Abstract
Catastrophizing is a potent psychological modulator of pain across several chronic pain populations; yet despite evidence that patients with sickle cell disease (SCD) catastrophize more than patients with other chronic pain conditions, prior research indicates that catastrophizing is not related to sickle cell pain after controlling for relevant covariates such as depression. Recent research suggests that pain-related catastrophizing should be assessed across pain contexts (e.g., dispositional and situational). Here, we measured disease-specific, general non-disease related, and situational catastrophizing and assessed the relationship between these contextual dimensions of catastrophizing and both laboratory and clinical pain among patients with SCD. Results revealed differential catastrophizing across pain contexts, with patients reporting greater catastrophizing about SCD-specific pain compared to non-SCD pain and laboratory pain. SCD-specific and non-SCD catastrophizing were associated with clinical pain outcomes, and situational catastrophizing with markers of central sensitization and laboratory pain. Further examination of the time course of laboratory responses revealed that increases in situational catastrophizing were associated with subsequent increases in laboratory pain sensitivity. Taken together, results demonstrate the relevance of catastrophizing in understanding pain in SCD, and suggest that context-specific anchors may be beneficial in predicting different aspects of the pain experience (e.g., chronic pain, pain sensitization).
Keywords: sickle cell disease, pain catastrophizing, quantitative sensory testing, chronic pain, central sensitization
Introduction
Catastrophizing is a potent psychological modulator of pain across several chronic pain populations [31]. Pain catastrophizing involves exaggerated negative affective and cognitive appraisals of pain, such as rumination, helplessness, and magnification of pain. Current evidence suggests a direct link between pain catastrophizing and physiological pain facilitation processes, such that increased catastrophizing is associated with centrally mediated pain enhancement (i.e., decreased conditioned pain modulation and increased temporal summation [24]) and enhanced pain-related brain response [27], and targeted therapeutic reduction in catastrophizing results in decreased clinical pain severity [28].
Patients with sickle cell disease (SCD) catastrophize more than patients with other chronic pain conditions, perhaps due to the lifelong and life-threatening nature of SCD, and this occurs more during periods of crisis relative to non-crisis [16]. However, this elevated catastrophizing is not associated with heightened crisis or non-crisis pain intensity, distress, or pain-related interference when symptoms of depression are controlled [7]. Therefore, others have concluded that inferences about catastrophizing and pain drawn from other populations should not be translated to sickle cell pain [7].
Recent work demonstrates substantial variability in the relationship between pain and catastrophizing [3]. One possible explanation for the lack of statistical association between pain and catastrophizing in SCD may be the specificity and proximity of measures of catastrophizing to specific pain experiences. Patients with SCD regularly experience a variety of different pains including severe episodic pain during periods of vaso-occlusive crisis (sudden onsets of acute pain typically lasting 4-7 days), neuropathic pain that includes hyperalgesia and allodynia, and other types of chronic pain with or without an identifiable pathology[9]. However, traditional assessments of catastrophizing measure dispositional responses to pain in general, and do not allow for differentiation of different types of pain [20]. Others have argued that catastrophizing in response to specific stimuli may more accurately predict corresponding stimulus-related pain [24]. Recent studies have demonstrated that measurement of situational catastrophizing (i.e., catastrophizing related to evoked laboratory pain) is a better predictor of laboratory-induced pain than dispositional catastrophizing [3]. However, unlike dispositional catastrophizing, situational catastrophizing has an inconsistent relationship with clinical pain. Studies have shown that situational catastrophizing in response to evoked pain is associated with laboratory pain in healthy adults [6] and post-elective surgical pain in healthy male adults[13], but not with later clinical pain in fibromyalgia [4]. Furthermore, situational catastrophizing can be poorly correlated with general dispositional catastrophizing, suggesting that an individual's engagement of catastrophic thinking toward pain may differ across contexts [3,20,24].
In this study we present data on a further refinement of the measurement of pain catastrophizing, introducing a disease-specific approach to measuring catastrophizing and comparing the relationship between disease-specific, general non-disease related, and situational pain catastrophizing in patients with SCD. Based on prior evidence, we hypothesized that general non-SCD catastrophizing would not be associated with clinical pain in SCD. Rather, we hypothesized that specifically anchored measures of catastrophizing would be associated with corresponding pain dimensions. We hypothesized that situational catastrophizing would be associated with laboratory pain intensity, and SCD-specific catastrophizing would be associated with clinical pain. Additionally, we examined changes in situational catastrophizing elicited during laboratory pain testing to explore the potential effects of acute changes in catastrophizing on pain sensitivity in a chronic pain population. We hypothesized based on prior studies among healthy controls, that patients with SCD would demonstrate increased psychophysical pain sensitivity associated with increases in situational catastrophizing.
Methods
Participants
Eighty-one volunteers (57 female, 78 African American/Black, 3 Multiracial) with SCD participated in this study (Table 1) as part of an ongoing larger study on pain in SCD (additional data on these participants has been published elsewhere [2,5,22,23]). Patients were recruited from the Sickle Cell Center for Adults at Johns Hopkins Hospital as well as through advertisements. Interested volunteers were included if they were >18 years old, had a formal diagnosis of SCD (hemoglobinopathy genotype (Hb SS, Hb SC, Hb S/β-thalassemia)), and were on a stable dose (if any) of NSAIDs, acetaminophen, or opioids one month prior to pain testing. Exclusion criteria included current alcohol or substance abuse/dependence and significant psychological impairment that would preclude completion of study measures (e.g., dementia, cognitive impairment, unstable psychiatric illness). Participants were free of any major medical conditions other than SCD and none reported having other chronic pain.
