Abstract
Studies in a variety of species have reported enhanced prosocial effects after an acute administration of the neuromodulating hormone, oxytocin (OT). Although the exact mechanisms underlying these effects are not fully understood, there is broad interest in developing OT into a treatment for social deficits. Only a few studies, however, have examined the effects of OT if given repeatedly during early development, the period when early intervention is likely to have the greatest benefits for reversing the progression towards social impairment. Those studies, exclusively in rodents, report mixed results. Some have shown enhancement of prosocial behavior, including increased social exploration, but others have shown anti-social effects, including increased aggression. In the present study, infant rhesus macaques were treated with a high-frequency (3x per week) or low-frequency (1x per week) dose of intranasal oxytocin (IN-OT) or placebo (IN-saline) between two and six months of age, after which their reactions to dynamic facial expressions (neutral, lipsmacking and threats) were measured. Results showed that IN-OT, compared to placebo, increased the time monkeys spent viewing the expression videos, but selectively reduced attention to the eyes in neutral faces in a dose dependent manner. The mechanism for this non-prosocial effect may be that repeated IN-OT administration down-regulates the expression of OT receptors in brain regions important for regulating social attention. Consequently, our results raise questions about the efficacy of implementing chronic IN-OT as a pharmacotherapy for the treatment of social deficits, particularly if given early in development. More work is needed, not only to identify optimal treatment schedules, but also to understand how IN-OT exerts its influences on the brain and behavior.
Keywords: Development, oxytocin, facial expression, infant macaque, gaze, eye-tracking
1. INTRODUCTION
Numerous studies in humans and nonhuman primates have reported that an acute dose of intranasal oxytocin (IN-OT) improves various aspects of social cognition and prosocial behavior, including interpreting emotion from faces, enhancing trust, increasing social memory, enhancing social reward, and modulating social attention (Chang et al., 2012; Ebitz et al., 2013; Guastella & MacLeod, 2012; Parr et al., 2013; Parr, 2014; Shamay-Tsoory & Abu-Akel, 2016). This has led to great excitement over the past decade that IN-OT may be an effective pharmacotherapy for enhancing prosocial functions in individuals with disorders characterized by social impairments, like autism spectrum disorders (ASD). However, there is still much to be learned about exactly how IN-OT is influencing behavior and where in the brain it might be acting (Churchland & Winkielman, 2012). In addition to these concerns, the majority of studies have measured the behavioral effects of OT after a single, acute administration in adults (mostly male). Understanding the impact of OT if given repeatedly during early development is of particular importance because many social disorders in humans, like ASD, are developmental in nature. Thus, the greatest potential to reverse or suspend the progression towards social impairment would be associated with early, repeated intervention.
Research has shown that the oxytocinergic system is functional early in development and has important organizing effects on the brain and behavior (Hammock, 2015; Miller & Caldwell, 2015). Early manipulation of the OT system, either through perturbations of early-life experience (for reviews, Alves et al., 2015; Veenema, 2012) or exogenous administration can, therefore, have long-lasting effects on social behavior and brain function (Bales & Perkeybile, 2012; Hammock, 2015). Several studies have now begun to explore the effects of chronic OT administration early in development and the results are often contradictory to the broad prosocial functions described in acute studies. Bales (Bales et al., 2013) gave IN-OT to male and female prairie voles (21 days of age) and measured its effects on social interaction into adulthood (42 days of age). Males that received a low (0.08 IU/kg) or medium dose (0.80 IU/kg) of OT were less likely to form their species-typical partner bonds, whereas no differences were found in those receiving a high OT dose (8.00 IU/kg) (Bales et al., 2013). In captive pigs, IN-OT (50 ug) or placebo was given over the first three days of life and social responsiveness was tested post-weaning (17 days of age). The pigs given IN-OT showed more aggression towards peers and were less interested in social interactions compared to placebo subjects (Rault et al., 2013). Finally, adult male mice received two doses of IN-OT (0.15 IU and 0.30 IU) or placebo per day for 7–21 days. Individuals given IN-OT, regardless of the dose, showed a reduction in social investigation, in addition to a reduction in oxytocin receptor (OXTR) expression throughout the brain (Huang et al., 2014). In contrast, an acute administration of IN-OT in the same species increased social behavior directed towards a novel female, but not a novel male. These studies suggest that chronic overstimulation of the oxytocinergic system in healthy animals, particularly early in development, may have unwanted and detrimental effects on social behavior.
The rhesus monkey is an excellent species in which to study the effects of chronic OT on the development of social behavior. Rhesus monkeys live in large, highly social groups in which mothers form strong, protracted bonds with their infants. They have large brains that are highly homologous with humans and individuals display advanced social cognitive skills, including sensitivity to a diverse range of facial expressions (Hinde & Rowell, 1962). Moreover, monkeys develop much faster than humans (often reported as 4x as fast, Boothe et al., 1982), providing an opportunity for longitudinal behavioral studies on a much faster time scale than is possible in humans. Although there are a growing number of studies on the effects of acute IN-OT in adult monkeys (Chang et al., 2012; Dal Monte et al., 2014a; Ebitz et al., 2013; Landman et al., 2014; Parr et al., 2013; Parr, 2014), only one study to date has examined the effects of acute IN-OT administration in infant monkeys. Simpson and colleagues (2014) gave a 25IU dose of IN-OT to 28 infant rhesus monkeys between 7 and 14 days of age. They found that IN-OT increased the facial gestures made by the infant monkeys in response to the same behavior displayed by an experimenter, e,g., facial mimicry. While these results suggest that IN-OT enhances prosocial behavior in infant monkeys, it should be noted that these monkeys were removed from their mothers at birth and nursery reared, and studies show that manipulation of the mother-infant relationship can have adverse consequences on the development of the OT system in a variety of species (e.g., see Hammock, 2015; Veenema, 2012).
The present study is the first to report on the effects of chronic IN-OT administration in 24 mother-reared infant rhesus macaques treated three times per week with either a high-frequency or low-frequency dose of IN-OT, or IN-saline between 2 and 6 months of age. Infants’ viewing behavior was then measured at 6 months of age in response to videos of conspecific facial expressions. If chronic IN-OT enhances prosocial behavior in infant monkeys, we expect that IN-OT will increase the time monkeys spend looking at the eyes in all facial expressions, consistent with previous findings (Dal Monte et al., 2014a; Guastella et al., 2008; Ebitz et al., 2013), and this will be greater when the eyes and heads are directed back at the viewer, rather than averted. Moreover, we hypothesize that IN-OT will reduce the aversive quality of the threat expressions (Parr et al., 2013), resulting in longer viewing times for these expressions after IN-OT treatment compared to placebo. We expect these effects to occur in a dose dependent manner.
