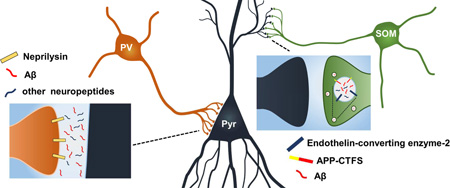
Abstract
Impaired clearance of amyloid-β peptide (Aβ) has been postulated to significantly contribute to the amyloid accumulation typical of Alzheimer’s disease. Among the enzymes known to degrade Aβ in vivo are endothelin-converting enzyme (ECE)-1, ECE-2, and neprilysin, and evidence suggests that they regulate independent pools of Aβ that may be functionally significant. To better understand the differential regulation of Aβ concentration by its physiological degrading enzymes, we characterized the cell and region-specific expression pattern of ECE-1, ECE-2, and neprilysin by in situ hybridization and immunohistochemistry in brain areas relevant to Alzheimer’s disease. In contrast to the broader distribution of ECE-1, ECE-2 and neprilysin were found enriched in GABAergic neurons. ECE-2 was majorly expressed by somatostatin-expressing interneurons and was active in isolated synaptosomes. Neprilysin mRNA was found mainly in parvalbumin-expressing interneurons, with neprilysin protein localized to perisomatic parvalbuminergic synapses. The identification of somatostatinergic and parvalbuminergic synapses as hubs for Aβ degradation is consistent with the possibility that Aβ may have a physiological function related to the regulation of inhibitory signaling.
Keywords: Alzheimer’s disease, Aβ degradation, endothelin-converting enzyme, GABA, interneuron, neprilysin, somatostatin, parvalbumin
Graphical abstract
1. Introduction
It is widely believed that impaired clearance of amyloid-β peptide (Aβ) contributes significantly to the abnormal accumulation and aggregation of Aβ characteristic of Alzheimer’s disease (AD) (Selkoe and Hardy, 2016). As a product of the physiological processing of amyloid-precursor protein (APP) by β- and γ-secretases, Aβ is produced continually throughout the lifespan and its concentration is tightly controlled by the activities of several proteases (Baranello, et al., 2015). The half-life of the peptide in brain is between 0.5 and 3 hours (Basak, et al., 2012) and disruption of the activities of Aβ degrading proteases through pharmacological inhibition or genetic inactivation results in increased steady-state levels of endogenous Aβ in the brains of mice (Pacheco-Quinto, et al., 2013). This complex regulation of both Aβ production and degradation strongly supports a key physiological function for the peptide and mounting evidence indicates that Aβ is important for synaptic plasticity and cognition (reviewed in (Puzzo, et al., 2015). The enzymes responsible for degrading Aβ could therefore be viewed as regulators of normal Aβ function and would be expected to concentrate in the microenvironments where Aβ fulfills its function. Therefore, establishing the specific cell-type distribution of these enzymes in the brain may yield insights on Aβ’s physiological role.
Among the enzymes known to degrade Aβ are several members of the M13 family of type II integral membrane zinc metalloproteases, including endothelin-converting enzyme (ECE)-1, ECE-2, neprilysin (NEP) and NEP-2 (reviewed in(Saido and Leissring, 2012). Mice deficient in each of these enzymes have elevated Aβ levels, and though they are highly related, the activities of the ECEs and NEP are unable to compensate for one another’s loss of function, suggesting that they regulate independent pools of Aβ (Eckman, et al., 2006). The apparent compartmentalization of Aβ degradation could be a reflection of the different subcellular localization of Aβ degrading enzymes. For instance, NEP localizes mainly to the plasma membrane, has a neutral pH optimum, and degrades Aβ in the extracellular space (Iwata, et al., 2000,Shirotani, et al., 2001) while ECEs are active in acidic Aβ-producing intracellular compartments, either in early endosomes before secretion or in the endosomal/lysosomal pathway (Eckman, et al., 2001,Pacheco-Quinto and Eckman, 2013). This compartmentalization of Aβ catabolism may also result from differences in the expression pattern of the enzymes. Therefore, to better understand the non-overlapping roles of the ECEs and NEP in regulating physiological Aβ concentration and preventing its aggregation in vivo, we characterized the cell and region-specific expression pattern of Ece1, Ece2, and Mme (neprilysin, also known as membrane metallo-endopeptidase) in brain areas relevant to AD.
2. Materials and methods
2.1 Mice
Mice were housed in ventilated micro-isolator cages with free access to food and water and were maintained at 25 °C with a 12/12 hour light/dark cycle. Three-month-old B6C3F1 mice (strain code 031, Charles River Laboratories) and TgCRND8 mice (kindly gifted by Dr. Paul Fraser, University of Toronto) were euthanized by CO2 asphyxiation as approved by the Institutional Animal Care and Use Committee at Rutgers University and consistent with AVMA guidelines.
2.2 Tissue processing
Immediately after euthanasia, brains were quickly dissected and frozen on dry ice or immersed in 10% neutral buffered formalin for 24 h and then dehydrated and embedded in paraffin blocks. Coronal sections, 5 µm thick, were air dried overnight, baked for 60 min at 60° C and stored at −20 °C until use.
2.3 Fluorescence in situ hybridization
ViewRNA™ dual ISH from Affymetrix (Santa Clara, CA, USA) was performed according to the manufacturer’s instructions with a 10 minute boil at 95 °C in pretreatment solution and 20 minute incubation with protease solution. The probe sets used for the study were Sst (NM_009215, region covered 20–549), Mme (NM_008604; region covered 114–1082), Ece-1 (NM_199307, region covered 1200–2161), Ece-2 (NM_177941, region covered 1501–2413), Gad1 (NM_008077, region covered 154–2441), Gfap (NM_010277, region covered 23–1144), and Gapd (NM_008084, region covered 109–1028)). The ISH probesets used to detect Mme, Ece1, and Ece2 are expected to bind to all known transcript variants, with the exception of Ece2 variant 3 (NM_025462.2), which lacks a protease domain and was deliberately excluded from the study. Nuclei were visualized with the fluorescent dye Hoechst 33258 (AnaSpec, Fremont, CA, USA). Specificity of Ece2 and Mme probesets was assessed using archived tissue from ECE-2 knockout mice (Yanagisawa, et al., 2000) and NEP knockout mice (Lu, et al., 1995), and their wild-type littermates (Supplementary Figure 1).
2.4 Immunohistochemistry
Tissue sections were blocked with 5% BSA containing 0.1% triton X-100 and then incubated sequentially with primary antibodies and AlexaFluor conjugated secondary antibodies (Invitrogen, Carlsbad, CA). Primary antibodies used included: rat monoclonal anti-somatostatin (1:100, Millipore MAB354), rabbit polyclonal anti-parvalbumin (1:1000, Abcam ab11427), rat monoclonal anti-neprilysin (1:100, R&D Systems mAB1126), mouse monoclonal anti-CD31/PECAM1 (Novus Biologicals NB100-64796), and mouse monoclonal anti-GFAP (1:100, Novus Biologicals NBP1-05197). Immunofluorescence was visualized with a Zeiss Axio Imager Z1 fluorescent microscope. For combined in situ hybridization and immunohistochemistry, immunodetection was conducted after in situ hybridization was completed.
2.5 Synaptosome preparation
Following a protocol developed by Dunkley et al. (Dunkley, et al., 2008) mouse brains were homogenized with ten strokes of a Dounce homogenizer in 0.32 mM sucrose, 5 mM Tris pH 7.4, 25 µM DTT containing 1× protease inhibitor cocktail without EDTA (Thermo Scientific), at a concentration of 20 mg/ml (w/v). Samples were centrifuged for 10 minutes at 2,000 rpm in a Beckman bench top centrifuge and 2.5 ml of the supernatant was layered on top of a discontinuous 0-3-10-15-23% Percoll® gradient and centrifuged for 5 minutes at 20,000 rpm using a Sorvall SS-34 rotor. Synaptosomes were collected from the interface between the 15% and 23% layers and pelleted by centrifugation at 13,000 rpm. Protein concentration was determined by BCA assay (Thermo Scientific Pierce).
