Abstract
MELF invasion has been associated with non-vaginal recurrences and lymph node (LN) metastases in multi-institutional case control studies, but has not been well examined in large single institution cohorts. Hysterectomy specimens with FIGO 1 endometrioid endometrial carcinoma (EEC) and lymphadenectomies from 2007 to 2012 were identified. Electronic medical records and histologic slides were reviewed. Of 464 identified cases, 163 (35.1%) were noninvasive, 60 (12.9%) had MELF, 222 (47.8%) had a component of the infiltrative invasion pattern without MELF, 13 (2.8%) had pure pushing borders of invasion, 5 (1.1%) had pure adenomyosis-like invasion, and 1 (0.2%) had pure adenoma malignum-like invasion. Sixteen cases had LN metastases. Significantly more MELF cases had positive LNs than non-MELF cases overall (18.3% vs 1.2%, p<0.001). The results were almost identical when invasive infiltrative cases with and without MELF were compared (18.3% vs 1.8%, p<0.001). The maximum number of MELF glands per slide did not differ between cases with and without LN metastases, p=0.137. A majority of positive LNs, even in MELF cases, demonstrated non-histiocyte-like metastases. Only five cases (all with MELF invasion) demonstrated micrometastatic lesions or isolated tumor cells only. MELF cases demonstrated a non-significant decrease in time to extra-vaginal recurrence (p=0.082, log-rank test), for which analysis was limited by low recurrence rates. In summary, MELF is associated with LN metastases, even when compared to other infiltrative cases, and shows multiple patterns of growth in positive LNs. MELF cases additionally trended toward decreased time to extra-vaginal recurrence.
Keywords: Endometrial carcinoma, MELF, non-vaginal recurrence, lymph node metastasis, FIGO grade 1
Introduction
Most FIGO grade 1 endometrioid endometrial carcinomas (EEC) present with early-stage disease and have an excellent prognosis.1–3 However, a minority with early-stage, low-grade disease will demonstrate a more aggressive clinical course. A priori identification of such cases could allow offering additional treatment to women who may benefit the most.4 EEC are histologically heterogeneous and the morphologic pattern of myometrial invasion may be related to biologic potential.5–6 Specifically, myometrial invasion with an infiltrative gland pattern has been recently associated with higher stage, lymphovascular invasion and recurrence.6 Additionally, a readily recognizable pattern of myometrial invasion characterized by microcystic elongated and fragmented (MELF) glands surrounded by myxoid and inflamed stroma has been associated with lymphovascular invasion7–8 and lymph node metastases.7,9–11 This morphologic pattern was initially recognized by Lee, Vacek and Belinson12 and the term MELF was later coined by Murray et al.13 Immunophenotypic changes including loss or reduction of CD147, MMP2,14 e-cadherin10 and Galectin-315 may indicate epithelial mesenchymal transition (EMT) in MELF. EMT, which results in loss of cell-cell adhesion and polarity, endows cells with migratory and invasive properties.16 EMT has been associated with poor prognostic parameters in breast, colorectal and ovarian cancers.16
Herein we investigate the clinical and pathologic associations of MELF in FIGO grade 1 EEC, including lymph node metastases, vaginal, and non-vaginal recurrences. By analyzing a large number of consecutive unselected single-institution grade 1 EEC we avoid case selection bias inherent in case-control designs. By excluding the clinically more variable and pathologically less reproducible FIGO grade 2 EEC, we seek to clarify the implications of MELF in women for whom it would matter the most: those in whom the standard of care may most frequently include forgoing adjuvant therapy. We report on the influence of MELF on grade 1 EEC overall as well as within grade 1 EEC that feature infiltrative invasion already thought to be associated with worse outcomes. Additionally we describe the pathologic features of MELF associated lymph node metastases which has been the subject of very scant and occasionally incomplete reports.