Table 1.
Demographics
| N | 81 |
| Age | 38.57 (11.88) |
| Female | 70.37% |
| Highest Education | |
| high school or less | 18.52% |
| some college | 43.21% |
| bachelor's degree | 27.16% |
| graduate degree | 11.11% |
| Depression | 14.59 (10.83) |
| Neuroticism | 4.88(.78) |
Data are reported as Mean (SD) or Percent
Procedure
Pain testing sessions were scheduled on days when patients were experiencing SCD pain at the level of 5 or lower on a 0-10 pain rating scale and when they had not had a vaso-occlusive crisis in the past three weeks. First, informed written consent was obtained from each participant. After the consent process, participants completed the surveys described below, and a psychophysical pain testing battery lasting approximately one hour. Participants were allowed to stop or refuse any procedure at any time. This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Survey Measures
Catastrophizing
To allow for direct comparison of the three catastrophizing measures, scores are reported and analyzed as average, rather than summed, scores. To allow for comparison to previous research on dispositional catastrophizing, summed scores are also reported in Table 2.
Table 2.
Correlation among Catastrophizing Scales
| Catastrophizing Scale | Mean (SD) | Correlations, R | ||
|---|---|---|---|---|
| Summed | Average | Non-SCD | SCD-specific | |
| Non-SCD | 13.23 (10.24) | 1.00 (.78) | - | - |
| SCD-specific | 26.65 (13.39) | 2.05 (1.03) | .33** | - |
| Situational | n/a | .90 (.81) | .21† | .32** |
p≤.005
p≤.10.
Situational catastrophizing was calculated as an average across psychophysical procedures. To allow for direct comparison of situational catastrophizing with the other measures, analyses were conducted on average scores.
Non-Sickle Cell Disease Catastrophizing
Non-SCD catastrophizing was assessed using the Pain Catastrophizing Scale (PCS)[30] that measures trait-like exaggerated negative cognitive and affective responses to pain. The PCS consists of 13 items rated on a 5-point scale (0 - not at all to 4 - all the time) with higher scores indicating greater pain catastrophizing. In the current study, we asked patients to base their answers on painful experiences other than sickle cell pain (“Please answer based on how you feel regarding painful situations other than sickle cell pain”). Total score was calculated as the average of all responses. Similar to previous reports[3, 30], internal reliability of this measure was found in our sample (α = .92).
Sickle Cell Disease-Specific Catastrophizing
Motivated by patients’ self-disclosure of differential responses to pain in general and sickle cell pain, we modified the PCS such that patients were directed to respond to the 13 items based on their sickle cell pain specifically (“Please answer based on how you feel about your sickle cell pain”). All other aspects of the instructions and questionnaire were identical to the PCS. Total score was calculated as the average of all responses, and responses revealed high internal reliability (α = .93).
Situational Catastrophizing
The situational catastrophizing scale[3] was assessed at four points during the pain testing session: after heat and pressure pain thresholds, thermal temporal summation, mechanical temporal summation, and conditioned pain modulation with hot water. The situational catastrophizing scale consists of 6 items modified from the PCS (e.g., “I worried about when it would end”) and was scored on the same 5-point scale as the PCS. The situational catastrophizing scale assesses responses directly after administration of noxious stimulation and directs participants to reference these procedures while answering (...“please indicate the degree to which you had these thoughts and feelings during this pain-testing session”). A total score was calculated as the average of responses after each pain testing procedure for use in the primary analyses. Secondary analyses examining the relationship between situational catastrophizing at the end of one procedure on pain sensitivity during subsequent procedures were run using individual procedure-specific situational catastrophizing scores. Internal reliability (α) was high within the scale across modalities (α: HPTh/PPTh = .84, TTS = .93, MTS = .94, CPM = .94).
Clinical Pain Severity and Pain-related Interference
Clinical pain severity was calculated as the average of four self-reported pain ratings (i.e., current pain and worst, least, and average pain over the past week) using an 11-point scale (0 – No Pain to 10 – Pain as bad as it could be) (α = .87).
Clinical pain interference was assessed using the ten-item extended[18,32] Brief Pain Inventory (BPI)[8] pain interference subscale. This extended subscale assesses functional interference caused by pain during the past week in the areas of mood, sleep, relationships with others, and various daily activities and is also scored on an 11-point scale (0 – Does not interfere to 10 – Completely interferes) (α = .97).
Depression
Depressive symptomatology was assessed as a potential confounding covariate of catastrophizing, and was measured using the Center for Epidemiological Studies Depression Scale (CES-D)[25], which assesses frequency of 20 feelings and experiences during the past week on a 4-point scale (0 - rarely/less than one day to 3 - most of the time/5-7 days). Total score was calculated as the sum of responses_(α = .78). Though this screening measure is not intended as a diagnostic tool, a score of ≥16 corresponds to “clinically significant” depressive symptomatology.
Neuroticism
Due to its known association with pain catastrophizing in other populations [12, 30], neuroticism was also assessed as a potential confounding variable and was measured using the neuroticism subscale of the Big Five Inventory (BFI) [19]. The BFI assesses characteristic behaviors and emotions associated with five personality traits (including extraversion, conscientiousness, openness to experience, agreeableness, and neuroticism) by asking participants to rate the extent to which they believe each item is self-characteristic on a 5-point scale (1 – disagree strongly to 5 – agree strongly). The neuroticism subscale is calculated as the average of the eight neuroticism items on the BFI (α = .77).