2.6 Big Endothelin-1 conversion assay
25 µg of protein from synaptosomal preparations were incubated with 100 nM recombinant human big endothelin (ET)-1 (American Peptide Company) in 0.1 M sodium citrate buffer (pH 5.5) containing 0.05% Triton X-114. Protease inhibitor cocktail without EDTA (Thermo Scientific) and 100 nM thiorphan were added to inhibit the degradation of ET-1 by NEP and other peptidases. After 1h incubation at 37°C, reactions were stopped by adding EDTA (5mM final concentration) and levels of ET-1 were measured by sandwich ELISA (R&D systems, Minneapolis, MN).
3. Results
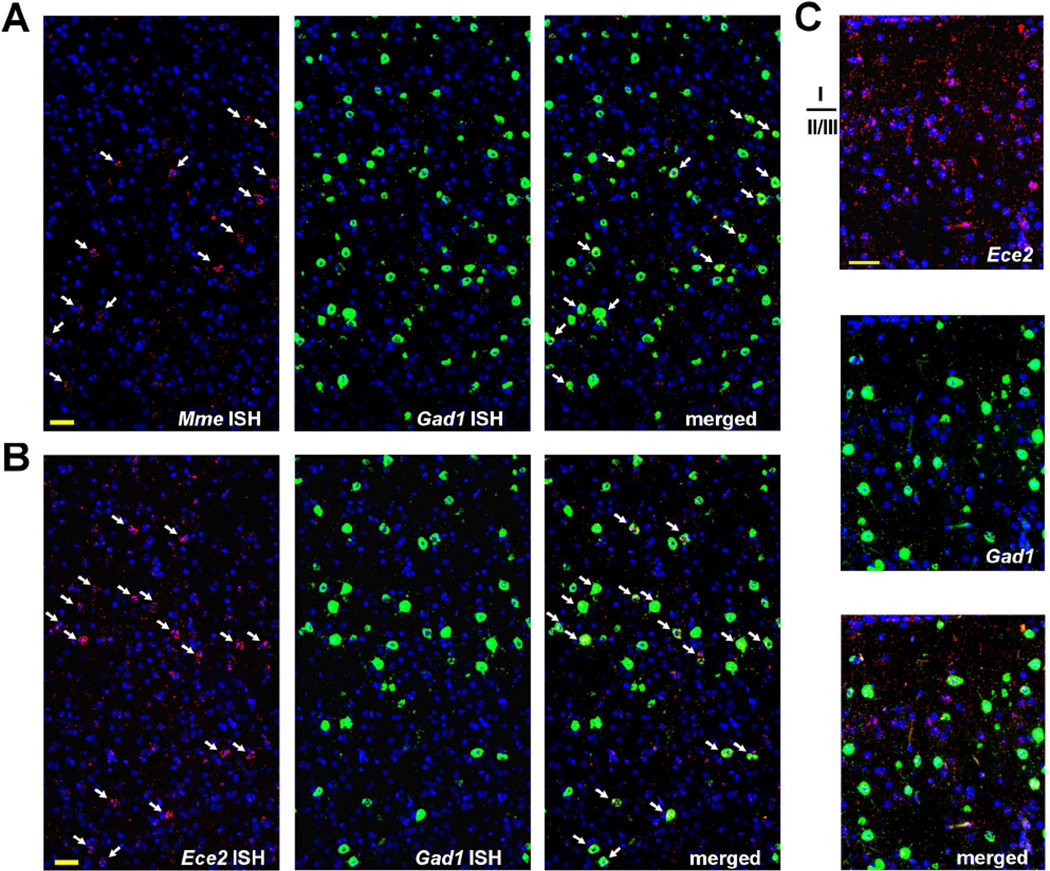
3.1 Ece2 and Mme, but not Ece1, are selectively expressed by GABAergic neurons
Single and multiplex in situ hybridization (ISH), employing branched DNA signal amplification for single-molecule sensitivity, were used to overcome several of the challenges associated with analysis of genes expressed at low levels in the brain. The initial ISH results for the ECEs and NEP (Mme) were quite striking and unexpected; despite the substantial contribution of ECE-2 and NEP to overall brain Aβ levels (Eckman, et al., 2006,Eckman, et al., 2003,Iwata, et al., 2001), the mRNA for both enzymes was found enriched in a sparse number of cells throughout the cortex as well as organized through the parenchyma in a pattern resembling neural processes (Figure 1). For Ece2, this non-somatic fiber-like pattern extended to layer I, where no Ece2+ cells were found, possibly indicating that ECE-2 mRNA travels through processes from deeper cortical layers. Based on the distribution of Ece2 and Mme positive cells, reminiscent of GABAergic interneurons, we performed dual ISH with the GABA-synthetic enzyme, glutamic acid decarboxylase-67 (GAD67, Gad1). Nearly all Ece2+ and Mme+ cells co-expressed Gad1, indicating that subpopulations of GABAergic interneurons are highly enriched in expression of these Aβ degrading enzymes.
Figure 1. Ece2 and Mme (neprilysin) co-localize with Gad1 in mouse cortex.
As detected by ISH, Mme (A) and Ece2 (B&C) transcripts, labeled red, were clustered mainly within a small number of cells (arrows) co-expressing the GABAergic marker Gad1, labeled green. Nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
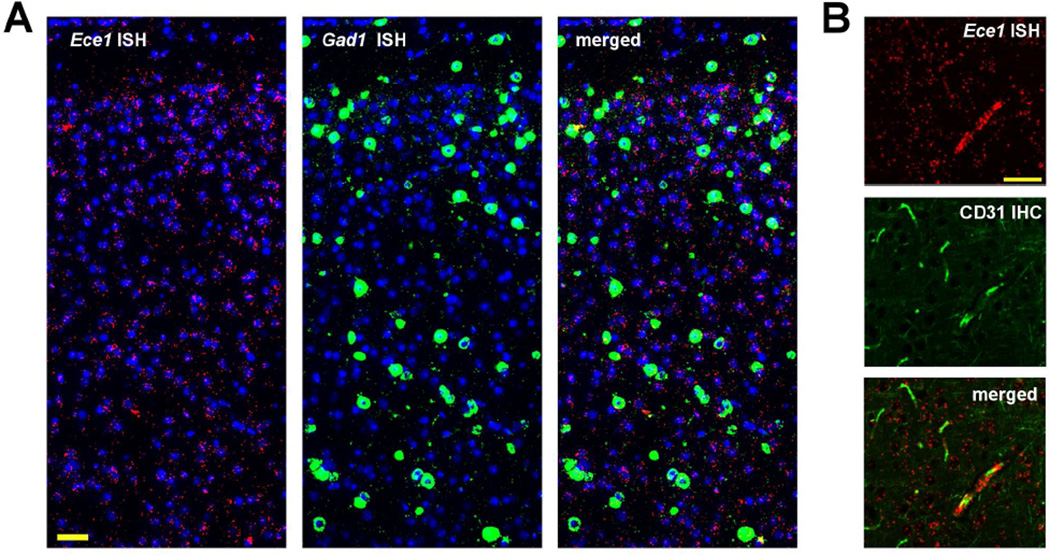
In contrast to Ece2 and Mme, Ece1 mRNA was not particularly enriched in the soma of neurons and co-localized with Gad1 only occasionally (Figure 2A). Consistent with ECE-1’s well-established function of generating the vasoactive peptide endothelin-1 (Xu, et al., 1994), a portion of Ece1 mRNA co-localized with cells immunopositive for platelet endothelial cell adhesion molecule 1 (PECAM-1, also known as CD31), an endothelial cell marker (Figure 2B). In addition to the vasculature, ECE-1 mRNA was also found in a neuronal process-like pattern. Since Ece1 did not appear to be expressed selectively by a certain cell type, subsequent experiments were focused on identifying the GABAergic neuron subtypes expressing Ece2 and Mme.
Figure 2. Distribution of Ece1 mRNA in mouse cortex.