2. METHODS
2.1 Subjects
Twenty-four, male infant rhesus macaques (Macaca mulatta) served as the subjects for this study. The study focused on males due to the increased prevalence of developmental social impairments, e.g., autism, in males compared to females. All infants were healthy, full-term, >450 g, offspring born into large social groups (~50–100 individuals) at the Yerkes National Primate Center field station (Lawrenceville, GA). Mothers consisted of both primiparous (N=3) and multiparous (N=21) females of all ranks. The infants were mother-reared and remained living in their social groups during the course of this study.
2.2 Treatment Groups and Dosing
The infants were assigned to one of three treatment groups at birth (placebo, high-frequency OT and low-frequency OT), balancing the group assignments for their mother’s rank, e.g., high (alpha or beta family), middle, or low (bottom two families). Due to the limited number of infant male subjects available for this study, maternal parity was unable to be balanced across treatment group. Subjects were dosed three times per week, where the placebo group received 3 doses of saline, the high-frequency OT group received 3 doses of OT, and the low-frequency group received 1 dose of OT and 2 doses of saline. Each treatment group was color coded and the weekly doses prepared in three separate vials, labelled 1–3, to be given each week. In this way, research staff remained blind as to which vial contained placebo and which contained OT. Each OT vial contained 0.12 ml of concentrated OT (Oxytocin acetate salt, Sigma-Aldrich, 0.821 mg/ml), while each placebo vial contained 0.12 ml of saline and these were stored at -80C until the day of use. All vials were prepared by individuals not involved in either administering the doses to subjects, or the behavioral testing. Prior to use, research staff blind to both treatment condition and the contents of each vial thawed and then diluted each vial with 4ml of sterile saline, so that it could be administered in aerosol form using a pediatric nebulizer. Subjects were nebulized for four minutes, which aerosolizes 2ml of fluid keeping 2ml in reserve to insure a steady stream of aerosol. Thus, each dose delivered approximately 0.049 mg of OT, equivalent to 24IU (1.71 ug/IU). These procedures have been shown to successfully deliver IN-OT to the central and peripheral nervous systems in both monkeys and humans (Chang et al., 2012; Dal Monte et al., 2014b; Freeman et al., 2016; Modi et al. 2014; Striepens et al., 2013).
Dosing began when the infants were two months of age. Using established protocols, researchers entered the social group and isolated the mother-infant pair, moving them to the indoor enclosure (e.g., see Muschinski et al., 2016). The pair was then boxed and moved to a smaller testing cage where the infant was removed from its mother’s ventrum. While one experimenter gently restrained the infant, another placed a mask connected to the nebulizer over the infant’s face so as not to obstruct breathing (see Figure 1). With the mask in place, the nebulizer was turned on and the infant passively breathed the aerosolized dose for four cumulative minutes (Modi et al., 2014). These procedures were used until the infants were 16 weeks of age, when it became more difficult to manually restrain them. After this time, all infants were dosed in a custom-made ‘dosing box’ that contained a clear front panel and several port openings for the nebulizers (see Figure 1). Once inside the dosing box, the research staff connected two nebulizers and delivered aerosol to the subjects for 2.5 minutes, quickly filling the box with aerosol, after which one nebulizer was turned off and the remaining one continued to deliver aerosol for another 2.5 minutes. All dosing was done either after behavioral testing, or on a different day. All of the results reported here involve chronic effects, not acute responses to dosing. All procedures were approved by the Institutional Animal Care and Use Committee of Emory University.
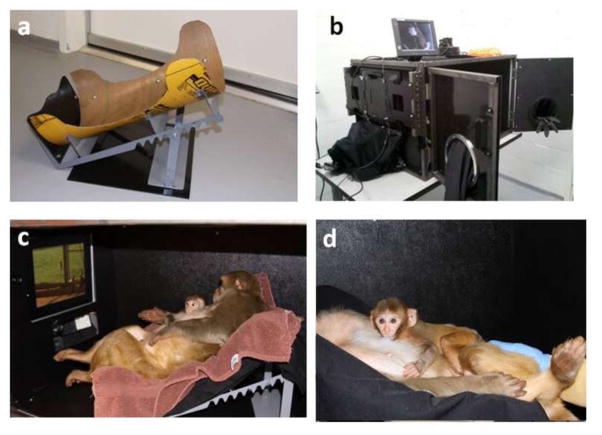
Figure 1.
Methods used for administering IN-OT to monkeys under 16 weeks of age (left image) and over 16 weeks of age using a custom designed dosing box. The middle image shows the older infant inside the dosing box, while the image on the right shows the rear of the dosing box where the nebulizers are connected.
2.3 Eye-tracking Procedure
The infants were tested at 25 weeks of age (mean 176 days) using an eye-tracking procedure1 that has been described in detail elsewhere (see Muschinski et al., 2016; Parr et al., 2016, see Supplementary Data section). In brief, the mother and infant were removed from their social group and moved to the testing room. The mother was then lightly anesthetized and the pair placed in a custom testing chamber containing an ISCAN (60 Hz) eye-tracking camera and monitor (see Figure 2). Once settled inside the testing chamber, the infant’s direction of gaze was calibrated using a 5-point system. The presentation of experimental stimuli (see Section 2.4) began immediately after the calibration routine and lasted no longer than 30 minutes to limit the mother’s anesthesia. After testing, the mother-infant pair was returned to the housing cage in the adjacent room and only when the mother was fully alert was the pair returned to their social group.
Figure 2.
Custom designed eye-tracking chamber. Figure 2a shows the chair where the anesthetized mother was positioned. Figure 2b shows an overview of the exterior of the testing chamber and controls. Figure 2c shows the infant lying quietly on their mom and the stimulus presentation monitor. Figure 2d shows the infant lying quietly on the mom while watching the stimulus presentation monitor.
2.4 Facial Expression Videos
Stimuli consisted of nine different 10 second videos depicting three unfamiliar conspecifics, each displaying three species-typical facial expressions, neutral, open mouth threats, and lipsmacks. Neutral videos showed a monkey looking passively around their enclosure. Open mouth threat videos showed a monkey making a series of open mouth threats which are highly ritualized signals used during aggressive and agonistic interactions. The lipsmack videos showed a monkey engaged in lipsmacking behavior, an affiliative and appeasing facial expression commonly shown by rhesus monkeys after fights, during approach, and in reciprocal face-to-face interactions with infants. Numerous studies have utilized these videos in other experiments (Gothard et al., 2007; Mosher et al., 2011; Putnam et al., 2016). Videos were presented in a pseudo-random order, in which trials were repeated if subjects were sleeping, moving, or not attentive. Therefore, each subject saw a different number and order of videos depending on their level of attention during the session. Figure S1 shows a representative screen shot from each category of video (see Supplementary Materials section).