Demographics
Participants also provided demographic information (including age, sex, race, and education) and completed a health history form.
Psychophysical Pain Testing
The pain testing procedures conducted here have been used extensively by our lab and others across chronic pain populations and have recently been demonstrated to be safe and effective in testing pain sensitivity among patients with SCD [5,10].
Pain Ratings
Pain ratings during each of the pain testing procedures were assessed using a numerical rating scale (NRS) ranging from 0 (no pain) to 100 (worst pain imaginable).
Thermal Stimuli
Heat stimuli were delivered to participants’ dominant ventral forearm using a Contact Heat-Evoked Potential Stimulator (CHEPS, Medoc Ltd., Ramat Yishai, Israel) system, a peltier-element-based stimulator with a 9 cm2 rapidly heating/cooling probe.
Heat Pain Threshold/Tolerance
Heat pain threshold (HPTh) and tolerance (HPTo) are reported in degrees Celsius and were calculated as the average of two corresponding trials administered using an ascending method of limits paradigm. On each trial, the contact thermode gradually increased in temperature, from a baseline of 30°C at a .5°C/second rate of increase, until the subject indicated via button press that the stimulus first felt painful (HPTh) or when the stimulus became intolerable (HPTo). Between trials, the thermode was moved up the arm slightly to avoid overlapping stimulation sites.
Pressure Pain Threshold
Pressure pain threshold was measured using an electronic algometer (SBmedic, Solna, Sweden) with a 1-cm2 probe covered with a 1-mm polypropylene material[17]. Pressure was applied to the muscle belly and increased steadily at a rate of 50kPa/sec until the subject verbally indicated the pressure first felt painful (PPTh). Thresholds were assessed twice at each of four body sites, bilaterally (trapezius muscle, interphalangeal joint of the thumb, the proximal third of the brachioradialis muscle (forearm), and middle of the quadriceps insertion point), for a total of 16 PPTh assessments. A minimum one minute interval was maintained between applications at the same site. An average PPTh was calculated for each site.
Thermal Temporal Summation
Ten repetitive thermal stimuli were applied rapidly in a series of identical pulses. Pain ratings were obtained for each pulse. The thermode remained in a fixed position during administration of each sequence of 10 heat pulses (0.5 sec each, with a 2.5-sec inter-pulse interval). A practice trial with pulses at participants’ warmth detection threshold was conducted to familiarize participants with the procedure. Experimental trials were conducted at tailored temperatures (HPTh and HPTh+2°C), and at a standard temperature of 45°C. The thermode was moved slightly between trials to avoid overlapping stimulation sites. Thermal temporal summation (TTS) was calculated as the difference between the maximum and first pain rating for each temperature. TTS after sensations were assessed 15 sec after the final stimulus at each temperature. An average TTS after sensation score was calculated across trials.
Mechanical Temporal Summation
Mechanical temporal summation (MTS) was calculated as the difference between pain ratings in response to a single punctuate stimulus compared to a sequence of ten identical punctuate stimuli. Weighted pinprick stimulators with a flat contact area of 0.2 mm diameter were used to deliver stimuli at a 1/sec rate to the middle phalange of the middle finger. A practice trial was conducted with a stimulator that produced 32mN force. Experimental trials were conducted at 128mN and 256mN.
Conditioned Pain Modulation and Hot Water Procedures
Conditioned pain modulation (CPM) was assessed using pressure applied to the trapezius as the test stimulus, and hot water bath as the conditioning stimulus. First, PPTh was again assessed (separate from PPTh above) twice at the non-dominant trapezius. The dominant hand was then submerged in a hot water bath for 20 seconds, at which time PPTh was reassessed (immediately before hand removal). If participants removed their hands before 20 seconds, PPTh was assessed immediately upon withdrawal. CPM was calculated as the difference between the PPThs during and before water submersion. This procedure was repeated a second time, and final scores reflect an average of both trials.
The hot water temperature used for CPM was determined early in the pain testing session as the temperature at which patients rate their pain as a 60-70 out of 100 after 20 seconds of hand submersion.
Hot water after sensations were assessed at 30 sec and 1 min after hand withdrawal. Final scores were calculated as an average of after sensations at both time points.
Order of Testing and Catastrophizing Measurements
Heat pain Threshold/Tolerance and Pressure Pain Thresholds were randomized, but always conducted at the beginning of the Psychophysical Pain Testing session, followed by either Mechanical or Thermal Temporal Summation (also randomized), with Conditioned Pain Modulation/Hot Water Procedures occurring last. Situational Catastrophizing was measured following thresholds (once following the thermal and pressure threshold testing), once following each of the temporal summation series (which were organized into the proper temporal order for cross-lag panel analyses purposes), and once following the CPM/Hot Water procedures.
Data Reduction
In order to reduce the number of comparisons examined, laboratory pain was quantified using two a priori defined composite summary scores: a central sensitization index and a QST (quantitative sensory testing) index. Category determination was based on previous work [1, 5, 14, 34] and was confirmed using factor analysis. Z-scores were first created for each variable and reverse scored where appropriate (by multiplying by - 1) such that higher scores correspond to greater pain sensitivity.
Central Sensitization Index
The central sensitization index was calculated as the average of the following seven individually z-scored values: MTS (128mN and 256mN), TTS (HPTh, HPTh+2°C, and 45°C), and the after sensations to TTS and hot water. Reliability analysis indicated internal consistency of this index (α = .74).