(A) Ece1 transcripts, in red, were only occasionally associated with Gad1+ cells, in green. (B) Within cerebral blood vessels, Ece1 transcripts colocalized with PECAM-1/CD-31, detected by IHC and labeled green. Nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
3.2 Ece2 is selectively expressed by somatostatin positive interneurons
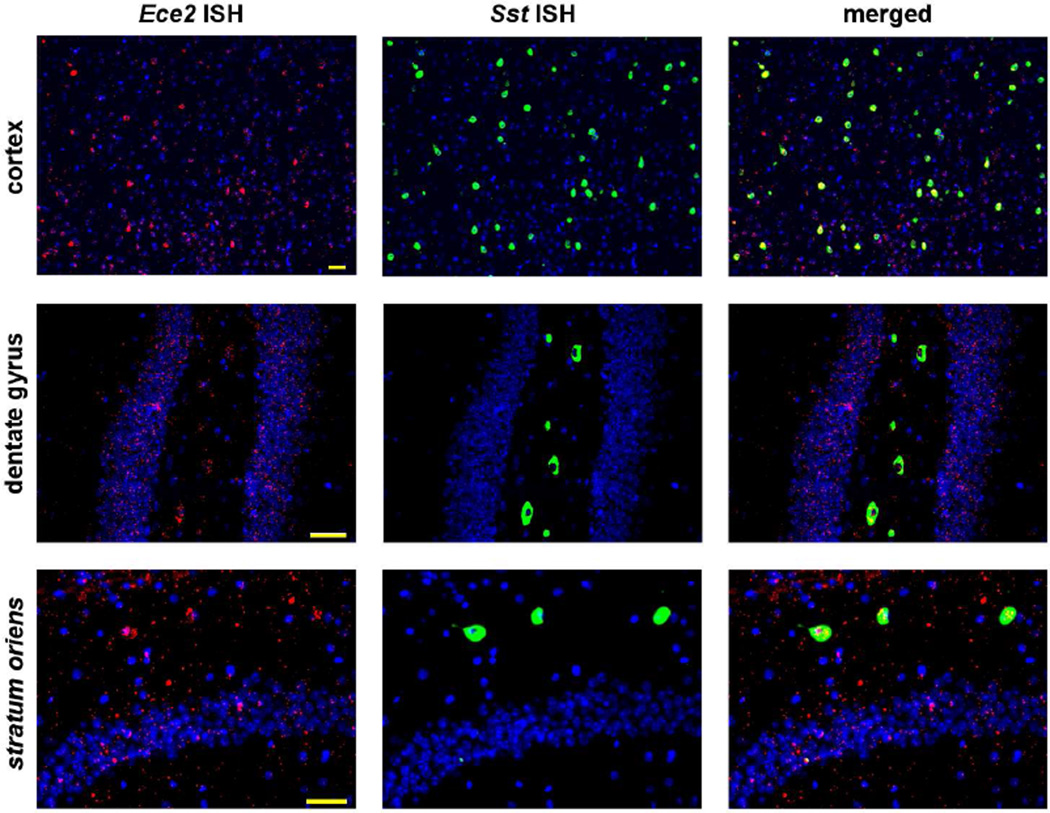
In rodents, GABAergic interneurons represent ~10–20% of all neurons in neocortex, with 40% expressing parvalbumin (PV, Pvalb), 30% expressing somatostatin (SOM, Sst), and most of the remaining cells expressing the ionotropic serotonin receptor 5HT3aR, with little overlap among the three categories (Rudy, et al., 2011). Using dual ISH, we found that the majority of Ece2 mRNA in the cortex concentrated in the soma of cells positive for somatostatin (Figure 3), and an estimated 92% of Sst+ cells (from a total count of 738 cells) co-expressed Ece2. Remarkably, although Sst was expressed at substantially higher levels than Ece2, Sst mRNA was nearly all concentrated in the soma versus the wider distribution of Ece2. In light of the selective expression of Ece2 by Sst+ neurons, and in agreement with the connectivity in layer I of ascending SOM+ axons with dendrites of pyramidal neurons (Kawaguchi and Kubota, 1996), the presence of Ece2 mRNA in layer I (Figure 1C) suggests that Ece2 transcripts are transported to the end terminals of SOM interneurons.
Figure 3. Ece2 is expressed selectively by somatostatin interneurons.
By dual ISH, Ece2, labeled red, was detected in the soma of somatostatin (Sst)+ cells, labeled green, as well as in a pattern resembling neuronal processes. Nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
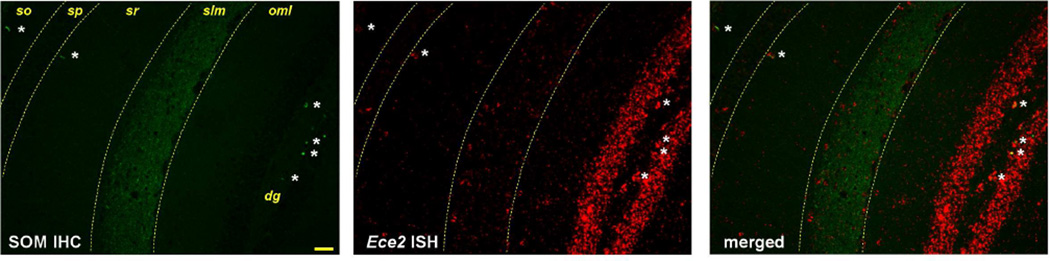
In hippocampus, different subpopulations of Sst+ cells exist, mainly residing in the stratum oriens of CA1–3 and the hilus of the dentate gyrus. Virtually all Sst+ cells in stratum oriens were positive for Ece2 (Figure 3). A particular subtype of SOM interneurons in this region, known as oriens-lacunosum-moleculare (O-LM) cells, have horizontal dendrites in stratum oriens and perpendicular axons that project to the stratum lacunosum-moleculare (Somogyi and Klausberger, 2005). Combining ISH for Ece2 and immunohistochemistry (IHC) for SOM showed fibers containing Ece2 mRNA extending to the stratum lacunosum-moleculare, where both Ece2 mRNA and SOM-immunopositive fibers were enriched (Figure 4). Of note, at the border of stratum radiatum and stratum lacunosum-moleculare we detected a few Ece2+ cells that did not co-express somatostatin. Interneurons in this region have not been thoroughly characterized neurochemically (Somogyi and Klausberger, 2005).
Figure 4. Ece2 transcripts are found in hippocampal regions where SOM interneurons form synaptic connections.
Ece2 mRNA, labeled red, was found in the soma of SOM immunopositive cells (labeled green, white asterisks) as well as in the outer molecular layer (oml) of the dentate gyrus (dg), the stratum pyramidale (sp), stratum radiatum (sr), and concentrated in the stratum lacunosum-moleculare (slm), a highly SOM-immunopositive region. Scale bar, 50 µm.
In hilus, as in stratum oriens, Ece2 was enriched in cells that were Sst+ (Figure 3) as well as immunopositive for SOM (Figure 4). Hilar SOM+ interneurons contact dendrites of pyramidal cells in the outer molecular layer, where Ece2 mRNA was also present. Interestingly, though the granule layer of the dentate gyrus was the area of hippocampus with the most Ece2 signal (Figure 4), the transcripts did not appear to surround the nuclei of granule cells and instead followed a neuronal process-like pattern indistinguishable from that observed for Gad1 (Supplementary Figure 2). This result suggests that Ece2 might not be expressed by granule cells per se, but rather by GABAergic neurons projecting to granule cells or through the granule layer towards the molecular layer.