2.5 Behavioral Ethogram and Analysis
All testing sessions were video recorded so that the behavioral reactivity of the subjects could be scored and analyzed in addition to the eye-tracking data. An ethogram was constructed that quantified the subject’s location in the testing chamber, their posture and alertness, facial expressions, and attention to the screen or the exterior of the testing box. The complete ethogram of behaviors and their definitions can be seen in the Supplementary Data section (Table S1). Once quantified, the proportion of session time that subjects engaged in the different categories of behavior was analyzed using multivariate ANOVAs (IBM SPSS, v 22) where treatment condition (high-frequency OT, low-frequency OT, and placebo) was a fixed factor. Prior to the analysis, research staff not involved in the coding of the behaviors or the analysis of the data blinded the scoring sheets, masking the subject and treatment group identifiers. All behavioral coding and analyses were done by individuals blind to the treatment conditions.
2.6 Eye-Tracking Data Analysis
Fixation data were extracted for analysis using GazeTracker software and custom MATLAB scripts. These procedures have been described in detail elsewhere (Muschinski et al., 2016). Specific regions of interest, called LookZones, were drawn by hand on each video frame (30 frames per second) using a polygon tracing tool in GazeTracker software (http://www.eyetellect.com/gazetracker/). Four regions were drawn corresponding to whether the head or eyes of the movie monkey were direct (oriented back to the camera) or averted. In addition, a separate LookZone was drawn around the entire video frame, referred to as the Screen LookZone. Fixations were extracted from these LookZones in a hierarchical manner, so that fixations on the eyes did not overlap with fixations on the rest of the head, and neither eye nor head fixations were included in Screen. Therefore, the videos were analyzed based on the occurrence of fixations within five, mutually exclusive LookZones; Direct Eyes, Averted Eyes, Direct Heads, Averted Heads, and Screen.
There were three dependent variables. Total Looking Duration was the total time subjects spent looking at a trial (max of 10 seconds) regardless of where the fixations occurred. The Proportion of Total Looking Duration was the proportion of time subjects spent looking at a particular LookZone divided by the Total Looking Duration, and Fixation Duration was the mean duration of each individual fixation. The data were analyzed using mixed linear modelling, where the LookZone orientation (direct versus averted), expression type (neutral, lipsmack and threat), and treatment condition were fixed factors. The data were modelled using an unstructured covariance matrix and subject was treated as a random factor using a random slope and intercept model (Field, 2013). The significance of each model was evaluated from tests of fixed effects, which provide an F-test and associated degrees of freedom. A chi-square likelihood ratio test was performed to identify the model that best fit the relationship between the variables. For this analysis, chi-square values were derived from the change in the log-likelihood ratios generated for each successive model level, e.g., simple main effects, 2-way and 3-way interactions. The deviance in the log-likelihood ratios (−2LL) has a chi-square distribution. The largest chi-square value for the change in degrees of freedom associated with each level of the model could then be identified (two-tailed). Both the F-test and the chi-squared values for the significant models are reported here. Follow-up analyses were performed using independent samples t-tests, adjusted for multiple comparisons using Bonferroni’s correction procedure, e.g., 0.05 / # comparisons. Effect sizes for follow-up analyses are reported as Cohen’s d.
3. RESULTS
3.1 Behavioral Analyses
Behavioral data were collected from all 24 subjects, regardless of whether they contributed to the eye tracking analysis. The mean duration of each testing session was 816.04 seconds (SEM = 52.65) and there were no significant differences between the length of the sessions for the different treatment groups, F(2,23) = 1.13, p= 0.34. There were also no significant differences in the behavioral reactivity of the monkeys during the testing sessions according to treatment group. More detail on the results of the behavioral analyses can be found in the Supplementary Materials section.
3.2 Eye-tracking Analyses
Prior to analysis, the data set was modified to remove any trial in which the total viewing time was less than 1 second (Muschinski et al., 2016). This resulted in the removal of 30 trials, totaling 160 fixations. The resulting analyses were performed on a data set containing 196 trials and 4,090 total fixations. Also, data were unable to be collected from five subjects, either because they failed to be calibrated or they slept during the session. Therefore, the eye-tracking data were analyzed from the remaining 19 subjects (7 placebo group, 6 high-frequency OT group, and 6 low-frequency OT group). Table S3 in the Supplementary Materials section lists the total number of video from each expression category that each subject contributed to the data analysis.
3.2.1 Chi-Square Analysis of Videos Viewed by Group
To ensure that the results reported below were not due to differences in the number and types of expression videos viewed by subjects in each treatment group, a chi-squared analysis was performed. Table 1 shows the total number of each expression video type that was viewed by subjects in each treatment group. Note that this number reflects the total per treatment group and not the total per individual within each treatment group. Overall, the chi-square analysis revealed no significant difference in the number of expression videos seen by subjects in each treatment group, X2(8) = 2.34, p= 0.67.
Table 1.
Total number of videos in each expression category that were viewed by the subjects in each treatment group. A Chi-square analysis showed no difference in the number of expression videos analyzed for each treatment group, X2(8) = 2.34, p= 0.67.
| Treatment Group | |||
|---|---|---|---|
| Expression Type | Placebo | High-freq OT | Low-freq OT |
| Neutral | 17 | 20 | 27 |
| Lipsmack | 20 | 25 | 21 |
| Threat | 22 | 19 | 25 |
3.2.2 Total Looking Duration
Analyses of the Total Looking Duration revealed significance for each level of the model: treatment group, F(2,17.82) = 5.57, p= 0.013; expression type, F(2,4080.51) = 5.18, p= 0.006, and treatment group x expression, F(4,4081.23) = 82.85, p< 0.001. Overall, subjects looked longest at the lipsmack expressions, followed by the threats, and finally the neutral videos. The longest looking was done by the low-frequency OT group, followed by the high-frequency OT group, and then the placebo group (see Supplementary Data for these analyses).