QST Index
The QST index was calculated as the average of the remaining psychophysical pain measures not included in the central sensitization index (i.e., heat and pressure thresholds, heat pain tolerance, hot water temperature, hot water pain rating and withdrawal time, and CPM). Reliability analysis indicated internal consistency of this index (α = .75).
Data Analysis
All data analyses were conducted using SPSS (version 21, Armonk, NY: IBM Corp.).
Correlation and Multivariate Regression Analyses
First, the relationship between each of the three catastrophizing measures and age, sex, education, depression and neuroticism were assessed. Second, intercorrelations among the three catastrophizing variables were measured. Third, the univariate relationships between all three catastrophizing measures and clinical and psychophysical pain responses were evaluated. Fourth, we investigated whether significant catastrophizing-pain relationships survived correction for covariates using multivariate hierarchical regression models. Demographics (e.g., age, sex, education) were entered in the first step, depression and/or neuroticism (if correlated) in the following step(s), and the correlated catastrophizing measure in the final step of the model. We chose the conservative approach of including correlated covariates for any catastrophizing measure in all models so as to not differentially parse the variance across models.
Cross-lagged Panel Analyses
Finally, we further interrogated the situational catastrophizing data to examine whether or not situational catastrophizing during one psychophysical pain testing procedure was associated with increased pain response to a subsequent procedure. We used a cross-lagged panel analysis design[6] in which standardized residualized change scores were calculated as an index of change between psychophysical tests. Situational catastrophizing was collected first after heat and pressure threshold (T1), next after either TTS or MTS (T2) which were presented in randomized order, then the remaining TS procedure (T3), and finally after CPM (T4). Average psychophysical pain sensitivity was calculated for each of these time points (e.g., T1 pain was calculated as the average standardized pain threshold for heat pain threshold as well as pressure pain threshold at all sites). These four time-points were the only ones assessed in the cross-lagged panel analysis. Hierarchical regression was used to determine whether changes in situational catastrophizing (e.g., between T1 and T2) predicted subsequent changes in psychophysical pain sensitivity (e.g., between T2 and T3) controlling for autocorrelations (e.g., correlations with changes in situational catastrophizing at different time points) and synchronous correlations (e.g., correlations with change in pain during the same time period). The reverse relationship examining whether changes in pain sensitivity precede changes in catastrophizing was also examined. This resulted in four models predicting the change in pain or situational catastrophizing between T2-T3 and T3-T4.
Missing Data
Participants were not excluded due to partially missing data and the majority of participants (n = 73; 90%) completed every component of all procedures. Missing psychophysical data are due to either participant choice to discontinue a certain procedure, or participant rating the maximum (100) before completion of a procedure. Composite scores were calculated using all available data for each participant. Missing values were left missing and not imputed. The central sensitization score could not be calculated for one participant (n=80), however, the QST index was calculated for all participants (n=81). Missing survey data values were due to participants leaving the questionnaire blank. All participants (n=81) completed the non-SCD catastrophizing questionnaire, 78 completed the SCD-specific questionnaire, and 80 completed situational questionnaires.
Results
Non-SCD catastrophizing was negatively associated with patient education (R = −.26, p = .02), positively associated with depression (R = .27, p = .02), and a sex-difference was found such that male patients reported greater non-SCD catastrophizing (M = 1.34, SD = .89) compared to females (M = .88, SD = .71, t(79) = 2.43, p = .02). Situational catastrophizing was significantly (R = .25, p = .03), and SCD-specific catastrophizing marginally (R = .20, p = .09), associated with depression, but neither was related to demographic variables. None of the catastrophizing measures were associated with patient age or neuroticism. Thus, sex, education and depression were used as covariates in subsequent multivariate models.
Participant responses to the three catastrophizing measures differed, suggesting these measures are at least partially independent (Table 2). Patients reported greater SCD-specific catastrophizing (M = 2.05, SD = 1.03) compared to non-SCD catastrophizing (M = 1.00, SD = .78, t(77) = 8.70, p <.001) and situational catastrophizing (M = .90, SD = .81, t(76) = 9.14, p <.001). SCD-specific catastrophizing significantly correlated with both non-SCD and situational catastrophizing. Situational and non-SCD catastrophizing were not significantly different in this sample and were marginally correlated.
Non-SCD and SCD-specific catastrophizing scores were significantly correlated with clinical pain severity and interference, but not the QST Index or the Central Sensitivity Index (Table 3). Situational catastrophizing was significantly correlated with both psychophysical pain indices as well as clinical pain severity and was marginally associated with clinical pain interference.
Table 3.
Relationship (R) between Catastrophizing and Pain
| Catastrophizing Scale | ||||
|---|---|---|---|---|
| Non-SCD | SCD-specific | Situational | ||
| Psychophysical Pain Indices | Central Sensitization | NS | NS | .62** |
| QST | NS | NS | .40** | |
| Clinical Pain Ratings | Severity | .42** | .34** | .25* |
| Interference | .39** | .37** | .20† | |
p≤.005
p≤.05
p≤.10
NS, p>.10.
Hierarchical multiple regression (Table 4) revealed that situational catastrophizing remained a significant predictor of both psychophysical pain indices even after controlling for education, sex, and depression. Situational catastrophizing was not associated with either clinical pain or pain interference when demographics and depression were covaried. SCD-specific catastrophizing remained marginally associated with clinical pain severity and significantly associated with clinical pain interference, even after controlling for the effects of education, sex, and depression. Non-SCD catastrophizing remained significantly associated with clinical pain severity and interference in these models.