Materials and Methods
Under an IRB-approved protocol (OSU 2014C0099), we searched The Ohio State University pathology database for patients with grade 1 EEC who underwent hysterectomy and lymphadenectomy between July 1, 2007 and April 15, 2012. Electronic medical records were reviewed for pathologic and clinical data including presence of LN metastases and recurrence data. Histologic slides were reviewed for presence or absence of myometrial invasion and pattern of invasion, if applicable. Histologic review was done blinded to clinical outcome. The descriptions of five myometrial invasion patterns in Cole et al5 were utilized to classify each invasive case: irregular or infiltrative invasion, MELF invasion, invasion with pushing borders (broad front), adenomyosis-like invasion, and adenoma malignum-like invasion. For cases with multiple patterns of invasion, each pattern and its relative proportion was recorded. If MELF pattern of invasion was present, a single gynecologic pathologist (AAS) selected the slide with the heaviest MELF burden. The slide was then reviewed by a second gynecologic pathologist (DWC) for confirmation and to count the number of MELF glands on that single slide. Intermixed cases without MELF were also given to the second gynecologic pathologist for quality assurance purposes, and in cases with disagreement as to the presence of MELF, joint review and consensus was attained.
Fisher Exact tests (two-tailed) were used to compare proportions of cases with LN metastases between MELF and non-MELF cases overall, and between MELF cases and the subset of infiltrative non-MELF cases. A Wilcoxon Rank Sum test was used to compare the number of MELF glands per slide with presence or absence of LN metastases. Median time to non-vaginal recurrence was planned using Kaplan-Meier estimates. The Kaplan-Meier curves for MELF and non-MELF were compared using a log-rank test. Time to recurrence was defined as the time from surgery date to first recurrence. For the recurrence analysis, patients were censored if they were alive without disease at the time of last follow up visit, were disease-free but died of other causes, or no outcome data was available. All statistical analyses were performed using Stata 13 (Statacorp LP, College Station, TX) or SAS 9.3 (SAS Institute Inc., Cary, NC).
Positive LN slides were reviewed for histologic patterns of metastases and to measure the diameter of largest metastasis in each case. Unless it was performed for clinical or pathologic suspicion at the time of the original case, no cytokeratin immunostains were used to routinely identify micrometastases or isolated tumor cells in LN sections. Confirmation of several measurements, including all micrometastatic cases, was performed by a breast pathologist (ZL) according to standard breast definitions.17
Results
464 consecutive hysterectomy specimens with FIGO 1 EEC and accompanying lymphadenectomies were identified and pulled for review. There were 163 (35.1%) noninvasive tumors and 301 (64.9%) myoinvasive tumors. Sixty-eight cases were initially identified as demonstrating MELF. After review of these cases plus controls by a second pathologist, fourteen cases required consensus microscopic review as to the presence of MELF (thirteen potential MELF cases, one negative control). Of these cases, five remained in the MELF group and the other nine were included in the infiltrative (n=8) or non-invasive (n=1) categories detailed above. Ultimately, 60 tumors (12.9%) were identified as having a component of MELF (Figure 1)One case with 12 MELF glands in the slide with the heaviest burden showed pure MELF pattern invasion while all of the remaining 59 cases showed overlap with the infiltrative or irregular invasion pattern. Of the remaining myoinvasive tumors, 222 (47.8%) had a component of infiltrative or irregular invasion pattern without MELF, 13 (2.8%) had pure pushing borders of invasion, 5 (1.1%) had pure adenomyosis-like invasion, and 1 (0.2%) showed adenoma malignum-like invasion. No cases of pushing borders, adenomyosis-like invasion, or adenoma malignum-like invasion showed any component of MELF pattern invasion. In the MELF cases, one to 17 (median = 2) MELF glands were identified on the glass slide with the highest count of MELF glands in each case.
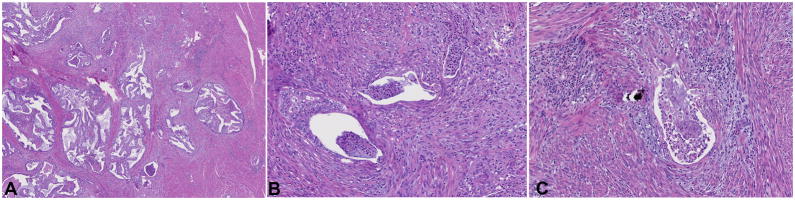
Figure 1.
The MELF pattern of invasion is often identified at low magnification by a characteristic inflammatory response and fibromyxoid stroma (A). At higher magnification, glands show the typical microcystic, elongated, and fragmented appearance (B,C).