3.3 ECE-2 is active in synaptosomes
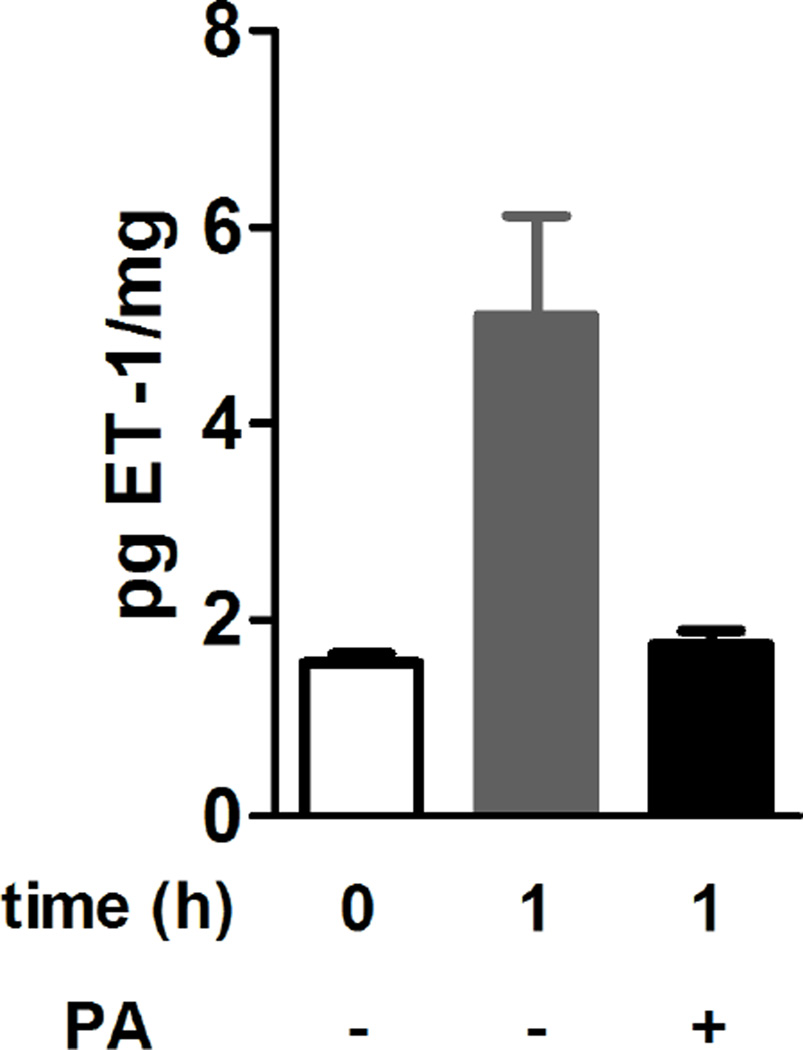
The apparent enrichment of Ece2 mRNA in somatostatinergic fibers, evidenced by the detection of abundant Ece2 mRNA in layer I and stratum lacunosum-moleculare, raises the possibility that Ece2 transcripts may be transported to synapses for local translation. To determine whether ECE-2 activity is present in synapses, we measured the conversion of big endothelin (ET)-1 to ET-1 by isolated brain synaptosomes at pH 5.5 (Emoto and Yanagisawa, 1995). The results of this selective assay for ECE-2 activity confirmed that the enzyme is present in synaptosomes (Figure 5).
Figure 5. ECE-2 is active in isolated brain synaptosomes.
ECE-2 activity was evaluated by measuring the conversion of big ET-1 to ET-1 by isolated brain synaptosomes at pH 5.5, in the presence or absence of the ECE inhibitor phosphoramidon (PA). Zero-time point represents background ET-1 present in synaptosomal preparations. The increase in ET-1 at 1 hr was statistically significant (P=0.0258, unpaired t test). Bars = mean ± SEM of 3 measurements.
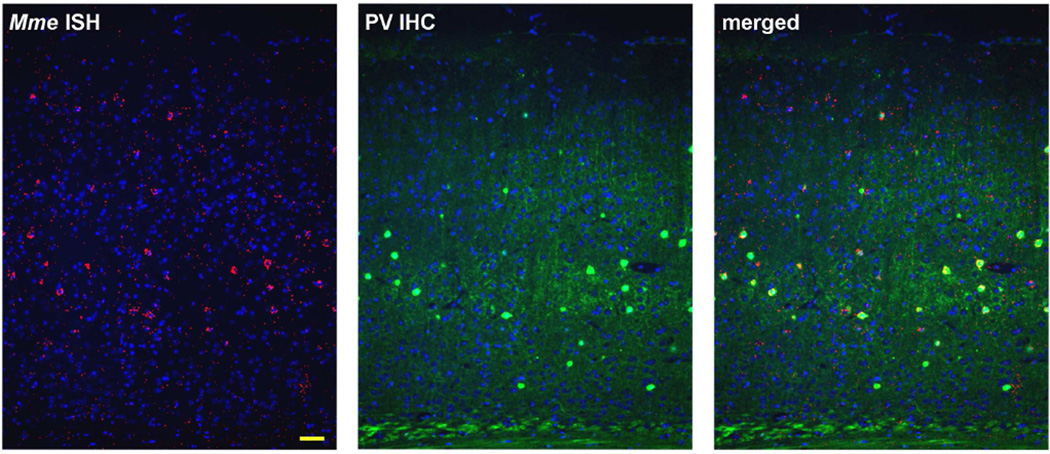
3.4 Neprilysin expression is enriched in parvalbumin positive interneurons
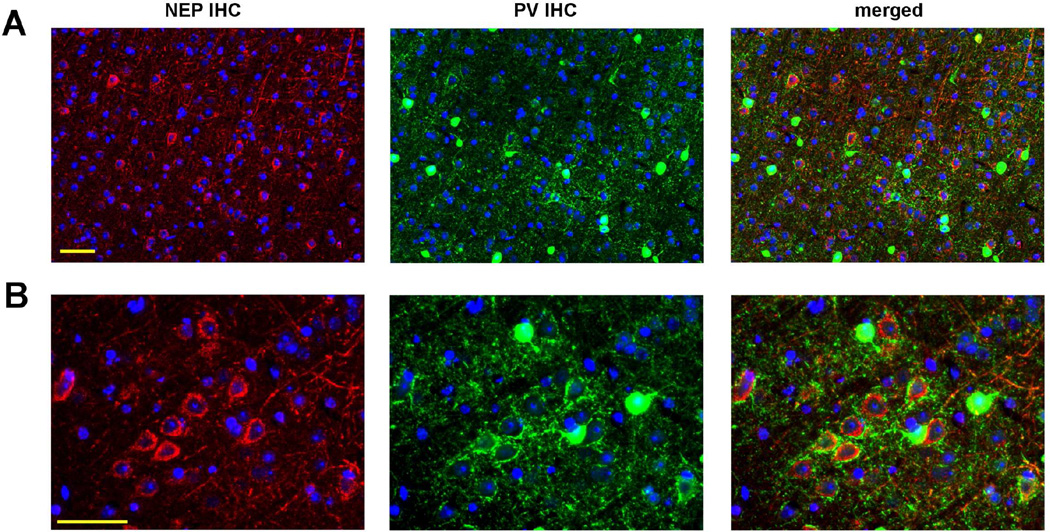
Early immunohistological studies revealed that NEP is expressed in brain, particularly in striatum, consistent with the enzyme’s role in neuropeptide metabolism (Matsas, et al., 1986,Wilcox, et al., 1989). Subsequent reports confirmed that NEP is present in multiple brain regions and is primarily neuronal (Facchinetti, et al., 2003,Fukami, et al., 2002,Gaudoux, et al., 1993,Li, et al., 1995). Though the specific neuronal subtypes expressing the enzyme were not determined, NEP immunoreactivity was found associated with both GABAergic and glutamatergic fibers (Fukami, et al., 2002). More recently, a study of metalloprotease expression in perineuronal net-enwrapped PV+ interneurons suggested the intriguing possibility that NEP may be enriched in cortical PV+ neurons (Rossier, et al., 2015). Guided by this, we combined ISH for Mme (neprilysin) with IHC for PV, and found that the cortical GABAergic cells expressing Mme were parvalbuminergic (Figure 6). Remarkably, we observed that in somatosensory cortex, NEP immunoreactivity was not prominently detected in PV+ neuronal cell bodies, but rather surrounding the soma of pyramidal cells, mainly in layer 5. PV+ neurons are the main type of perisomatic inhibitory interneurons (Freund and Katona, 2007), and as shown in Figure 7, PV+ fibers surrounding the soma of pyramidal cells were found associated with NEP immunoreactivity. Thus, based on the known presynaptic localization of NEP (Fukami, et al., 2002), restricted expression of Mme mRNA by PV+ neurons, and the enrichment of NEP protein around pyramidal cells with strong PV+ perisomatic innervation, it is likely that NEP is selectively enriched in presynaptic endings of PV axons. The fact that little-to-no NEP protein was detected in the soma of cortical PV+ interneurons indicates that the protein is specifically transported to the synaptic region (Iwata, et al., 2004) and/or that Mme transcripts are translated there.