Table 4.
Hierarchical Regression Models
| Central Sensitization Index | B | SE | β | t | R2 | Δ R2 |
|---|---|---|---|---|---|---|
| Step 1: Demographics | .05 | .05 | ||||
| Sex | .10 | .15 | .08 | .66 | ||
| Education | −.09 | .05 | −.21† | −1.83 | ||
| Step 2: Depression | .01 | .01 | .18 | 1.55 | .08 | .03 |
| Step 3: Situational Catastrophizing | .47 | .07 | .63* | 6.66 | .44* | .36* |
|
QST Index | ||||||
| Step 1: Demographics | .09* | .09* | ||||
| Sex | .22 | .13 | .20† | 1.76 | ||
| Education | −.09 | .04 | −.25* | −2.22 | ||
| Step 2: Depression | −.01 | .01 | −.14 | −1.18 | .11* | .02 |
| Step 3: Situational Catastrophizing | .32 | .07 | .47* | 4.54 | .31* | .20* |
|
Clinical Pain Severity | ||||||
| Step 1: Demographics | .02 | .02 | ||||
| Sex | .03 | .94 | .01 | .07 | ||
| Education | −.16 | .15 | −.13 | −1.06 | ||
| Step 2: Depression | .07 | .02 | .44* | 3.95 | .19* | .18* |
| Step 3a: Non-SCD Catastrophizing | .61 | .27 | .26* | 2.24 | .25* | .05* |
| Step 3b: SCD-specific Catastrophizing | .35 | .19 | .21† | 1.87 | .23* | .04† |
| Step 3c: Situational Catastrophizing | .28 | .24 | .13 | 1.13 | .21* | .02 |
|
Clinical Pain Interference | ||||||
| Step 1: Demographics | .02 | .02 | ||||
| Sex | −.36 | .63 | −.07 | −.57 | ||
| Education | −.23 | .21 | −.13 | −1.10 | ||
| Step 2: Depression | .12 | .02 | .50* | 4.71 | .26* | .23* |
| Step 3a: Non-SCD Catastrophizing | .73 | .37 | .22* | 1.97 | .30* | .04* |
| Step 3b: SCD-specific Catastrophizing | .56 | .25 | .23* | 2.22 | .30* | .05* |
| Step 3c: Situational Catastrophizing | .20 | .33 | .07 | .60 | .26* | <.01 |
p≤.05
p≤.10.
Statistics are presented in sequential fashion, such that the first step includes coefficients when only step 1 is executed. Subsequent steps show adjusted coefficients controlling for the predictors entered previous steps. As all three catastrophizing scales were at least marginally associated with clinical pain outcomes, individual models entering a different scale in step 3 were compared. Multiple catastrophizing measures were not entered into the same model. B, unstandardized coefficient; β, standardized beta coefficient
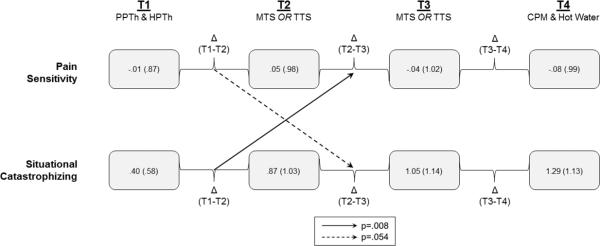
Results from the cross-lagged panel analyses (Table 5) revealed that increases in situational catastrophizing during the first period (T1-T2) were positively associated with increases in pain sensitivity during the second period (T2-T3). However, change in situational catastrophizing during the second period did not significantly predict change in pain sensitivity during the third period (T3-T4). The reverse model revealed that increased pain during the first period also showed a tendency toward predicting later change in situational catastrophizing during the second period. Changes during the second period did not predict changes during the third. These effects remained when depression was controlled for in the models.
Table 5.
Cross-lagged Regression Models
| Δ Pain Sensitivity: Second Period (T2-T3) | B | SE | B | t | R2 | Δ R2 |
|---|---|---|---|---|---|---|
| Step 1: Controlling for auto- and synchronous correlations | .27* | .27* | ||||
| Δ Pain Sensitivity (T1-T2) | −.09 | .10 | −.09 | −.93 | ||
| Δ Situational Catastrophizing (T2-T3) | .50 | .10 | .52* | 5.16 | ||
| Step 2: Δ Situational Catastrophizing (T1-T2) | .29 | .10 | .27* | 2.75 | .34* | .07* |
|
Δ Pain Sensitivity: Third Period (T3-T4) | ||||||
| Step 1: Controlling for auto- and synchronous correlations | .21* | .21* | ||||
| Δ Pain Sensitivity (T2-T3) | −.07 | .11 | −.07 | −.66 | ||
| Δ Situational Catastrophizing (T3-T4) | .49 | .12 | .44* | 4.18 | ||
| Step 2: Δ Situational Catastrophizing (T2-T3) | .11 | .12 | .12 | .95 | .22* | .01 |
|
Δ Situational Catastrophizing: Second Period (T2-T3) | ||||||
| Step 1: Controlling for auto- and synchronous correlations | .30* | .30* | ||||
| Δ Situational Catastrophizing (T1-T2) | −.22 | .11 | −.20* | −2.01 | ||
| Δ Pain Sensitivity (T2-T3) | .57 | .10 | .55* | 5.48 | ||
| Step 2: Δ Pain Sensitivity (T1-T2) | .20 | .10 | .19† | 1.96 | .33* | .04† |
|
Δ Situational Catastrophizing: Third Period (T3-T4) | ||||||
| Step 1: Controlling for auto- and synchronous correlations | .24* | .24* | ||||
| Δ Situational Catastrophizing (T2-T3) | −.15 | .09 | −.17 | −1.68 | ||
| Δ Pain Sensitivity (T3-T4) | .41 | .09 | .45 | 4.38 | ||
| Step 2: Δ Pain Sensitivity (T2-T3) | −.03 | .11 | −.03 | −.23 | .24* | <.01 |
p≤.05
p=.54.