Lymph Node Involvement
Lymph node metastases were found in 16 cases (3.4%). The clinicopathologic details of these cases are outlined in Table 1. The proportion of MELF cases with positive lymph nodes was significantly higher than the proportion of non-MELF cases (18.3% vs 1.2%, p<0.001, Fisher’s exact test). A similar difference was found in a subset of cases (n=282) comparing the MELF cases to infiltrative invasive cases without MELF (18.3% vs 1.8%, p<0.001, Fisher’s exact test). Although the number of MELF glands per slide was higher in cases with lymph node metastases than in cases without LN metastases, the difference was not statistically significant (average 5.82 vs 3.41 glands, p=0.137, Wilcoxon Rank Sum test). Additional variables, such as pT stage, could not be controlled for in a multivariable analysis due to the overall low rate of lymph node involvement.
Table 1.
Clinical and pathologic details of sixteen cases with positive lymph nodes.
| Case | Age | pT stageo | MELF | No. MELF glands per slide | No. positive lymph nodes | Positive lymph node locations* | Largest metastatic focus size (mm) | Morphology† |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 16 | 58 | 1b | Y | 1 | 4 | 1,2 | 7 | 2 |
| 101 | 50 | 1b | N | 0 | 5 | 2 | 13 | 2 |
| 103ϒ | 62 | 1b | Y | 13 | 1 | 1 | 5 | 2,3 |
| 109 | 47 | 3a | Y | 4 | 2 | 1,2 | 1.5 | 2,3 |
| 110 | 60 | 1b | Y | 1 | 1 | 1 | ITC‡ | 1 |
| 176ϒ | 62 | 3a | Y | 5 | 10 | 1,2 | 16 | 2,3 |
| 178ϒ | 57 | 2 | N | 0 | 1 | 1 | 4 | 2 |
| 199 | 67 | 1a | Y | 5 | 3 | 1 | 5 | 1 |
| 206 | 54 | 1b | Y | 3 | 3 | 1 | 1.5 | 3 |
| 213 | 47 | 3a | Y | 12 | 5 | 1,2 | 10 | 1 |
| 253 | 77 | 1b | Y | 2 | 1 | 1 | 1 | 2 |
| 261 | 59 | 1b | Y | 17 | 1 | 1 | 2.5 | 1,3 |
| 317ϒ | 57 | 3a | N | 0 | 9 | 1,2 | 8 | 1,2,3 |
| 328 | 64 | 3a | N | 0 | 2 | 1,2 | 7 | 2 |
| 363ϒ | 58 | 2 | N | 0 | 2 | 1 | 3 | 1,3 |
| 444 | 66 | 1a | Y | 1 | 2 | 1,2 | 1.5 | 1 |
According to guidelines outlined by the AJCC Cancer Staging Handbook 2010
1=pelvic, 2=paraaortic
Morphologic description of lymph node metastases: 1=sinus histiocyte-like, 2=solid glandular, 3=cystic glandular
ITC, isolated tumor cells
These patients are also represented in Table 2.
Review of positive lymph node slides demonstrated multiple histologic patterns. Metastases were grouped into three categories: sinus histiocyte-like, solid and glandular, and cystic glandular (Figure 2). Multiple patterns were observed in separate lymph nodes of the same case, as well as in different areas or sections of the same lymph node. Out of the 11 MELF cases with positive lymph nodes, 5 showed sinus histiocyte-like metastases, 5 had solid glandular metastases, and 5 had cystic glandular metastases. In the 5 non-MELF cases with positive lymph nodes, 2 showed sinus histiocyte-like metastases, 4 showed solid glandular metastases, and 2 showed cystic glandular metastases. Five cases had only low volume lymph node metastases including isolated tumor cells and micrometastases (cases 109, 110, 206, 253, and 444; see Table 1). Low volume lymph node metastases, including isolated tumor cells and micrometastases, were only seen in cases with MELF. Interestingly, not all of these low volume metastases were of the sinus-histiocyte like pattern. However, the remaining MELF cases had larger nodal metastases overlapping with those of non-MELF tumors.
