells and fibers in the rat brain: immunohistochemical studies. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing Factor: Basic and Clinical Studies of a Neuropeptide. Boca Raton, FL: CRC Press; 1989. pp. 29–51. [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gould LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioural manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC, Sphor HL. Long-term outcome of children with fetal alcohol syndrome: psychopathology, behavior, and intelligence. Alcohol. Clin. Exp. Res. 1998;22:334–338. doi: 10.1111/j.1530-0277.1998.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Kokka N. Long-term effects of fetal ethanol exposure on pituitary-adrenal response to stress. Pharmacol. Biochem. Behav. 1982;16:585–589. doi: 10.1016/0091-3057(82)90420-8. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JMHM, Stalla GK, Blanquer V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat. Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez A, Sandi C. Stress during adolescence increases novelty seeking and risk-taking behaviors in male and female rats. Front. Behav. Neurosci. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J. Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Guterman A, Richter-Levin G. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: potential relevance for mood and anxiety disorders. Neuropsychopharmacology. 2008;33:378–393. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- Uban KA, Comeau WL, Ellis LA, Galea LAM, Weinberg J. Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology. 2013;38:1953–1966. doi: 10.1016/j.psyneuen.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Veenit V, Riccio O, Sandi C. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. J Psychiat Res. 2014;53:1–7. doi: 10.1016/j.jpsychires.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KGC. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J. Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Raineki C, Weinberg J, Galea LAM. Alcohol and pregnancy: effects in maternal care, HPA axis function, and hippocampal neurogenesis in adult females. Psychoneuroendocrinology. 2015;57:37–50. doi: 10.1016/j.psyneuen.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Wick-Carlson S, Packard BA, Morano R, Herman JP. Adolescent chronic stress causes hypothalamic-pituitary-adrenal hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology. 2016;65:109–117. doi: 10.1016/j.psyneuen.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.