Statistics are presented in sequential fashion, such that the first step includes coefficients when only step 1 is executed. Subsequent steps show adjusted coefficients controlling for the predictors entered in previous steps. B, unstandardized coefficient; β, standardized beta coefficient. Time points of situational catastrophizing assessment within the psychophysical pain testing session are indicated by T1, T2, T3, and T4. T1: Pressure and thermal pain threshold testing, T2: either thermal or mechanical temporal summation (whichever was presented first – randomized across participants), T3: the remaining temporal summation modality, T4: conditioned pain modulation
Discussion
Here we demonstrate that catastrophizing in sickle cell disease (SCD) varies across pain contexts, with patients reporting greater catastrophizing about SCD pain compared to general non-SCD pain experiences and laboratory pain. Both disease-specific and general non-disease-related catastrophizing are associated with clinical pain outcomes, whereas situational catastrophizing is associated with sensitization to laboratory pain. Furthermore, our results suggest that situational catastrophizing and pain sensitivity may mutually facilitate one another in SCD, such that increases in catastrophizing predict later increases in pain sensitivity and early increases in pain sensitivity predict slight (marginal) increases in catastrophizing.
While SCD patients’ non-SCD catastrophizing was similar to, or lower than, average PCS scores in other chronic pain populations (e.g., [3,33]), SCD-specific catastrophizing scores were higher than PCS averages. This suggests that prior findings that SCD patients tend to catastrophize more than other chronic pain patients [7] may be driven by a number of disease-related factors that deserve future investigation. The nature of SCD pain as related to a life-long and life-threatening disease may promote disease-related catastrophizing. Furthermore, the severe episodic and chronic pain experienced by SCD patients is often undertreated [29], which reasonably may further exacerbate SCD-specific pain rumination, magnification, and helplessness. One consideration for future studies is that disease-specific pain may be more prevalent and salient, and therefore may lead to greater disease-specific catastrophizing. Future studies are also needed to tease apart growing evidence of greater catastrophizing in SCD patients from demonstrated racial differences in catastrophizing [11].
Despite differential patterns of SCD-specific and non-SCD pain catastrophizing responses, both measures were associated with clinical pain severity and interference. This may reflect the “trait” component of catastrophizing that has been conceptualized as not being disease-specific but a general response to many types of pain [26,30,31]. The pattern of findings suggests that catastrophic cognitions in this population are not isolated to disease-related pain, and that such cognitions in response to pain unrelated to the disease may in turn be associated with worsened disease-related pain and the impact of pain on daily activities. The relationships between SCD-specific and non-SCD catastrophizing with clinical pain severity and pain interference largely remained even after controlling for known covariates of pain-related catastrophizing including sex, education, and depression (though the SCD-specific catastrophizing/pain severity correlation was marginal). Depression is common in SCD, and is associated with chronic pain [21] and catastrophizing [7]. Citero et al. [7] found that the relationship between general catastrophizing and daily pain in SCD was not significant when controlling for depression. Here, though we find a relationship between depression and our three catastrophizing measures, we find that the association between catastrophizing and pain is independent of the effects of depression. Important differences between this and the prior study are the instruments used to assess the primary outcomes of interest: catastrophizing and pain. Citero and colleagues (2007) [7] used the catastrophizing subscale of the Coping Strategies Questionnaire (CSQ) and we used a slightly modified version of the PCS (referencing non-SCD pain specifically). Though the CSQ-catastrophizing subscale and the PCS are correlated [15], the CSQ scale includes only the helplessness dimension of pain catastrophizing, whereas the PCS added the components of pain magnification and rumination [31]. Additionally, pain assessment differed between studies (multi-item assessment cross-sectionally vs. single-item assessment using daily diaries). Both studies confirm the significance of depressive symptoms and our finding suggests that continued investigation of the role of pain catastrophizing is warranted, particularly as rumination and/or magnification may be important components for sickle cell patients.
Our findings further demonstrate that situational catastrophizing is strongly associated with indices of central sensitization and laboratory pain in SCD, but not clinical pain severity or interference after controlling for sex, education and depression. This pattern suggests that acute catastrophic cognitions may enhance pain sensitivity. Indeed, cross-lagged panel analyses demonstrate changes in situational catastrophizing were associated with subsequent changes in laboratory pain sensitivity, a finding our group has previously demonstrated in healthy controls [6]. Changes in catastrophizing precede heightened pain sensitivity. However, unlike our findings among healthy controls, these results in SCD suggest that there is a possible bidirectional relationship between situational catastrophizing and pain. Early changes in psychophysical pain sensitivity were associated with marginal increases in subsequent situational catastrophizing, suggesting that situational catastrophizing and pain sensitivity may mutually facilitate one another in SCD. Future work is needed to further explore the temporal dynamics of this relationship. If a mutually facilitatory relationship between situational catastrophizing and pain sensitization exists in SCD, catastrophizing may be especially harmful in this population, triggering a pattern of ever increasing sensitization. Furthermore, in SCD, this pattern may be particularly important for understanding the longitudinal effects of episodic crisis pain. Over years, pain-related catastrophizing may lower the individual patient's threshold for crisis and/or increase the frequency or severity of crises.