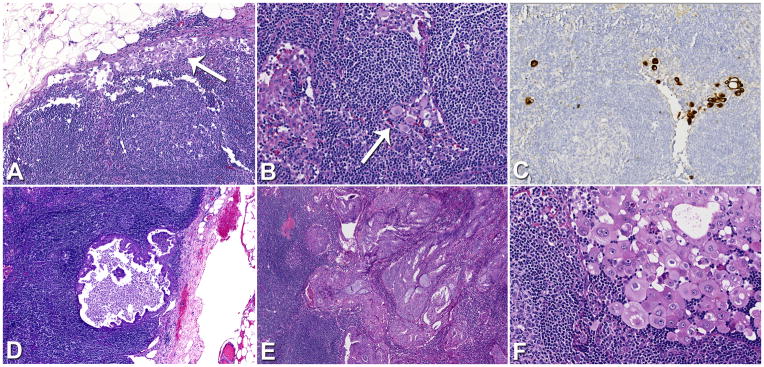
Figure 2.
Lymph nodes in MELF cases often showed the sinus histiocyte-like pattern of metastasis (A, B), which was occasionally confirmed at the time of the original pathologic diagnosis with cytokeratin immunostaining (C). Numerous larger glandular metastases were identified showing cystic (D) or solid patterns (E). Glandular metastases in MELF cases also occasionally displayed discohesive areas reminiscent of the sinus histiocyte-like pattern (F).
Recurrence and Other Adverse Outcomes
Patients had a median of 35 months of follow up, ranging from less than one month to 90 months. Twenty (4.3%) patients developed recurrent disease over a range of 5 to 53 months, of which nine (45.0%) had an isolated vaginal recurrence and eleven (55.0%) had extra-vaginal recurrence. Clinicopathologic details of all patients with recurrences are outlined in Table 2. Of note, no patients with the MELF pattern of invasion experienced vaginal-only recurrences. Additionally, no recurrences occurred in patients that demonstrated lymph nodes with isolated tumor cells or micrometastases only. Extra-vaginal recurrences occurred in 3 (5.0%) MELF cases (at 8, 10, and 47 months follow-up time) and in 9 (2.0%) non-MELF cases (at 5 to 53 months follow-up time). Kaplan-Meier curves were constructed and no significant difference in non-vaginal recurrence was found between MELF and non-MELF cases, p = 0.082 (log-rank test). The median time to recurrence could not be estimated due to the low absolute number of events. A subgroup analysis comparing the MELF cases (n=60) and non-MELF infiltrative/irregular invasion patterns only (n=222), where 7 non-vaginal recurrences occurred, similarly showed no statistically significant difference, p=0.392 (log-rank test).
Table 2.
Clinical and pathologic details of twenty cases with recurrent disease.
| Case | Age | MELF | pN stageo | pT stageo | FIGO stage | Extra-vaginal | Months to recurrence | Patient Status† |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 12 | 61 | N | 0 | 1a | IA | Y | 38 | AWD |
| 37 | 72 | N | 0 | 1a | IA | Y | 11 | D-UNK |
| 66 | 64 | N | 0 | 1b | IB | Y | 24 | DOD |
| 67 | 52 | N | 0 | 2 | II | N | 19 | AWD |
| 94 | 67 | Y | 0 | 1a | IA | Y | 10 | NED |
| 103‡ | 62 | Y | 1 | 1b | IIIC1 | Y | 47 | AWD |
| 168 | 56 | N | 0 | 1a | IA | N | 9 | NED |
| 176‡ | 62 | Y | 2 | 3a | IIIC2 | Y | 8 | DOD |
| 178‡ | 57 | N | 1 | 2 | IIIC1 | Y | 28 | NED |
| 189 | 50 | N | 0 | 1a | IA | N | 48 | NED |
| 212 | 56 | N | 0 | 1a | IA | N | 25 | DOD |
| 291 | 86 | N | 0 | 1a | IA | N | 39 | NED |
| 294 | 49 | N | 0 | 1a | IA | Y | 53 | AWD |
| 295 | 50 | N | 0 | 3a | IIIA | N | 45 | NED |
| 317‡ | 57 | N | 2 | 3a | IIIC2 | Y | 11 | DOD |
| 332 | 67 | N | 0 | 2 | II | N | 14 | NED |
| 351 | 60 | N | 0 | 1a | IA | N | 13 | NED |
| 363‡ | 58 | N | 1 | 2 | IIIC1 | Y | 50 | DOD |
| 378 | 57 | N | 0 | 1a | IA | N | 20 | AWD |
| 384 | 55 | N | 0 | 1b | IB | Y | 5 | AWD |
According to guidelines outlined by the AJCC Cancer Staging Handbook 2010
AWD = alive with disease; DOD = dead of disease; NED = no evidence of disease; D-UNK = dead of unknown causes
These patients are also represented in Table 1.