Figure 6. Neprilysin mRNA is enriched in cortical parvalbumin interneurons.
Neprilysin (Mme) mRNA, in red, was found mostly in the soma of PV immunopositive neurons. Nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
Figure 7. Neprilysin protein localizes to the presynaptic endings of cortical parvalbumin interneurons.
(A) By double immunofluorescence, NEP protein was found enriched at the end terminals of PV+ neurons surrounding the soma of principal cells. The area shown corresponds to layer 4/5 of somatosensory cortex. (B) Higher magnification image of a similar area. Nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
In hippocampus, we detected a population of PV-immunopositive Mme-expressing cells along the stratum pyramidale, following the expected distribution for PV+ interneurons (Figure 8). In addition to the soma of PV+ cells, Mme transcripts were also detected in a fiber-like pattern concentrated especially in areas with non-somatic PV immunolabeling. Using double immunofluorescence, we found an enrichment of NEP protein immunoreactivity within the stratum pyramidale, an area with strong PV innervation, as well as the stratum lacunosum moleculare (Figure 9), confirming earlier results from Fukami et al. (Fukami, et al., 2002). While the stratum lacunosum moleculare is innervated by certain weakly PV-immunopositive O-LM neurons (Klausberger, et al., 2003), NEP positive fibers in this region may also belong to other undetermined neuronal populations.
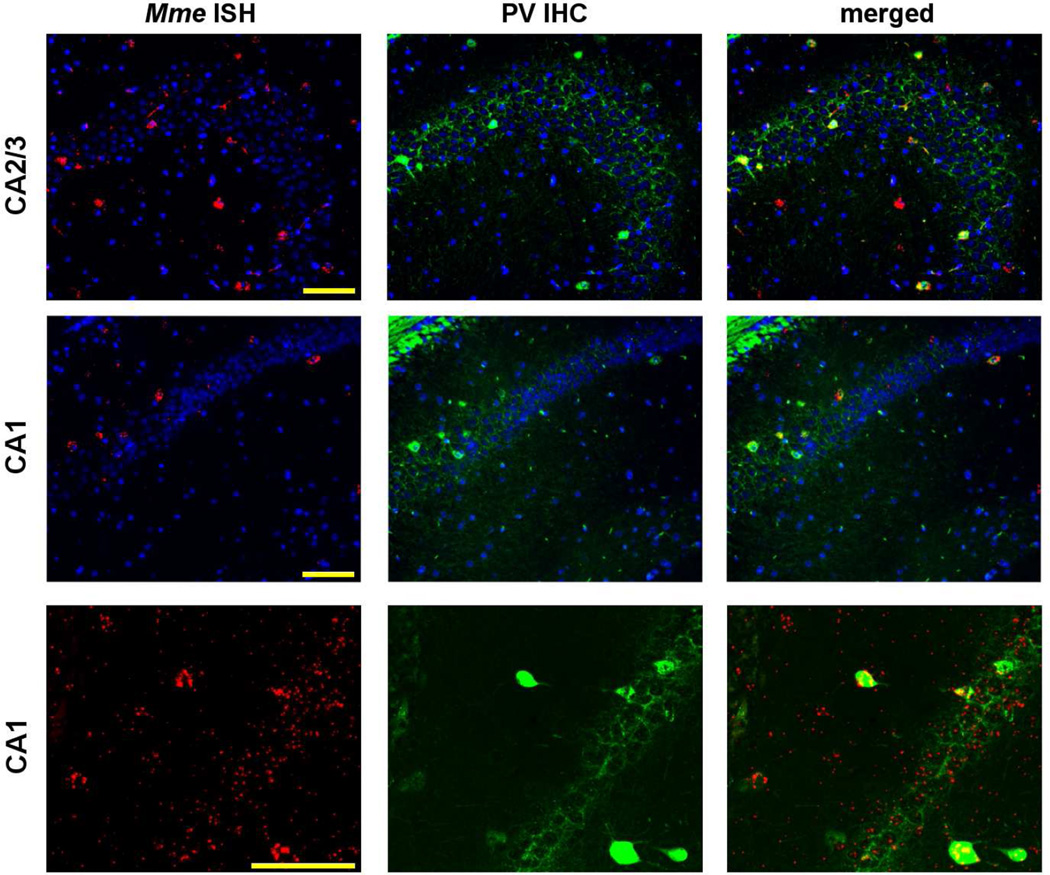
Figure 8. Mme expression in hippocampus.
Mme+ cells (in red) were found adjacent to the pyramidal layer of the CA1 and CA2/3 and the majority were immunoreactive for PV (in green). At higher magnification (bottom panels), Mme mRNA could be observed in the soma of PV+ cells as well as in PV+ processes surrounding the CA1 pyramidal cell layer. Nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
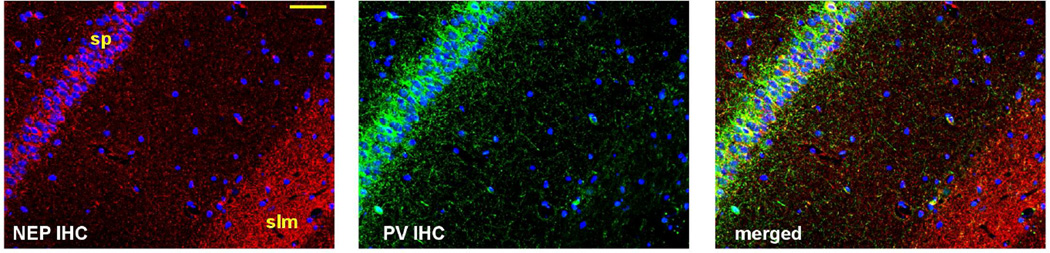
Figure 9. NEP protein localization in hippocampus.