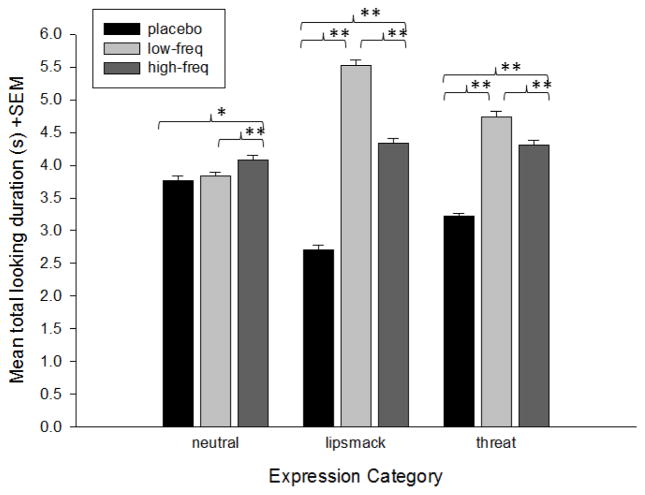
The change in the -2LL values revealed that the most significant result was the treatment group x expression interaction, X2(4) = 318.65, p< 0.0001. Follow-up analyses showed that for the neutral expressions, the high-frequency OT group looked significantly longer than the low-frequency, t(945) = 2.57, p= 0.01, Cohen’s d = 0.17, and placebo groups, t(821) = 3.29, p= 0.001, Cohen’s d = 0.23. The placebo and low-frequency OT groups did not differ. For the lipsmack and threat expressions, all treatment groups differed with the longest viewing times shown by the low-frequency OT group, followed by the high-frequency OT group, and then the placebo group; lipsmacks; placebo vs high-frequency, t(847) = 14.55, p< 0.001, Cohen’s d = 1.0; placebo vs low-frequency, t(783) = 25.97, p< 0.001, Cohen’s d = 1.86; and high-frequency vs low-frequency, t(986) = 10.97, p< 0.001, Cohen’s d = 0.70, and threats; placebo vs high-frequency, t(934) = 12.61, p< 0.001, Cohen’s d = 0.83; placebo vs low-frequency, t(976) = 16.28, p< 0.001, Cohen’s d = 1.04, and high-frequency vs low-frequency, t(990) = 4.10, p< 0.001, Cohen’s d = 0.26. Figure 3 shows the mean Total Looking Duration at each expression type by the three treatment groups.
Figure 3.
Mean total looking duration (+SEM) by monkeys in each treatment condition for each of the facial expression video categories. *p< 0.01; **p< 0.001
3.2.3 Proportion of Total Looking Duration
Initial inspection of the histograms and q-q plots of the residuals for the Proportion of Total Looking Duration revealed concerns about homoscedasticity and normality, e.g., Head LookZone, skew = 1.63; Eye LookZone, skew = 2.91. These issues were addressed by log-transforming the data. Analyses of the log-transformed data for the eyes LookZone revealed a significant main effect of expression type, F(2,182) = 34.03, p< 0.001. The interactions between treatment group x expression type, F(4,182) = 2.52, p= 0.043, and expression type x LookZone orientation were also significant, F(2,182) = 3.91, p= 0.022. The change in the −2LL values revealed that the main effect of expression type was the most significant effect, X2(2) = 57.82, p< 0.0001. Subjects looked longer at the eyes of neutral faces, followed by lipsmacks, and then threats (see Supplementary Material section). Both interaction terms also significantly improved the model, treatment group x expression type interaction, X2(4) = 9.80, p< 0.05, and expression type x LookZone orientation, X2(2) = 7.66, p< 0.03. None of the other main effects or interactions reached significance. All p-values > 0.05.
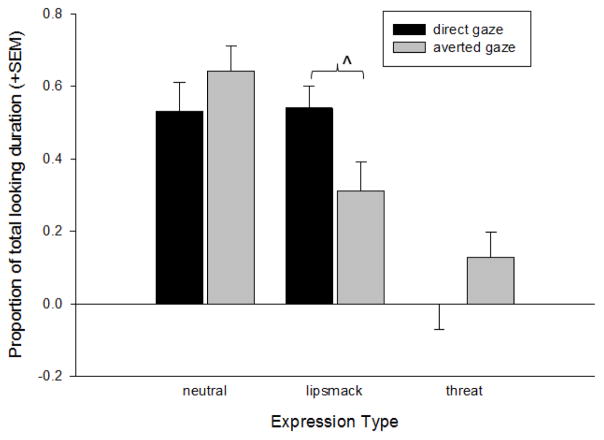
Follow-up analyses for the significant treatment group x expression type interaction revealed that for the neutral expressions, the high-frequency OT group looked at the eyes significantly less than the placebo group, t(36) = 3.81, p= 0.001, Cohen’s d = 1.27. There were no significant differences in the Proportion of Total Looking Duration between the treatment groups for the lipsmack or threat expressions. Figure 4 shows the mean Proportion of Total Looking Duration by each treatment group for each expression type. Follow-up analyses for the significant expression type and LookZone orientation interaction revealed that subjects looked at the direct eyes of the lipsmack expressions significantly longer than the averted eyes, t(58) = 2.24, p= 0.029, Cohen’s d = 0.59. There were no significant differences in the Proportion of Total Looking Duration at direct or averted eyes for either the neutral faces or the threats. Figure 5 shows the mean Proportion of Total Looking Duration at direct and averted eyes for each expression type.
Figure 4.
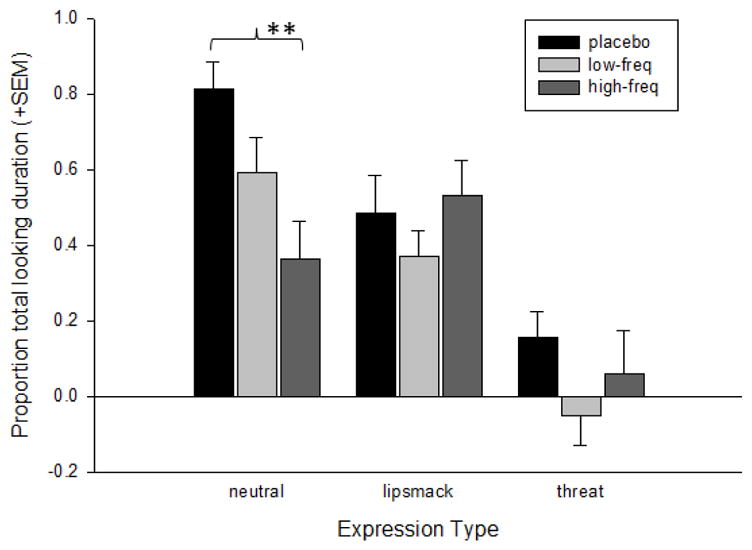
The mean log-transformed (+SEM) proportion of total looking duration directed at the eyes LookZones by monkeys in each treatment condition for each of the facial expression video categories. **p< 0.001
Figure 5.