The univariate Cox proportional hazards model showed no significant difference in rate of non-vaginal recurrences in MELF vs. non-MELF cases (hazard ratio = 3.06, p=0.099). Further analysis using a multivariable Cox proportional hazards model was not possible due to the small absolute number of recurrences in the cohort. Additionally, too few recurrences in the MELF cases precluded statistical comparisons involving the maximum number of MELF glands per slide.
Other adverse outcomes noted in the cohort included five (1.1%) patients who died of disease (DOD), 12 (2.6%) patients who died of other known causes, and nine (1.9%) patients who died of unknown causes. As detailed in Table 2, six patients with recurrences were alive with disease (AWD) at the time of submission, after varying follow-up periods (range 5–53 months). While treatment variables are not a component of our analysis, it is of note that nine of 11 patients with extra-vaginal recurrence received chemotherapy with or without radiation therapy, with the two remaining patients receiving hormonal therapy (tamoxifen, megestrol) or not returning to follow up appointments, respectively. Among the nine vaginal recurrences, four patients received a combination of chemotherapy and radiation therapy, three received radiation therapy alone, and one received radiation and hormonal therapies. One patient did not return for follow up appointments. Of the patients with positive lymph nodes, 13 of 16 patients received chemotherapy with or without radiation therapy. Information about clinical follow up and adjuvant treatments was not available on the remaining three patients, as their care was transferred back to referring oncologists.
Discussion
We studied lymph node metastases and non-vaginal recurrences amongst 464 single-institution, consecutive cases of FIGO grade 1 EEC, for which patients underwent hysterectomy with staging lymphadenectomies. We confirmed previous reports that patients whose tumors demonstrated MELF invasion have an increased propensity to also demonstrate LN metastases. We also demonstrated that this association between MELF and LN metastases remained significant when only myoinvasive tumors with an irregular, infiltrative pattern and no MELF (n=222) were compared with MELF cases (n=60) (1.8 vs. 18.3%, p<0.001, Fisher’s exact test).
Several investigators have studied the MELF pattern of invasion in relation to lymph node metastases. In fact, a case of pT1a grade 1 EEC with positive lymph nodes was reported by Young and Clement along with an early description of this pattern of myometrial invasion before the term MELF had even been coined.18 McKenney, Kong and Longacre followed with a report of well differentiated endometrioid carcinomas with positive lymph nodes including two cases with MELF.19
More recently, a few larger series have been published trying to clarify MELF associations with lymphovascular invasion7–8 and lymph node metastases.7,9–11,20 Stewart et al and Hertel et al reported strong associations with lymphovascular invasion.7–8 Pavlakis et al,9 Han et al,10 Hertel et al7 and Dogan Altunpulluk et al11 reported lymph node metastases in 54%, 67%, 67% to 100%, and 71% of their respective cases, while Euscher et al20 reported MELF in 70% of their tumors with lymph node metastases or extrauterine disease. However, putting these high percentages into perspective and comparing these series is a nuanced exercise due to varying methods and case selection. Han et al10 in their study of eighteen stage 1 FIGO grade 1 endometrioid carcinomas with “occult” lymph node metastases and thirty-six controls reported MELF to be univariately but not multivariately associated with lymph node metastases. Hertel et al7 studied eighty pT1 low grade endometrioid adenocarcinomas with lymphovascular invasion and documented higher rates of nodal metastasis amongst those with MELF. Interestingly, Hertel et al also reported that the amount of MELF correlated with the rate of lymph node metastases,7 while in our current study there was no association of MELF glands per slide with lymph node metastases. Dogan Altunpulluk et al11 reported MELF in 28 (including 8 FIGO grade 3 tumors) out of 121 consecutive hysterectomies with endometrial cancer and documented an association with lymph node metastases on univariate and multivariate analyses. However, FIGO grading and clinical stage were not included in the multivariable model, despite significant associations of these variables with lymph node metastases and presence of MELF.11 Finally, a multi-institution case-control study of 304 tumors by Euscher et al showed MELF as a univariate but not multivariate predictor of lymph node metastases or extrauterine disease.20 However, the large proportion of FIGO grade 2 cases in the latter study may reflect selection bias by more lymphadenectomies being performed in higher grade cases.20
