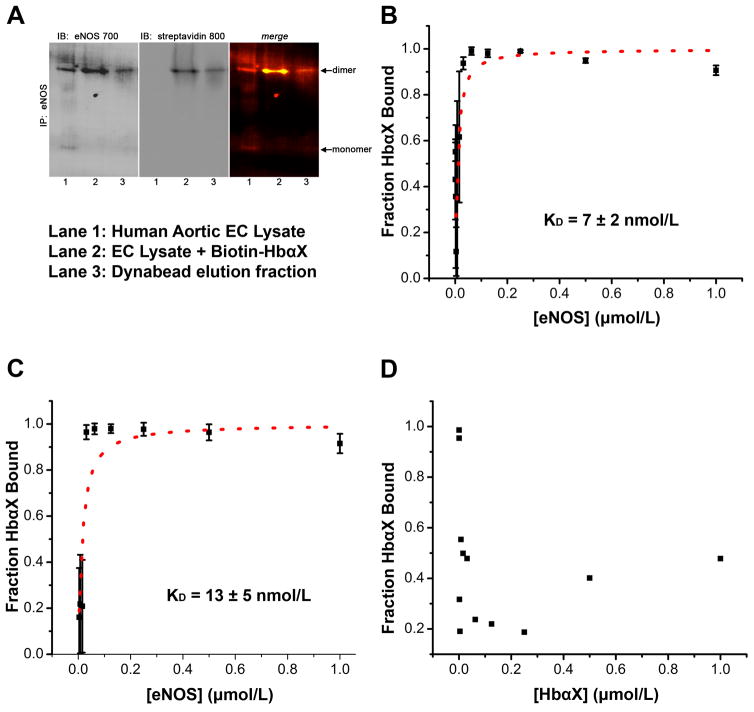
Figure 4. An alpha globin mimetic peptide and eNOS dimers bind with high affinity.
(A) Co-immunoprecipitation of eNOS and biotin-labeled HbαX from hAoECs shows that HbαX binds to the dimeric form of eNOS. Non-denaturing IP and electrophoresis conditions were used to preserve the conformational state of eNOS. Lane 1 shows EC lysate as a control for eNOS species. Lane 2 is from beads incubated with EC Lysate and biotin-conjugated HbαΧ peptide. Lane 3 is sample eluted from dynabeads with low pH. (B) Fluorescence polarization assays show nanomolar binding affinity between fluorescein isothiocyanate (FITC) – labeled HbαX and the oxygenase domain of eNOS. The concentration of FITC-HbαX is 5 nmol/L in (B). The total concentration of peptide in (C) was held constant, but only half (2.5 nmol/L) was fluorescently tagged. This effectively will double the observed binding affinity because of the 1:1 competition for binding site. (D) shows no curve because no binding was observed between HbαX peptides. The concentration of labeled peptide was held constant (5 nmol/L) and was used to serially dilute unlabeled HbαX. In B–D, the points represent mean and bars indicate standard deviation, samples were run in triplicate in one experimental preparation.