NEP immunostaining (in red) was detected in PV immunopositive fibers (in green) in the stratum pyramidale (sp) and was highly enriched in stratum lacunosum moleculare (slm). Nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
3.5 Ece2 and Mme are not upregulated by reactive glia
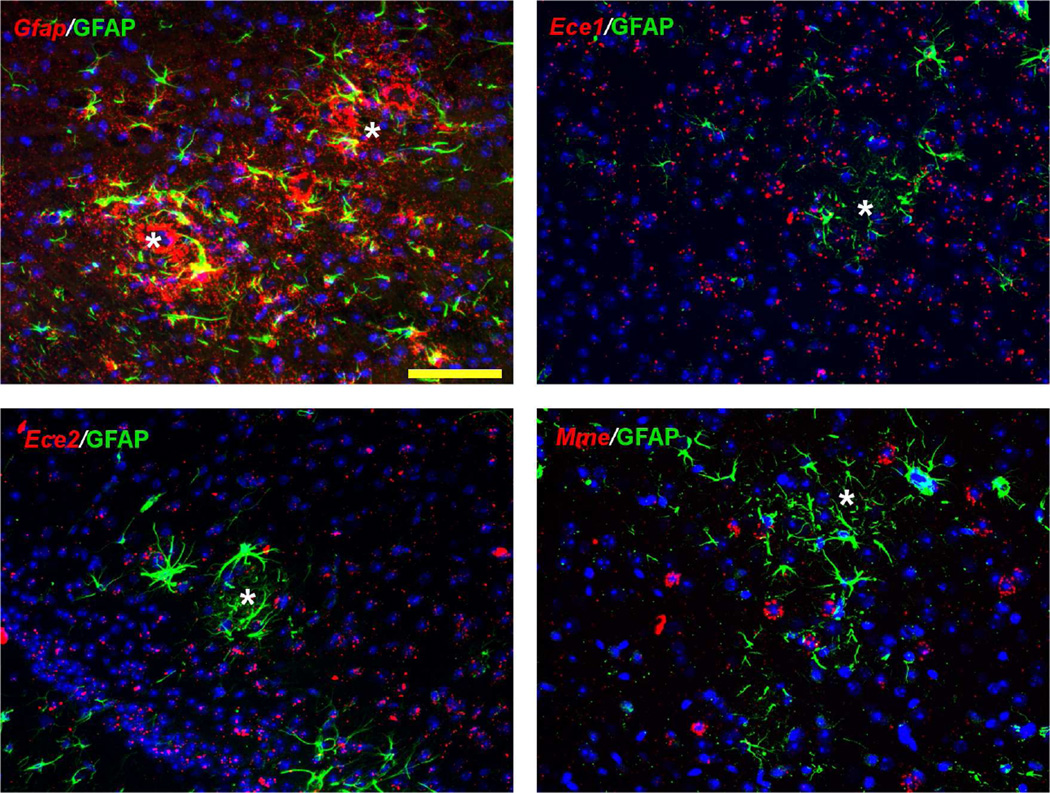
Reactive astrocytes and microglia are a common finding surrounding amyloid plaques and are considered contributors to Aβ removal. Despite the specific expression of Ece2 and Mme by interneurons, we questioned whether the ECEs and NEP may also be upregulated by activated glia. Thus, we evaluated expression of the enzymes in areas with active gliosis in brains from TgCRND8 APP transgenic mice with significant amyloid deposition. As expected for a marker of reactive astrocytes, increased Gfap expression was found surrounding amyloid plaques and coincided with increased immunoreactivity for GFAP (Figure 10). However, there was no indication of increased expression of Ece1, Ece2 or Mme in peri-plaque areas with high GFAP immunoreactivity.
Figure 10. Expression of Aβ degrading enzymes in peri-plaque areas with gliosis.
In brains of TgCRND8 APP transgenic mice, Gfap mRNA (red, upper left panel) was prominent surrounding amyloid plaques (white asterisks) and coincided with increased GFAP immunoreactivity (labeled green in all panels). Expression of Ece1, Ece2, and Mme (in red) was not apparently increased compared to surrounding areas. Nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
4. Discussion
Our histological study of hippocampus and neocortex reveals that two of the major Aβ degrading enzymes, ECE-2 and NEP, are expressed almost exclusively by two non-overlapping populations of GABAergic interneurons. Aside from being the likely explanation for our previous observation that these enzymes cannot compensate for one another (Eckman, et al., 2006), our results underscore the role that somatostatinergic and parvalbuminergic neurons may have in normal Aβ physiology, as well as in the pathophysiology of AD.
SOM+ interneurons compose ~5–8% of the total neuronal population and although they vary greatly in electrophysiological properties, morphology and connectivity (Ma, et al., 2006), their inhibitory activity is fundamental for cognitive function. Demonstrated impairments in learning and memory in ECE-2 knockout mice (Rodriguiz, et al., 2008) suggest that ECE-2 activity may be critical for SOM+ interneuron function, though further studies are required to determine whether disturbances in the homeostasis of Aβ, and/or other potential ECE-2 substrates, can directly alter somatostatinergic neurotransmission.
In AD, there is a clear loss of SOM innervation (reviewed in (Martel, et al., 2012) and several studies with APP transgenic mice support that SOM+ cells may be highly vulnerable to pathological Aβ accumulation (Albuquerque, et al., 2015,Ma and McLaurin, 2014,Perez-Cruz, et al., 2011,Ramos, et al., 2006). The sensitivity of these specific interneurons to AD pathology may relate to their putatively high capacity for intracellular Aβ metabolism via expression of ECE-2. Given that ECE-2 degrades only intracellular Aβ (Pacheco-Quinto and Eckman, 2013), the ~30% increase in endogenous Aβ measured in whole brain homogenates from ECE-2 knockout mice (Eckman, et al., 2003) may be derived from locally high accumulation within SOM+ cells. Any impairment in ECE-2 activity, or imbalance between intracellular production and degradation, could conceivably result over time in the formation of intraneuronal Aβ aggregates, considered a first step in the amyloid cascade (Wirths, et al., 2004).
One interesting subgroup of SOM+ cells to consider, with respect to intracellular Aβ accumulation and the potential to propagate amyloid pathology, is long-range-projecting interneurons. Whereas interneurons, by definition, mainly modulate the function of local principal cells, a specialized group of GABAergic inhibitory neurons project to distant areas of the brain helping to coordinate activity across regions. More than 90% of hippocampal long-range interneurons projecting to the basal forebrain are SOM+ positive cells preferentially distributed along stratum oriens and hilus of dentate gyrus (Jinno and Kosaka, 2002). Due to their long projections, this type of interneuron must rely on efficient retrograde transport to ensure the optimal traffic of synaptic cargo to the soma. Since ECE-2 resides mainly in endosomal/lysosomal vesicles of the retrograde transport pathway, disturbances in ECE-2 expression, or impairments in vesicular trafficking that prevent proper localization of ECE-2 activity, could lead to the formation and interregional dissemination of Aβ aggregates.
There is currently no consensus as to whether ECE-2 expression or activity are disrupted in AD. In a microarray study of gene expression in inferior parietal lobe, ECE2 was found to be the single most down-regulated gene, with average decreases of ~75% in AD compared to non-demented controls (Weeraratna, et al., 2007). A subsequent study of ECE2 expression in temporal lobe found increased expression in AD (Palmer, et al., 2009). The contrasting results of these studies could reflect regional differences in SOM+ interneuron pathology in AD (Chan-Palay, 1987,Kowall and Beal, 1988). They also highlight the difficulties in interpreting the pathophysiological significance of markers detected in end-stage diseased tissue.
Clear evidence for impaired NEP expression or activity in AD is similarly lacking, with studies reporting conflicting results (Carpentier, et al., 2002,Gahete, et al., 2010,Miners, et al., 2009,Wang, et al., 2005). However, as for SOM+ interneurons, mounting evidence supports the link between PV+ interneuron dysfunction and AD. PV+ cells are the largest group of GABAergic interneurons and work as a network generating high-frequency oscillatory currents, known as gamma oscillations, which give temporal reference to pyramidal firing, thus synchronizing the activity of large neuronal groups (Cardin, et al., 2009). Disturbances in gamma oscillations are the suspected culprits of the spontaneous neuronal discharges, cognitive impairment and premature death typical of APP transgenic mice (Minkeviciene, et al., 2009,Verret, et al., 2012), and may be associated with the excitation-inhibition imbalance and susceptibility to seizures in AD (Goutagny and Krantic, 2013).
Studies of physiological Aβ regulation have shown that synaptic activity-mediated increases in Aβ may serve as a negative feedback mechanism to reduce neuronal firing (Cirrito, et al., 2005,Kamenetz, et al., 2003). Given the 2-fold increase in endogenous Aβ in NEP knockout mice (Iwata, et al., 2001), the large pool of Aβ degraded at PV+ synapses may represent an important component of this inhibitory feedback. Interestingly, a pool of endogenous Aβ regulated by NEP was recently shown to positively modulate synaptic vesicle release probability in the hippocampus (Abramov, et al., 2009). Considering that this specific Aβ pool may act locally, and that Aβ can influence gamma oscillatory power (Nerelius, et al., 2009), it is conceivable that Aβ’s physiological function at PV+ synapses might be the modulation of PV+ synaptic transmission. As a consequence, dysfunction in NEP activity, or other disturbances that abnormally elevate Aβ in PV+ synapses, could disrupt gamma rhythms.