The mean log-transformed (+SEM) proportion of total looking duration directed at either the direct or averted eyes LookZones for each of the facial expression video categories. ^p< 0.05
For the Proportion of Total Looking Duration at the head LookZone, the effects of expression type, F(2,347) = 33.88, p< 0.001, LookZone orientation, F(1,347) = 4.95, p= 0.027, and expression type x LookZone orientation interactions, F(2,339.09) = 24.77, p= 0.001, were significant. The change in the -2LL values revealed that the largest improvement in the model came from the main effect of expression type, X2(2) = 61.88, p< 0.0001. Subjects look significantly longer at neutral heads, followed by lipsmacks and then threats (see Supplementary Material section). However, the interaction also significantly improved the model, X2(2) = 46.16, p< 0.0001. Follow-up comparisons revealed that subjects spent significantly longer looking at averted heads in neutral expressions, t(107) = 5.91, p< 0.001, Cohen’s d = 1.14, but direct heads in lipsmacking expressions, t(112) = 4.19, p< 0.001, 0.79. There was no difference in the Proportion of Total Looking Duration at the direct or averted heads in the threat faces.
3.2.4 Fixation Duration
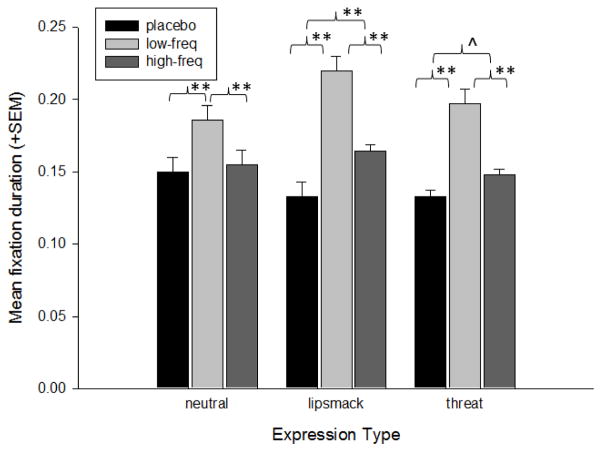
The analysis of Fixation Duration revealed a significant main effect of treatment group, F(2,18.68) = 3.86, p= 0.04, and a significant interaction between expression type and treatment group, F(4,4088.25) = 4.16, p= 0.002. The change in the −2LL values revealed that the only significant improvement in the model came from the significant interaction between expression type and treatment group, X2(4) = 16.61, p< 0.005. Follow-up analyses revealed that for neutral expressions, significantly longer fixations were made by the low-frequency OT group compared to the placebo, t(880) = 3.87, p< 0.001, Cohen’s d = 0.26, and high-frequency OT group, t(945) = 3.5, p< 0.001, Cohen’s d = 0.23. For the lipsmack and threat expressions, there were significant differences between all of the treatment groups. The low-frequency OT group had the longest fixations, followed by the high-frequency OT group and then placebo; lipsmacks; low-frequency OT group vs placebo, t(783) = 8.62, p< 0.001, Cohen’s d = 0.62, and high-frequency OT group, t(986) = 6.50, p< 0.001, Cohen’s d = 0.41, and high-frequency OT group vs placebo, t(847) = 4.25, p< 0.001, Cohen’s d = 0.29; threats; low-frequency OT group vs placebo, t(976) = 8.40, p< 0.001, Cohen’s d = 0.54, and high-frequency OT group, t(990) = 6.42, p< 0.001, Cohen’s d = 0.41, and high-frequency OT group vs placebo, t(934) = 2.49, p=0.013, Cohen’s d = 0.16. Figure 6 shows the mean fixation durations for each treatment group for each expression type.
Figure 6.
Mean fixation duration (+SEM) by monkeys in each treatment condition for each of the facial expression video categories. ^p< 0.05; **p< 0.001
A separate analysis examined the effects of treatment condition on the duration of fixations made to each of the three main LookZones, the head, eyes and screen. This analysis revealed a main effect of treatment condition, F(2,18.68) = 3.86, p< 0.05. All treatment groups were significantly different from each other and followed the same pattern described above. Fixation duration was longest for the low-frequency OT group (mean = 0.201, SEM = 0.004), followed by the high-frequency OT group (mean = 0.156, SEM = 0.003), and then the placebo group (mean = 0.138, SEM = 0.003). There was also a main effect of the LookZone type, F(2,4083.83) = 4.81, p< 0.01, where fixation duration was longest for the eyes (mean = 0.178, SEM = 0.007), followed by the head (mean = 0.167, SEM = 0.003), and finally the screen (mean = 0.164, SEM = 0.003). However, none of the follow-up comparisons reached statistical significance; head vs eye, t(2466) = 1.52, p= 0.13; head vs screen, t(3686) = 0.67, p= 0.50; and eye vs screen, t(2022) = 1.91, p= 0.057. The interaction between treatment condition and LookZone type did not reach significance, F(4,4083.9) = 1.95, p= 0.10.
4. DISCUSSION
This study revealed several interesting findings resulting from chronic IN-OT treatment in infant monkeys. First, IN-OT increased the overall time monkeys spent watching the videos, particularly the expression videos. This increased viewing was not, however, dose-dependent. Monkeys in the low-frequency group spent significantly longer viewing the lipsmack and threat videos, followed by the high-frequency group, and then the placebo group. In contrast, monkeys in the high-frequency OT group spent significantly longer watching the neutral videos compared to either the low-frequency or placebo groups. Therefore, IN-OT specifically increased the time monkeys spent watching expression videos, whereas monkeys in the placebo group spent more time viewing the neutral expressions. This is consistent with previous findings that IN-OT may function to reduce social vigilance in rhesus monkeys, thus releasing any inhibition in their social attention and enabling them to view unfamiliar individuals engaged in expressive behavior, regardless of valence (Ebitz et al., 2013; Parr et al., 2013).
Second, the monkeys also spent a greater proportion of time looking at the eyes of neutral faces, followed by lipsmacks and then threats, and treatment condition significantly influenced this looking pattern. Specifically, IN-OT administration significantly reduced the proportion of time subjects spent looking at the eyes in neutral faces, with the high-frequency OT group looking significantly less than the placebo group. There were no differences among the treatment groups for the proportion of time spent looking at the eyes in either the lipsmack or threat videos. When viewing the lipsmack expressions, all subjects spent a significantly greater proportion of time looking when the eyes were oriented directly back at the viewer, compared to averted. In contrast, when looking at neutral faces the subjects preferred to look when the heads were averted.