Limitations of this study include that both measures of trait-like catastrophizing (SCD-specific and non-SCD) and clinical pain reports were cross-sectional, limiting causal inferences. We also do not have qualitative data on the specific aspects of these types of pain that patients considered in their ratings. Additionally, in creating the SCD-specific measure, we slightly altered the commonly used dispositional catastrophizing measure to be specific to only non-SCD pain. Though the development of the SCD-specific measure was motivated by spontaneous patient reports, our separation of these two targets (SCD pain and non-SCD pain) may encourage separation of these constructs by patients, and should be considered when employing with other patient groups. It is likely that patients combine both disease specific and non-disease pain as targets when responding to the standard PCS. This assumption is supported by a recent finding that healthy research participants refer to various types of pain experiences when completing the PCS [20]. Importantly, the context of the pain referent influenced PCS scores. Future studies are needed to determine whether providing specific referents for catastrophizing measures captures the various types of pain spontaneously referenced by patients, as well as to further explore the utility of these more specific catastrophizing measures in better predicting specific aspects and contexts of pain. Finally, our cross-lagged analysis of the temporal relationship between situational catastrophizing and laboratory pain sensitivity involved sensitivity to four different types of pain. The strength of this analysis is that it provides insight into how catastrophizing may influence pain responding, and vice versa, in SCD; however this analysis is limited by available data and the fact that pain modality and assessment varied across procedures. Future research is needed to probe specific mechanisms of sensitization and their temporal aspects that may be influenced by pain catastrophizing.
Taken together, the current findings suggest 1) that sickle cell patients catastrophize most about their SCD pain rather than demonstrating greater catastrophic thinking about pain in general, but that 2) catastrophizing across pain contexts - even about pain not related to SCD – may predict greater clinical pain severity and interference and that 3) greater situational catastrophizing may both influence and be influenced by greater sensitization to pain. Our findings suggest, somewhat contrary to early research on the topic, that catastrophizing may play an important role in the severity and exacerbation of sickle cell disease pain, as well as its impact on daily life.
Figure 1. Cross-lagged Panel Analysis.
Mean (SD). Time points of assessment within the psychophysical pain testing session are indicated by T1, T2, T3, and T4. Pain sensitivity scores are reported as averaged Z-scores to allow for comparison across pain tests. Specifically, the Z-scored PPTh and Z-scored HPTh were averaged together to create T1. The Z-scored temporal summation index (max or peak minus 1st rating) was used for T2 from either MTS or TTS, whichever was randomized first. MTS or TTS that was completed second, was termed T3. The Z-scored CPM and hot water ratings were averaged to create T4. Pain catastrophizing scores are mean values reported by patients after corresponding pain tests. A solid arrow depicts a significant relationship, and a dashed arrow a marginal relationship, after controlling for auto- and synchronous correlations. PPTh: Pressure Pain Threshold; HPTh: Heat Pain Threshold; MTS: Mechanical Temporal Summation; TTS: Thermal Temporal Summation; CPM: Conditioned Pain Modulation.
Perspective.
Patients with sickle cell disease report greater catastrophizing about sickle cell specific pain relative to other pains. Disease-specific and non-disease related pain catastrophizing were associated with clinical pain, and situational catastrophizing predictive of subsequent laboratory pain. Evaluation of context-specific catastrophizing may more accurately predict different aspects of the pain experience.
Highlights.
Catastrophizing (CAT) was assessed referencing three different pain contexts in SCD
SCD-specific CAT was greater than CAT about non-SCD or laboratory pain
SCD-specific and non-SCD CAT were associated with clinical pain
Situational CAT was associated with central sensitization markers & laboratory pain
Changes in situational CAT predicted later changes in laboratory pain sensitivity
Acknowledgments
This research was supported by the National Institutes of Health R01HL98110 (J.A.H.) and T32 NS070201 (V.A.M.). S.L has received research funds unrelated to this project from Pfizer, Selexys, Prolong, and Astra Zeneca and is a consultant for Apopharma and Global Blood Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All other authors declare that they have no conflicts of interest.