The concentration of NEP activity at PV+ synapses may be functionally important for cognition beyond regulation of Aβ. As indicated by one of its original names (enkephalinase), NEP participates in the termination of enkephalin signaling (Matsas, et al., 1984) as well as in the hydrolysis of other neuropeptides relevant for interneuron function including neuropeptide Y (Rose, et al., 2009), somatostatin (Barnes, et al., 1995,Sakurada, et al., 1990), cholecystokinin (Matsas, et al., 1984), and vasoactive intestinal peptide (Duggan, et al., 1994). Among known NEP substrates, the metabolism of enkephalins in brain is particularly interesting in light of our results. Enkephalins are important facilitators of long term potentiation by lowering GABA release from PV+ neurons, via activation of μ-opioid receptors, which are enriched in hippocampal PV+ synapses (Drake and Milner, 2006).
The finding that NEP is expressed in the microenvironment where enkephalins are most active agrees well with the biology of neuropeptide signaling and reinforces our working hypothesis that Aβ may actually work as a neuropeptide important for interneuron function, with proteolysis terminating its activity in situ. Furthermore, due to the probable modulation of several neuropeptides by NEP in PV+ synapses, it is possible that elevations in substrates with higher affinity for NEP could negatively impact the levels of other neuropeptides through competitive inhibition.
5. Conclusion
Our results provide a plausible explanation for the compartmentalization of Aβ catabolism and the existence of distinct and independent pools of Aβ in the brain. Analogous to the biology of other neuropeptides, proteolysis of Aβ at inhibitory synapses may be an important mechanism to terminate its activity and curb the spill of Aβ from synapses where Aβ is active. Moreover, with 1.3- and 2-fold increases in endogenous steady-state Aβ observed in ECE-2 and NEP knockout mice, respectively, physiological levels of Aβ within these microenvironments are likely orders of magnitude higher than in the interstitial fluid, potentially reaching concentrations that may facilitate Aβ aggregation.
Exploring whether Aβ has a direct role in modulating inhibitory signaling, and whether alterations in these pools of Aβ can affect synaptic function, Aβ aggregation and neurotoxicity may lead to the identification of more precise and effective therapeutic targets for AD and may help to explain some of the recent failures in drug development for AD.
Supplementary Material
In cortex, ISH for Ece2 and Mme mRNA in ECE-2 (−/−) mice and NEP (−/−) mice, respectively, gave negligible signal as compared to wild type (+/+) tissue. mRNA labeled red, nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
Ece2 transcripts in the dentate gyrus granule cell layer appear to be concentrated in processes and at synaptic connections. While Gapdh mRNA (right panels) surrounds the nuclei of granule cells of the dentate gyrus, Ece2 (left panels) and Gad1 (center panels) transcripts were detected in a fiber-like pattern crossing over the soma of granule cells. mRNA labeled red, nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
Highlights.
Two major Aβ degrading enzymes are selectively expressed by GABAergic interneurons.
ECE-2 is expressed by somatostatin interneurons and is active in synapses.
Neprilysin is expressed by parvalbumin interneurons.
Neprilysin protein localizes to perisomatic parvalbuminergic synapses.
Acknowledgments
We thank Maria Geraci-Erck and Mazen Albadri for expert technical assistance. We also thank Dr. Ariel Agmon for many helpful discussions and for critical reading of the manuscript. Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS073512. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- Aβ
amyloid-β peptide
- AD
Alzheimer’s disease
- NEP
neprilysin
- ECE
endothelin-converting enzyme
- ISH
in situ hybridization
- IHC
immunohistochemistry
- PV
parvalbumin
- SOM
somatostatin
- GAD67
glutamic acid decarboxylase-67
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
J.P.Q and E.A.E designed the study, J.P.Q performed the experiments, and J.P.Q, C.B.E, and E.A.E interpreted the findings and wrote the paper.
References
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nature neuroscience. 2009;12(12):1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- Albuquerque MS, Mahar I, Davoli MA, Chabot JG, Mechawar N, Quirion R, Krantic S. Regional and sub-regional differences in hippocampal GABAergic neuronal vulnerability in the TgCRND8 mouse model of Alzheimer's disease. Front Aging Neurosci. 2015;7:30. doi: 10.3389/fnagi.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranello RJ, Bharani KL, Padmaraju V, Chopra N, Lahiri DK, Greig NH, Pappolla MA, Sambamurti K. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer's disease. Curr Alzheimer Res. 2015;12(1):32–46. doi: 10.2174/1567205012666141218140953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K, Doherty S, Turner AJ. Endopeptidase-24.11 is the integral membrane peptidase initiating degradation of somatostatin in the hippocampus. J Neurochem. 1995;64(4):1826–1832. doi: 10.1046/j.1471-4159.1995.64041826.x. [DOI] [PubMed] [Google Scholar]
- Basak JM, Kim J, Pyatkivskyy Y, Wildsmith KR, Jiang H, Parsadanian M, Patterson BW, Bateman RJ, Holtzman DM. Measurement of apolipoprotein E and amyloid beta clearance rates in the mouse brain using bolus stable isotope labeling. Mol Neurodegener. 2012;7:14. doi: 10.1186/1750-1326-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier M, Robitaille Y, DesGroseillers L, Boileau G, Marcinkiewicz M. Declining expression of neprilysin in Alzheimer disease vasculature: possible involvement in cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61(10):849–856. doi: 10.1093/jnen/61.10.849. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Somatostatin immunoreactive neurons in the human hippocampus and cortex shown by immunogold/silver intensification on vibratome sections: coexistence with neuropeptide Y neurons, and effects in Alzheimer-type dementia. J Comp Neurol. 1987;260(2):201–223. doi: 10.1002/cne.902600205. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are extensively co-localized with parvalbumin, but not somatostatin, in the dentate gyrus. Neurosci Lett. 2006;403(1–2):176–180. doi: 10.1016/j.neulet.2006.04.047. [DOI] [PubMed] [Google Scholar]
- Duggan KA, Jones DM, Davis RE, Macdonald GJ. Effect of neutral endopeptidase on plasma and tissue concentrations of vaso-active intestinal peptide. Clin Exp Pharmacol Physiol. 1994;21(4):307–309. doi: 10.1111/j.1440-1681.1994.tb02517.x. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc. 2008;3(11):1718–1728. doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Adams SK, Troendle FJ, Stodola BA, Kahn MA, Fauq AH, Xiao HD, Bernstein KE, Eckman CB. Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem. 2006;281(41):30471–30478. doi: 10.1074/jbc.M605827200. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Reed DK, Eckman CB. Degradation of the Alzheimer's amyloid beta peptide by endothelin-converting enzyme. J Biol Chem. 2001;276(27):24540–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer's disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003;278(4):2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon- sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270(25):15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- Facchinetti P, Rose C, Schwartz JC, Ouimet T. Ontogeny, regional and cellular distribution of the novel metalloprotease neprilysin 2 in the rat: a comparison with neprilysin and endothelin-converting enzyme-1. Neuroscience. 2003;118(3):627–639. doi: 10.1016/s0306-4522(02)01002-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56(1):33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fukami S, Watanabe K, Iwata N, Haraoka J, Lu B, Gerard NP, Gerard C, Fraser P, Westaway D, St George-Hyslop P, Saido TC. Abeta-degrading endopeptidase, neprilysin, in mouse brain: synaptic and axonal localization inversely correlating with Abeta pathology. Neurosci Res. 2002;43(1):39–56. doi: 10.1016/s0168-0102(02)00015-9. [DOI] [PubMed] [Google Scholar]
- Gahete MD, Rubio A, Duran-Prado M, Avila J, Luque RM, Castano JP. Expression of Somatostatin, cortistatin, and their receptors, as well as dopamine receptors, but not of neprilysin, are reduced in the temporal lobe of Alzheimer's disease patients. J Alzheimers Dis. 2010;20(2):465–475. doi: 10.3233/JAD-2010-1385. [DOI] [PubMed] [Google Scholar]
- Gaudoux F, Boileau G, Crine P. Localization of neprilysin (EC 3.4.24.11) mRNA in rat brain by in situ hybridization. J Neurosci Res. 1993;34(4):426–433. doi: 10.1002/jnr.490340407. [DOI] [PubMed] [Google Scholar]