Many studies have shown that the eyes are one of the most salient features in primate faces (Gothard et al., 2004; Guo et al., 2003; Leonard et al., 2012; Mosher et al., 2011; Nahm et al. 1997). As early as 4 weeks of age, infant monkeys engage in bouts of spontaneous mutual gaze with their mothers, including reciprocal lip-smacking (Ferrari et al., 2009), and Muschinski and colleagues (2016) have recently shown that infant monkeys’ preference for direct compared to averted gaze faces increases developmentally over the first four months of life. Additionally, greater attention to the eyes in faces after acute administration of IN-OT has been one of the most well replicated findings in the OT literature in both human and non-human primates (Ebitz et al., 2013; Guastella et al., 2008; Dal Monte et al., 2014a). These studies suggest that IN-OT enhances the salience of the eye region, leading to a greater proportion of looking, in addition to enhanced emotion recognition from the eye region alone (Domes et al., 2007a). One study has even shown that acute IN-OT administration can improve attention to the eyes in autistic subjects (Andari et al., 2010). Therefore, the data overwhelmingly suggest an enhancing effect of IN-OT for attention to the eye region and processing relevant social information from the eyes. Despite these findings, the present study found that chronic administration of IN-OT between two and six months of life reduced infant monkeys’ visual preference for the eyes of neutral faces in a dose dependent manner. This reduction of time spent looking at the eyes could not be explained by general decreased interest in the videos themselves, as IN-OT increased the time monkeys spent viewing the expression videos and specifically, monkeys in the high-frequency OT group spent the longest amount of time looking at the neutral compared to expressive faces. Moreover, both the low-frequency and high-frequency OT groups had longer fixations to the videos than the placebo group. The results are also not explained by general differences in the behavioral reactivity of subjects in the different treatment groups in response to the videos as there were no treatment group differences for any behavioral category. It should be noted that the studies mentioned above utilized different analysis strategies to assess time spent looking at specific facial features. Specifically, Ebitz and colleagues (2013) reported that OT increased fixation duration to the eye region in faces. In their study, they adjusted fixation duration to the eyes by dividing by the fixation duration to the mouth. Although we could not replicate an effect of OT on fixation duration to specific LookZones, we did find that fixation duration was longest for the low-frequency treatment group, and when fixations occurred on the eyes, compared to the head or screen, although this latter finding failed to reach significance.
There are two possible explanations for the present findings. First, compared to the placebo condition, IN-OT may reduce the salience of the eye region, thus reducing monkeys’ attention to this preferred facial feature. Although this is in contrast to previous findings in adults showing increased attention to the eyes after acute IN-OT administration (however see Lischke et al., 2012), the studies reviewed above suggest that chronic, repeated administration of OT can produce non-prosocial effects, including decreased interest in social interactions (see Section 1). Alternatively, although our results did not support a role for OT in regulating attention to eyes based on gaze orientation, previous studies have suggested that attention to averted eyes may be extremely arousing for monkeys (Hoffman et al., 2007). If the eyes are averted, the viewer monkey does not know what the focus of the stimulus monkey’s attention is which creates uncertainty and increased arousal. Second, it’s plausible that a high-frequency dose of IN-OT may increase the salience of the eyes to the point that attending to this feature becomes overly aversive resulting in the monkeys looking less. Regardless of what the underlying explanation may be, the current data appear to indicate that chronic administration of IN-OT in infant monkeys has the undesired effect of reducing attention to an important facial feature in a manner that may create a social impairment in healthy individuals (Lefevre & Sirigu, 2016).
Both of the explanations reported above involve OT altering the salience of the eyes and, thus, affecting attention to the eye region. These explanations are consistent with what is currently known about the function of brain regions that contain OXTR in rhesus monkeys, although OT can also bind to vasopressin receptors, so any functional consequence of IN-OT administration may not be restricted to the location of OXTR in the brain. Freeman and colleagues (2014) reported binding of OXTR in brain regions involved in visual processing, shifting gaze direction, and the allocation of attention to visual stimuli. In particular, OXTR binding was found in the superior colliculus and pulvinar, regions involved in attention to faces, gaze direction, and the salience of visual stimuli; the oculomotor and pedunculopontine tegmental nucleus, two brainstem motor nuclei involved in controlling movements of the eye; and the nucleus basalis of Meynert (NBM). This latter region is particularly interesting because it is involved in regulating attention to visual stimuli and it is the only region that contains selective OXTR in all primate species studied thus far (Freeman & Young, 2016). Additionally, the NBM is the primary source of cholinergic input to the basolateral amygdala which contains cells that are, not only selective for faces and facial expressions (Gothard et al., 2007), but also for eye contact (Mosher et al., 2014). It may be that a chronic, as opposed to acute, administration of IN-OT, functions to down-regulate OXTR in the NBM, reducing input to the amygdala. When monkeys are confronted with important social stimuli, like faces, the reduced OXTR binding in the NBM results in decreased attentional orienting towards the eyes and impaired amygdala activity. This may affect the monkey’s ability to shift its visual attention in response to changing social and environmental conditions (Freeman & Young, 2016). Ten days of continuous OT infusion into the rat brain, for example, led to widespread down-regulation of OXTR that persisted for at least 24 hours post-delivery (Insel et al., 1992). These results are also consistent with findings from human neuroimaging studies showing that acute IN-OT reduces amygdala activation in response to aversive imagery (Domes et al., 2007b; Kirsch et al., 2005). However, other studies that report positive effects of chronic OT on prosocial behavior in adolescent and adult rats, including reduced aggression and enhanced prosocial exploration, suggest that exogenous OT can upregulate the endogenous OT system (Bowen et al., 2011; Calcagnoli et al., 2014; 2015). Because our finding of reduced attention to the eyes of neutral faces was found only in the high-frequency OT group, it is likely that both chronic exposure and dose played a factor in these findings.
The reduced attention to the eyes of neutral faces, but not facial expressions, after IN-OT treatment may be related to several factors. First, consistent with previous reports in adult monkeys using the identical stimuli, our infants showed the greatest attention to the eyes of neutral faces, followed by lipsmacks and threats (Mosher et al., 2011). Moreover, studies have shown that feature salience varies depending on the quality of the expression type (Mosher et al., 2011). Because IN-OT appears to influence the salience of eyes, it follows that the effect of IN-OT treatment would be greatest for the category of expression in which the eyes were the most salient. Additionally, studies reporting the effects of IN-OT on processing facial expressions in humans are mixed. While some studies have reported greater effects for negative expressions, e.g., faster detection and improved recognition, other studies have reported OT-mediated enhancement of positive expressions (for a review see Guastella & MacLeod, 2012). Moreover, Lischke and colleagues (2012) reported that acute IN-OT increased the perceived salience of some facial emotions without altering the duration of fixations to specific facial features. Based on previous reports that IN-OT reduced the aversive quality of negative facial expressions in nonhuman primates (Parr et al., 2013), we predicted that IN-OT may enhance attention to threat faces. However, our results showed that the high-frequency OT group spent the longest time viewing the neutral expressions compared to either the low-frequency or placebo groups, whereas the low-frequency OT group spent the longest time viewing lipsmack videos compared to either the high-frequency OT or placebo group. Therefore, IN-OT did not appear to selectively increase attention to negative expressions. Moreover, there were no effects of treatment group on the proportion of time spent viewing the eyes or the heads of the lipsmack or threat expressions. Alternatively, previous studies in humans have suggested that the effects of IN-OT may have a saturation point depending on the salience of the stimuli, like arousing facial expressions (Shamay-Tsoory & Abu-Akel, 2016). For example, acute IN-OT was shown to enhance attention biases to emotional faces in control subjects, suggesting increased salience, but not in highly anxious subjects for whom the faces were already extremely salient (Clark-Elford et al., 2014). In fact, for the socially anxious individuals, the trend was for IN-OT to reduce the attentional bias to emotional faces, similar to the findings of Parr and colleagues in monkeys (Parr et al., 2013).