Some investigators have reported on the size10 and histologic features7,9–10,20 of lymph node metastases in carcinomas with MELF. Subtle sinus histiocyte-like morphology has been emphasized7,10 with one institution even reporting it as the sole pattern of lymph node metastasis.9 The use of cytokeratin immunostains in otherwise negative lymph nodes may have contributed very significantly to these data.7,9–10 We did find sinus histiocyte-like deposits as the sole morphologic pattern in 4 out of 11 MELF cases with positive nodes. However, other patterns were seen in the majority. Han et al, using terminology better validated in the breast cancer lymph node literature, reported “isolated tumor cells only” in 12 of their cases with MELF and positive nodes.10 In our study, we found lymph node metastases measuring less than 2mm (n=5) only amongst cases with MELF. Of note, four of these patients were alive with no evidence of disease at their last follow up. Last follow up visit was at three and five years for two of the patients; unfortunately it was limited to their last visit for chemotherapy in the other two. These four patients had received standard six-cycle chemotherapy regimens with carboplatin and paclitaxel; and two of them had bevacizumab as part of a clinical trial. No treatment details or follow up information were available on one of the patients with metastases less than 2mm. Six out of 11 cases with MELF had larger metastases, up to 16mm. While it is becoming increasingly prevalent in the endometrial cancer literature and has been included in the current College of American Pathologists endometrial cancer checklist,21 the breast cancer constructs of “isolated tumor cells” and “micrometastasis” should probably be employed with caution in endometrial cancer. Currently, there is limited and conflicting evidence as to whether micrometastases are clinically significant in various stages and grades of endometrial adenocarcinoma,22–25 with additional larger and long term studies required. Thus, whether additional therapy may benefit women with low grade EEC, MELF, and lymph node tumor deposits within these size ranges (micrometastases, isolated tumor cells) is a pressing matter.
Thus, we contribute data to dispel the impression that lymph node metastases in low grade endometrial carcinomas with MELF are mostly small and subtle. The significant association of MELF with lymph node metastases is important, especially since lymphadenectomy may not be performed in all patients with grade 1 EEC by biopsy. Reporting MELF in hysterectomy specimens with EEC may aid treating oncologists in deciding further therapy or completion lymphadenectomy when lymphadenectomy is not performed concurrent with the hysterectomy. Similarly, the possibility of reporting MELF on frozen sections to influence an intraoperative decision regarding lymphadenectomy may be worth exploring.
In the present study of consecutive cases there were no statistically significant differences between cases with and without MELF in extra-vaginal recurrences but this analysis is limited by the low number of events: 3 and 9 recurrences, respectively. MELF was identified in 53% of tumors that led to extravaginal recurrences compared to 30% in vaginal recurrences and 33% in cases without recurrence in a retrospective multi-institutional study.26 Although the association was statistically significant in that study, the case-control design with many FIGO grade 2 tumors are of note.26
Our strengths include a large number of consecutive patients with accompanying lymphadenectomies. Additionally, the inclusion of only FIGO grade 1 tumors focuses the analysis on those tumors for which, other variables being equal, an indolent behavior is most reasonably expected. Therefore, we have optimized the study to detect and isolate a negative impact of MELF on the variables reported while also avoiding the weaknesses of case-control designs and maximizing the potential clinical impact of MELF. This was done at the expense of having lower counts (16 cases with positive nodes, 5 DOD, 6 AWD) than could otherwise easily be obtained at our institution.2 As a result, our analysis of non-vaginal recurrences and other adverse outcomes is very limited, without thorough multivariable conclusions. Moreover, a majority (13/16) of patients with positive nodes received adjuvant chemotherapy and or radiotherapy, complicating extrapolation of our findings to women who may have had only hysterectomy and for whom additional therapy is being considered. However, the relative rarity of adverse outcomes is a main difficulty faced by all research attempting to identify those grade 1 EEC that will go on to behave more aggressively.