References
- 1.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, Robinson M, Edwards RR, Smith MT. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015;67:1387–96. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell CM, Carroll CP, Kiley K, Han D, Haywood C, Jr, Lanzkron S, Swedberg L, Edwards RR, Page GG, Haythornthwaite JA. Quantitative sensory testing and pain-evoked cytokine reactivity: Comparison of patients with sickle cell disease to healthy matched controls. Pain. 2016;157:949–956. doi: 10.1097/j.pain.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell CM, Kronfli T, Buenaver LF, Smith MT, Berna C, Haythornthwaite JA, Edwards RR. Situational versus dispositional measurement of catastrophizing: associations with pain responses in multiple samples. J Pain. 2010;11:443–453. doi: 10.1016/j.jpain.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell CM, McCauley L, Bounds SC, Mathur VA, Conn L, Simango M, Edwards RR, Fontaine KR. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res Ther. 2012;14:R231. doi: 10.1186/ar4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell CM, Moscou-Jackson G, Carroll CP, Kiley K, Haywood C, Lanzkron S, Hand M, Edwards RR, Haythornthwaite JA. An Evaluation of Central Sensitization in Patients with Sickle Cell Disease. J Pain. 2016;17:617–627. doi: 10.1016/j.jpain.2016.01.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell CM, Quartana PJ, Buenaver LF, Haythornthwaite JA, Edwards RR. Changes in situation-specific pain catastrophizing precede changes in pain report during capsaicin pain: a cross-lagged panel analysis among healthy, pain-free participants. J Pain. 2010;11:876–884. doi: 10.1016/j.jpain.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Citero VdA, Levenson JL, McClish DK, Bovbjerg VE, Cole PL, Dahman BA, Penberthy LT, Aisiku IP, Roseff SD, Smith WR. The role of catastrophizing in sickle cell disease–The PiSCES project. PAIN. 2007;133:39–46. doi: 10.1016/j.pain.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Cleeland C, Ryan K. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed] [Google Scholar]
- 9.Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol. 2014;93:89–95. doi: 10.1111/ejh.12340. [DOI] [PubMed] [Google Scholar]
- 10.Ezenwa MO, Molokie RE, Wang ZJ, Yao Y, Suarez ML, Pullum C, Schlaeger JM, Fillingim RB, Wilkie DJ. Safety and Utility of Quantitative Sensory Testing among Adults with Sickle Cell Disease: Indicators of Neuropathic Pain? Pain Practice. 2016;16:282–293. doi: 10.1111/papr.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsythe LP, Thorn B, Day M, Shelby G. Race and sex differences in primary appraisals, catastrophizing, and experimental pain outcomes. J Pain. 2011;12:563–572. doi: 10.1016/j.jpain.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Goubert L, Crombez G, Van Damme S. The role of neuroticism, pain catastrophizing and pain-related fear in vigilance to pain: a structural equations approach. Pain. 2004;107:234–41. doi: 10.1016/j.pain.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Grosen K, Vase L, Pilegaard HK, Pfeiffer-Jensen M, Drewes AM. Conditioned pain modulation and situational pain catastrophizing as preoperative predictors of pain following chest wall surgery: a prospective observational cohort study. PloS one. 2014;9:e90185. doi: 10.1371/journal.pone.0090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hastie BA, Riley JL, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–37. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh AT, George SZ, Bialosky JE, Robinson ME. Fear of pain, pain catastrophizing, and acute pain perception: relative prediction and timing of assessment. J Pain. 2008;9:806–812. doi: 10.1016/j.jpain.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollins M, Stonerock GL, Kisaalita NR, Jones S, Orringer E, Gil KM. Detecting the emergence of chronic pain in sickle cell disease. J Pain Symptom Manage. 2012;43:1082–1093. doi: 10.1016/j.jpainsymman.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen K, Andersen HØ, Olesen J, Lindblom U. Pressure-pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain. 1986;25:313–323. doi: 10.1016/0304-3959(86)90235-6. [DOI] [PubMed] [Google Scholar]
- 18.Jensen MP, Ehde DM, Hoffman AJ, Patterson DR, Czerniecki JM, Robinson LR. Cognitions, coping and social environment predict adjustment to phantom limb pain. Pain. 2002;95:133–142. doi: 10.1016/s0304-3959(01)00390-6. [DOI] [PubMed] [Google Scholar]
- 19.John OP, Srivastava S. The Big Five trait taxonomy: History, measurement, and theoretical perspectives. Handbook of personality: Theory and research. 1999;2:102–138. [Google Scholar]
- 20.Kapoor S, Thorn B, Bandy O, Clements K. Pain referents used to respond to the Pain Catastrophizing Scale. European Journal of Pain. 2015;19:400–407. doi: 10.1002/ejp.561. [DOI] [PubMed] [Google Scholar]
- 21.Levenson JL, McClish DK, Dahman BA, Bovbjerg VE, de A Citero V, Penberthy LT, Aisiku IP, Roberts JD, Roseff SD, Smith WR. Depression and anxiety in adults with sickle cell disease: the PiSCES project. Psychosom Med. 2008;70:192–196. doi: 10.1097/PSY.0b013e31815ff5c5. [DOI] [PubMed] [Google Scholar]
- 22.Mathur VA, Kiley KB, Haywood C, Jr, Bediako SM, Lanzkron S, Carroll CP, Buenaver LF, Pejsa M, Edwards RR, Haythornthwaite JA, Campbell CM. Multiple Levels of Suffering: Discrimination in Health-Care Settings is Associated With Enhanced Laboratory Pain Sensitivity in Sickle Cell Disease. Clin J Pain. 2016 doi: 10.1097/AJP.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moscou-Jackson G, Finan PH, Campbell CM, Smyth JM, Haythornthwaite JA. The Effect of Sleep Continuity on Pain in Adults with Sickle Cell Disease. J Pain. 2015;16:587–593. doi: 10.1016/j.jpain.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert review of neurotherapeutics. 2009;9:745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1:385–401. [Google Scholar]
- 26.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 27.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524. [Google Scholar]
- 31.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Tyler EJ, Jensen MP, Engel JM, Schwartz L. The reliability and validity of pain interference measures in persons with cerebral palsy. Arch Phys Med Rehabil. 2002;83:236–239. doi: 10.1053/apmr.2002.27466. [DOI] [PubMed] [Google Scholar]
- 33.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96:319–324. doi: 10.1016/S0304-3959(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 34.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]