Several studies in humans have now evaluated the effect of chronic IN-OT as a treatment for social impairments seen in individuals with autism spectrum disorders (ASD) using double-blind, cross over designs. Yatawara and colleagues (2015), for example, examined the effects of IN-OT or placebo in 3–8 year old children on the ASD spectrum. Treatment consisted of 24IU per day of IN-OT or IN-P, 12IU given in the morning and evening, for a period of 5 weeks. The authors report that IN-OT compared to IN-P significantly improved scores on the caregiver-rated Social Responsiveness Scale, and borderline improvement as measured by the Autism Diagnostic Observation Schedule (ADOS), and a caregiver-rated measure of repetitive behaviors (Yatawara et al., 2015). Moreover, Watanabe and colleagues (2015) evaluated the effects IN-OT versus placebo in autistic adults over a 6 week administration period. The authors report significant improvement in ADOS scores, their primary outcome measure. Subjects also performed a social evaluation task where they made personal judgements about emotional faces while undergoing a resting state fMRI scan. IN-OT improved the participants’ performance on the social judgement task, including increasing the time spent fixating on the eyes in the facial stimuli, and enhanced functional connectivity between the anterior cingulate and the dorso-medial prefrontal cortex, suggesting a possible mechanism for the improved social perception and behavior (Watanabe et al., 2015). While these studies showed improvements in social perception and behavior, including changes in brain function, other studies in humans have failed to identify any behavioral improvements in subjects after repeated IN-OT treatment (Anagnostou et al., 2014; Dadds et al., 2014; Guastella et al., 2015). Guastella and colleagues (2015) failed to find improvement in primary or secondary outcome measures in a group of 12–18 year olds with ASD who received either IN-OT or IN-P twice a day for a period of 8 weeks, and Dadds and colleagues (2014) failed to find any improvement in 7–16 year olds with ASD after daily treatments with IN-OT over a 4 day period.
Inconsistencies both within and across the human and animal literatures with regard to the effects of IN-OT treatment strongly highlight the need for further study, particularly in light of the growing body of published literature reporting positive effects of IN-OT on prosocial behavior in a variety of mammals. Most studies note that IN-OT is likely to have its greatest therapeutic effects in alleviating social impairments if given repeatedly early in development. To this end, researchers must identify the most optimal time frame in which to administer IN-OT, however, to date, it is unclear whether IN-OT produces different effects if given in infants, children or adolescents. In addition, the majority of studies, including the present one, have studied only males, in large part due to the greater frequency of males on the autism spectrum. Several studies in adults have now reported sex differences in both the behavioral and neural responses to acute doses of IN-OT (references, domes xxx). Similarly, researchers must identify the most optimal duration of treatment, frequent enough to have a positive and lasting effect on social development, but not so frequent as to functionally alter the endogenous OT system. Because numerous studies suggest that acute IN-OT can alter brain circuitry and function, this would best be done in an animal model where repeated longitudinal assessments using behavioral measurement, neuroimaging, or other methods, can be employed to track the consequences of chronic IN-OT treatment on brain and behavioral development. Finally, in additional to identifying an optimal age and time course for chronic IN-OT treatment, it is important to understand the most effective dose for improving social attention and prosocial interactions. A 24IU dose is typical for most human studies, however, the range can vary widely and the dose is not typically adjusted for the subject’s body weight. Although the present study is the first to report dose-related differences in nonhuman primate behavior, the 6 month old subjects weight on average only 1.4 kg, but they were given the same 24IU dose typically used in studies of adult males weighing ~80kg.
4.1 Conclusion
In conclusion, the present study reports a reduction in the proportion of time spent looking at the eyes in neutral faces after chronic IN-OT administration in infant monkeys. This is in contrast to numerous studies that report enhanced attention to the eyes after acute and chronic IN-OT administration in adults, and in patients with autism (Andari et al., 2010; Guastella et al., 2008; Watanabe et al., 2015). These results, in combination with studies of chronic OT administration in rodents (see Section 1), suggest major differences in the prosocial effects of OT based on whether delivery is chronic vs acute, and its age of delivery. These results have important consequences for the potential use of OT to improve social impairments in humans and re-emphasize the need to probe further into the mechanisms by which IN-OT has its prosocial effects in humans. Particular attention should be paid to differences between doses, administration schedules, and the subject or patient’s age.
Supplementary Material
Highlights.
A longitudinal study explored the effect of chronic oxytocin on social attention
The study was done on infant rhesus monkeys
Several doses of oxytocin were used
Results showed that oxytocin reduced attention to the eyes of neutral faces
Acknowledgments
Role of funding source
This project was funded by the National Institutes of Health MH104534 to L.A. Parr. Additional support was provided by the National Center for Research Resources P51RR000165 to the Yerkes National Primate Research Center, currently the Office of Research Infrastructure Programs / OD P51OD011132.
Special thanks to Hilary Smith, Aaron Gray and Jessica Johnson for assistance with animal testing, Jamie La Prairie for assistance with drug preparation, and Katalin Gothard for the use of the video images. Helpful comments on an earlier version of this manuscript were provided by Erin Hecht and Katalin Gothard.
Footnotes
These data represent one cross-sectional time point from a larger longitudinal study in which the subjects were tested every other week starting at 2 weeks of age. These earlier time points involved different stimuli than the ones described here, but readers should be aware that the subjects were very familiar and comfortable with the eye-tracking procedures by the time they reached 25 weeks of age.
Conflict of interest statement: All authors report no biomedical financial conflicts of interests.