We conclude women with FIGO grade 1 EEC and MELF are at a significantly increased risk of regional lymph node metastases. This justifies the inclusion of this finding in pathology reports, especially in women who have not had staging lymphadenectomies. While molecular data are increasingly incorporated into clinical thinking and practice,27–28 histologic findings, including MELF, will continue to provide a main framework for pathology reports. It is important to put them into a clear and clinically useful perspective.
Acknowledgments
Source of funding: Julie Stephens was supported by Award Number Grant UL1TR001070 from the National Center For Advancing Translational Sciences. For the remaining authors, no funding sources are declared.
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
References
- 1.Chan JK, Wu H, Cheung MK, et al. The outcomes of 27,063 women with unstaged endometrioid uterine cancer. Gynecol Oncol. 2007;106:282–8. doi: 10.1016/j.ygyno.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 2.Joehlin-Price AS, Stephens JA, Zhang J, et al. Endometrial cancer insulin-like growth factor 1 receptor (IGF1R) expression increases with body mass index and is associated with pathologic extent and prognosis. Cancer Epidemiol Biomarkers Prev. 2015;25:438–45. doi: 10.1158/1055-9965.EPI-15-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves KW, Carter GC, Rodabough RJ, et al. Obesity in relation to endometrial cancer risk and disease characteristics in the Women’s Health Initiative. Gynecol Oncol. 2011;121:376–82. doi: 10.1016/j.ygyno.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SGO Clinical Practice Endometrial Cancer Working Group. Burke WM, Orr J, et al. Endometrial cancer: a review and current management strategies: Part II. Gynecol Oncol. 2014;134:393–402. doi: 10.1016/j.ygyno.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Cole AJ, Quick CM. Patterns of myoinvasion in endometrial adenocarcinoma: recognition and implications. Adv Anat Pathol. 2013;20:141–7. doi: 10.1097/PAP.0b013e31828d17cc. [DOI] [PubMed] [Google Scholar]
- 6.Quick CM, May T, Horowitz NS, et al. Low grade, low stage endometrioid endometrial adenocarcinoma: a clinicopathologic analysis of 324 cases focusing on frequency and pattern of myoinvasion. Int J Gynecol Pathol. 2012;31:337–43. doi: 10.1097/PGP.0b013e31823ff422. [DOI] [PubMed] [Google Scholar]
- 7.Hertel JD, Huettner PC, Pfeifer JD. Lymphovascular space invasion in microcystic elongated and fragmented (MELF)-pattern well differentiated endometrioid adenocarcinoma is associated with a higher rate of lymph node metastasis. Int J Gynecol Pathol. 2014;33:127–34. doi: 10.1097/PGP.0b013e318285657b. [DOI] [PubMed] [Google Scholar]
- 8.Stewart CJR, Brennan BA, Leung YC, et al. MELF pattern invasion in endometrial carcinoma: association with low grade, Myoinvasive endometrioid tumors, focal mucinous differentiation and vascular invasion. Pathol. 2009;41:454–9. doi: 10.1080/00313020903041135. [DOI] [PubMed] [Google Scholar]
- 9.Pavlakis K, Messini I, Vrekoussis T, et al. MELF invasion in endometrial cancer as a risk factor for lymph node metastasis. Histopathol. 2011;58:966–73. doi: 10.1111/j.1365-2559.2011.03802.x. [DOI] [PubMed] [Google Scholar]
- 10.Han G, Lim D, Leitao MM, Jr, et al. Histologic features associated with occult lymph node metastasis in FIGO clinical stage I, grade I endometrioid carcinoma. Histopathol. 2014;64:389–98. doi: 10.1111/his.12254. [DOI] [PubMed] [Google Scholar]
- 11.Dogan Altunpulluk M, Kir G, Topal CS, et al. The association of the microcystic, elongated, and fragmented (MELF) invasion pattern in endometrial carcinomas with deep myometrial invasion, lymphovascular space invasion and lymph node metastasis. J Obstet Gynaecol. 2015;35:397–402. doi: 10.3109/01443615.2014.960827. [DOI] [PubMed] [Google Scholar]