- Goutagny R, Krantic S. Hippocampal oscillatory activity in Alzheimer's disease: toward the identification of early biomarkers? Aging Dis. 2013;4(3):134–140. [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B, Gerard NP, Gerard C, Ozawa K, Saido TC. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J Neurosci. 2004;24(4):991–998. doi: 10.1523/JNEUROSCI.4792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292(5521):1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6(2):143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Immunocytochemical characterization of hippocamposeptal projecting GABAergic nonprincipal neurons in the mouse brain: a retrograde labeling study. Brain Res. 2002;945(2):219–231. doi: 10.1016/s0006-8993(02)02804-4. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16(8):2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421(6925):844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Beal MF. Cortical somatostatin, neuropeptide Y, and NADPH diaphorase neurons: normal anatomy and alterations in Alzheimer's disease. Ann Neurol. 1988;23(2):105–114. doi: 10.1002/ana.410230202. [DOI] [PubMed] [Google Scholar]
- Li C, Booze RM, Hersh LB. Tissue-specific expression of rat neutral endopeptidase (neprilysin) mRNAs. J Biol Chem. 1995;270(11):5723–5728. doi: 10.1074/jbc.270.11.5723. [DOI] [PubMed] [Google Scholar]
- Lu B, Gerard NP, Kolakowski LF, Jr, Bozza M, Zurakowski D, Finco O, Carroll MC, Gerard C. Neutral endopeptidase modulation of septic shock. J Exp Med. 1995;181(6):2271–2275. doi: 10.1084/jem.181.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, McLaurin J. alpha-Melanocyte stimulating hormone prevents GABAergic neuronal loss and improves cognitive function in Alzheimer's disease. J Neurosci. 2014;34(20):6736–6745. doi: 10.1523/JNEUROSCI.5075-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26(19):5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel G, Dutar P, Epelbaum J, Viollet C. Somatostatinergic systems: an update on brain functions in normal and pathological aging. Front Endocrinol (Lausanne) 2012;3:154. doi: 10.3389/fendo.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R, Kenny AJ, Turner AJ. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R, Kenny AJ, Turner AJ. An immunohistochemical study of endopeptidase-24.11 ("enkephalinase") in the pig nervous system. Neuroscience. 1986;18(4):991–1012. doi: 10.1016/0306-4522(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Miners JS, Baig S, Tayler H, Kehoe PG, Love S. Neprilysin and insulin-degrading enzyme levels are increased in Alzheimer disease in relation to disease severity. J Neuropathol Exp Neurol. 2009;68(8):902–914. doi: 10.1097/NEN.0b013e3181afe475. [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29(11):3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerelius C, Sandegren A, Sargsyan H, Raunak R, Leijonmarck H, Chatterjee U, Fisahn A, Imarisio S, Lomas DA, Crowther DC, Stromberg R, Johansson J. Alpha-helix targeting reduces amyloid-beta peptide toxicity. Proc Natl Acad Sci U S A. 2009;106(23):9191–9196. doi: 10.1073/pnas.0810364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Quinto J, Eckman EA. Endothelin-converting enzymes degrade intracellular beta-amyloid produced within the endosomal/lysosomal pathway and autophagosomes. J Biol Chem. 2013;288(8):5606–5615. doi: 10.1074/jbc.M112.422964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Quinto J, Herdt A, Eckman CB, Eckman EA. Endothelin-converting enzymes and related metalloproteases in Alzheimer's disease. J Alzheimers Dis. 2013;33(Suppl 1):S101–S110. doi: 10.3233/JAD-2012-129043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JC, Baig S, Kehoe PG, Love S. Endothelin-converting enzyme-2 is increased in Alzheimer's disease and up-regulated by Abeta. Am J Pathol. 2009;175(1):262–270. doi: 10.2353/ajpath.2009.081054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cruz C, Nolte MW, van Gaalen MM, Rustay NR, Termont A, Tanghe A, Kirchhoff F, Ebert U. Reduced spine density in specific regions of CA1 pyramidal neurons in two transgenic mouse models of Alzheimer's disease. J Neurosci. 2011;31(10):3926–3934. doi: 10.1523/JNEUROSCI.6142-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Gulisano W, Arancio O, Palmeri A. The keystone of Alzheimer pathogenesis might be sought in Abeta physiology. Neuroscience. 2015;307:26–36. doi: 10.1016/j.neuroscience.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos B, Baglietto-Vargas D, del Rio JC, Moreno-Gonzalez I, Santa-Maria C, Jimenez S, Caballero C, Lopez-Tellez JF, Khan ZU, Ruano D, Gutierrez A, Vitorica J. Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1xAPP transgenic model of Alzheimer's disease. Neurobiol Aging. 2006;27(11):1658–1672. doi: 10.1016/j.neurobiolaging.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Gadnidze K, Ragnauth A, Dorr N, Yanagisawa M, Wetsel WC, Devi LA. Animals lacking endothelin-converting enzyme-2 are deficient in learning and memory. Genes Brain Behav. 2008;7(4):418–426. doi: 10.1111/j.1601-183X.2007.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JB, Crews L, Rockenstein E, Adame A, Mante M, Hersh LB, Gage FH, Spencer B, Potkar R, Marr RA, Masliah E. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer's disease. J Neurosci. 2009;29(4):1115–1125. doi: 10.1523/JNEUROSCI.4220-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier J, Bernard A, Cabungcal JH, Perrenoud Q, Savoye A, Gallopin T, Hawrylycz M, Cuenod M, Do K, Urban A, Lein ES. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry. 2015;20(2):154–161. doi: 10.1038/mp.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71(1):45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido T, Leissring MA. Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med. 2012;2(6):a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada C, Yokosawa H, Ishii S. The degradation of somatostatin by synaptic membrane of rat hippocampus is initiated by endopeptidase-24.11. Peptides. 1990;11(2):287–292. doi: 10.1016/0196-9781(90)90084-i. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016 doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotani K, Tsubuki S, Iwata N, Takaki Y, Harigaya W, Maruyama K, Kiryu-Seo S, Kiyama H, Iwata H, Tomita T, Iwatsubo T, Saido TC. Neprilysin degrades both amyloid beta peptides 1–40 and 1–42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J Biol Chem. 2001;276(24):21895–21901. doi: 10.1074/jbc.M008511200. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562(Pt 1):9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Lipton RB, Katz MJ, Davies P, Buschke H, Kuslansky G, Verghese J, Younkin SG, Eckman C, Dickson DW. Decreased neprilysin immunoreactivity in Alzheimer disease, but not in pathological aging. J Neuropathol Exp Neurol. 2005;64(5):378–385. doi: 10.1093/jnen/64.5.378. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Kalehua A, Deleon I, Bertak D, Maher G, Wade MS, Lustig A, Becker KG, Wood W, 3rd, Walker DG, Beach TG, Taub DD. Alterations in immunological and neurological gene expression patterns in Alzheimer's disease tissues. Exp Cell Res. 2007;313(3):450–461. doi: 10.1016/j.yexcr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox JN, Pollard H, Moreau J, Schwartz JC, Malfroy B. Localization of enkephalinase mRNA in rat brain by in situ hybridization: comparison with immunohistochemical localization of the protein. Neuropeptides. 1989;14(2):77–83. doi: 10.1016/0143-4179(89)90062-0. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Bayer TA. A modified beta-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide--the first step of a fatal cascade. J Neurochem. 2004;91(3):513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78(3):473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, Clouthier DE, Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105(10):1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In cortex, ISH for Ece2 and Mme mRNA in ECE-2 (−/−) mice and NEP (−/−) mice, respectively, gave negligible signal as compared to wild type (+/+) tissue. mRNA labeled red, nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.
Ece2 transcripts in the dentate gyrus granule cell layer appear to be concentrated in processes and at synaptic connections. While Gapdh mRNA (right panels) surrounds the nuclei of granule cells of the dentate gyrus, Ece2 (left panels) and Gad1 (center panels) transcripts were detected in a fiber-like pattern crossing over the soma of granule cells. mRNA labeled red, nuclei (by Hoechst staining) are labeled blue. Scale bar, 50 µm.