Contributors: LAP designed the study. TJ, SM and JMB collected the data. LAP, TRH and JOJ analyzed the data. LAP, TJ, SM, JMB and TRH contributed to writing and editing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves E, Fielder A, Ghabriel N, Sawyer M, Buisman-Pijlman FT. Early social environment affects the endogenous oxytocin system: a review and future directions. Frontiers in Endocrinology. 2015;6:32. doi: 10.3389/fendo.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, Jacob S. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Research. 2014;1580:188–198. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM. Developmental experiences and the oxytocin receptor system. Hormones and Behavior. 2012;61:313–319. doi: 10.1016/j.yhbeh.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biological Psychiatry. 2013;74:180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe RG, Kiorpes L, Regal DM, Lee CP. Development of visual resonsiveness in Macaca nemestrina monkeys. Developmental Psychology. 1982;18:665–670. [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PloSONE. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnoli F, Kreutzmann JC, de Boer SF, Althaus M, Koolhaas JM. Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology. 2015;51:112–121. doi: 10.1016/j.psyneuen.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM. Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Hormones and Behavior. 2014;65:427–433. doi: 10.1016/j.yhbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self-reinforcement in rhesus macaques (Macaca mulatta) Proceedings of the National Academy of Sciences of the United States of America. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Hormones and Behavior. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Elford R, Nathan PJ, Auyeung B, Mogg K, Bradley BP, Sule A, Muller U, Dudas RB, Sahakian BJ, Baron-Cohen S. Effects of oxytocin on attention to emotional faces in healthy volunteers and highly socially anxious males. The international journal of Neuropsychopharmacology. 2014;18:1–7. doi: 10.1093/ijnp/pyu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. Journal of Autism and Developmental Disorders. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Costa VD, Averbeck BB. Oxytocin enhances attention to the eye region in rhesus monkeys. Frontiers in Neuroscience. 2014a;8:41. doi: 10.3389/fnins.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PloSONE. 2014b;9:e103677. doi: 10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry. 2007b;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves "mind-reading" in humans. Biological Psychiatry. 2007a;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ebitz RB, Watson KK, Platt ML. Oxytocin blunts social vigilance in the rhesus macaque. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11630–11635. doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Paukner A, Ionica C, Suomi SJ. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Current biology : CB. 2009;19:1768–1772. doi: 10.1016/j.cub.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. Discovering Statistics Using IBM SPSS. Sage; Los Angeles: 2013. [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GG, Roberts JA. Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology. 2016;66:185–194. doi: 10.1016/j.psyneuen.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative Perspectives on Oxytocin and Vasopressin Receptor Research in Rodents and Primates: Translational Implications. Journal of Neuroendocrinology. 2016;28 doi: 10.1093/ijnp/pyu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Animal Cognition. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Hormones and Behavior. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, Keating CM, Cacciotti-Saija C, Einfeld SL. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2015;56:444–452. doi: 10.1111/jcpp.12305. [DOI] [PubMed] [Google Scholar]
- Guo K, Robertson RG, Mahmoodi S, Tadmor Y, Young MP. How do monkeys view faces? A study of eye movements. Experimental Brain Research. 2003;150:363–374. doi: 10.1007/s00221-003-1429-1. [DOI] [PubMed] [Google Scholar]
- Hammock EA. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology. 2015;40:24–42. doi: 10.1038/npp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde RA, Rowell TE. Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta) Proceedings of the Zoological Society of London. 1962;138:1–21. [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Current Biology : CB. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni ML, Papaleo F. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Winslow JT, Witt DM. Homologous regulation of brain oxytocin receptors. Endocrinology. 1992;130:2602–2608. doi: 10.1210/endo.130.5.1315251. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman R, Sharma J, Sur M, Desimone R. Effect of distracting faces on visual selective attention in the monkey. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18037–18042. doi: 10.1073/pnas.1420167111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Sirigu A. The two fold role of oxytocin in social developmental disorders: A cause and a remedy? Neuroscience and Biobehavioral Reviews. 2016;63:168–176. doi: 10.1016/j.neubiorev.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Leonard TK, Blumenthal G, Gothard KM, Hoffman KL. How macaques view familiarity and gaze in conspecific faces. Behavioral Neuroscience. 2012;126:781–791. doi: 10.1037/a0030348. [DOI] [PubMed] [Google Scholar]
- Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, Domes G. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology. 2012;37:475–481. doi: 10.1016/j.psyneuen.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Miller TV, Caldwell HK. Oxytocin during Development: Possible Organizational Effects on Behavior. Frontiers in Endocrinology. 2015;6:76. doi: 10.3389/fendo.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. 2014;45:49–57. doi: 10.1016/j.psyneuen.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, Gothard KM. Videos of conspecifics elicit interactive looking patterns and facial expressions in monkeys. Behavioral Neuroscience. 2011;125:639–652. doi: 10.1037/a0024264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, Gothard KM. Neurons in the monkey amygdala detect eye contact during naturalistic social interactions. Current Biology. 2014;24:2459–2464. doi: 10.1016/j.cub.2014.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschinski J, Feczko E, Brooks J, Heitz TR, Parr LA. The development of visual preferences for direct versus averted gaze in infant macaques (Macaca mulatta) Developmental Psychobiology. 2016 doi: 10.1002/dev.21421. [DOI] [PubMed] [Google Scholar]
- Nahm FKD, Perret A, Amaral DG, Albright TD. How do monkeys look at faces? Journal of Cognitive Neuroscience. 1997;9:611–623. doi: 10.1162/jocn.1997.9.5.611. [DOI] [PubMed] [Google Scholar]
- Parr LA. Oxytocin enhances socially reinforced learning in rhesus macaques. Frontiers in Behavioral Neuroscience. 2014;8:1–8. [Google Scholar]
- Parr LA, Modi M, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys' attention to negative facial expressions. Psychoneuroendocrinology. 2013;38:1748–1756. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Murphy L, Feczko E, Brooks J, Collantes J, Heitz TR. Experience shapes the development of social attention in infant rhesus monkeys. Developmental Psychobiology. 2016 doi: 10.1002/dev.21434. [DOI] [PubMed] [Google Scholar]
- Putnam PT, Roman JM, Zimmerman PE, Gothard KM. Oxytocin enhances gaze-following responses to videos of natural social behavior in adult male rhesus monkeys. Psychoneuroendocrinology. 2016;72:47–53. doi: 10.1016/j.psyneuen.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rault JL, Carter CS, Garner JP, Marchant-Forde JN, Richert BT, Lay DC., Jr Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiology & Behavior. 2013;112–113:40–48. doi: 10.1016/j.physbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A. The Social Salience Hypothesis of Oxytocin. Biological Psychiatry. 2016;79:194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Simpson EA, Sclafani V, Paukner A, Hamel AF, Novak MA, Meyer JS, Suomi SJ, Ferrari PF. Inhaled oxytocin increases positive social behaviors in newborn macaques. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6922–6927. doi: 10.1073/pnas.1402471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH. Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Hormones and Behavior. 2012;61:304–312. doi: 10.1016/j.yhbeh.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, Takao H, Nippashi Y, Kawakubo Y, Kunimatsu A, Kasai K, Yamasue H. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–3412. doi: 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Molecular Psychiatry. 2015 doi: 10.1038/mp.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.