- 12.Lee KR, Vacek PM, Belinson JL. Traditional and nontraditional histopathologic predictors of recurrence in uterine endometrioid adenocarcinoma. Gynecol Oncol. 1994;54:10–8. doi: 10.1006/gyno.1994.1158. [DOI] [PubMed] [Google Scholar]
- 13.Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. Int J Gynecol Pathol. 2003;22:324–33. doi: 10.1097/01.pgp.0000092161.33490.a9. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CJR, Crook ML. CD147 (EMMPRIN) and matrix metalloproteinase-2 expression in uterine endometrioid adenocarcinoma. Pathol Res Pract. 2011;207:30–6. doi: 10.1016/j.prp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Stewart CJR, Crook ML. Galectin-3 expression in uterine endometrioid adenocarcinoma: comparison of staining in conventional tumor glands and in areas of MELF pattern myometrial invasion. Int J Gynecol Pathol. 2010;29:555–61. doi: 10.1097/PGP.0b013e3181e4ee4ea. [DOI] [PubMed] [Google Scholar]
- 16.Thiery JP, Aclogue H, Huang RY, et al. Epithelial-mesenchymal transitions. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Lester SC, Bose S, Chen Y, et al. [Accessed April 26, 2016];Protocol for the examination of specimens from patients with invasive carcinoma of the breast [College of American Pathologists website] 2016 Jan; Available at: www.cap.org/cancerprotocols.
- 18.Clement PB, Young RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002;9:145–84. doi: 10.1097/00125480-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 19.McKenney JK, Kong CS, Longacre TA. Endometrial adenocarcinoma associated with subtle lymph-vascular space invasion and lymph node metastasis: a histologic pattern mimicking intravascular and sinusoidal histiocytes. Int J Gynecol Pathol. 2005;24:73–78. [PubMed] [Google Scholar]
- 20.Euscher E, Fox P, Bassett R, et al. The pattern of myometrial invasion as a predictor of lymph node metastasis or extrauterine disease in low grade endometrial carcinoma. Am J Surg Pathol. 2013;37:1728–36. doi: 10.1097/PAS.0b013e318299f2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Movahedi-Lankarani S, Gilks CB, Otis CN, et al. [Accessed April 16, 2016];Protocol for the examination of specimens from patients with carcinoma of the endometrium [College of American Pathologists website] 2016 Jan; Available at www.cap.org/cancerprotocols.
- 22.Erkanli S, Bolat F, Seydaoglu G. Detection and importance of micrometastases histologically negative lymph nodes in endometrial carcinoma. Eur J Gynaec Oncol. 2011;32:619–25. [PubMed] [Google Scholar]
- 23.Ferraioli D, Chopin N, Beurrier F, et al. The incidence and clinical significance of micrometastases in the sentinel lymph nodes during surgical staging for early endometrial cancer. Int J Gynecol Cancer. 2015;25:673–80. doi: 10.1097/IGC.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 24.McCoy A, Finan MA, Boudreaux FT, et al. The incidence and clinical significance of lymph node micrometastases determined by immunohistochemical staining in stage I – lymph node negative endometrial cancer. Histol Histopathol. 2012;27:181–5. doi: 10.14670/HH-27.181. [DOI] [PubMed] [Google Scholar]
- 25.Todo Y, Kato H, Okamoto K, et al. Isolated tumor cells and micrometastases in regional lymph nodes in stage I to II endometrial cancer. J Gynecol Oncol. 2016;27:e1. doi: 10.3802/jgo.2016.27.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschiano EJ, Barbuto DA, Walsh C, et al. Risk factors for recurrence and prognosis of low-grade endometrial adenocarcinoma; vaginal versus other sites. Int J Gynecol Pathol. 2014;33:268–73. doi: 10.1097/PGP.0b013e31829c6757. [DOI] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, et al. Nature. 2013;497:67–73. [Google Scholar]
- 28.Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer. 2015;113:299–310. doi: 10.1038/bjc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]