Abstract
Background
Experimental evidence correlates anesthetic exposure during early development with neuronal and glial injury and death as well as behavioral and cognitive impairments in young animals. Several, although not all, retrospective human studies of neurocognitive and behavioral disorders following childhood exposure to anesthesia suggest a similar association. Few studies have specifically investigated the effects of infant anesthesia exposure on subsequent neurobehavioral development. Using a highly translational nonhuman primate model, we investigated the potential dose-dependent effects of anesthesia across the first year of development.
Methods
We examined effects of single or multiple early postnatal isoflurane exposures on subsequent behavioral development in 24 socially reared rhesus macaques. Infants were exposed to 5-h of isoflurane anesthesia either once, three times, or not at all (control). We assessed reflex development and anxiety using standardized tests. At approximately one year, infants (n=23) were weaned and housed indoors with 5-6 other subjects. We recorded their response to this move and re-assessed anxiety.
Results
Compared to controls, animals exposed to repeated isoflurane (ISO-3) presented with motor reflex deficits at 1 month (median, range: ISO-3= 2 [1–5] versus control= 5 [3–7], p<0.005) and responded to their new social environment with increased anxiety (median, range: ISO-3=0.4 bouts/minute [0.2–0.6]; control= 0.25 [0.1–0.3], p,0.05) and affiliative/appeasement behavior (median, range: ISO-3=0.1 bouts/min [0–0.2]; control= 0 [0–0.1], p<0.01) at 12 months. There were no statistically significant behavioral alterations after single isoflurane exposure.
Conclusions
Neonatal exposure to isoflurane, particularly when repeated, has long-term behavioral consequences affecting both motor and socio-emotional aspects of behavior.
Introduction
A large body of experimental evidence associates anesthetic exposure with neural cell injury and death in the developing brain. These exposures have been associated with impaired behavioral and cognitive development in young animals (e.g.,1–5). Several retrospective human studies of neurocognitive and behavioral disorders following childhood exposure to anesthesia suggest a similar association; results indicate that the effects may be more profound with repeated general anesthetic events, longer anesthesia duration,6–9 or cumulative exposures to multiple types of anesthetics, independent of comorbidities.10,11 Moreover, repeated anesthetic exposure has been associated with an increased risk for the development of attention deficit hyperactivity disorder (ADHD) later in life.12
It is less clear whether a single anesthetic exposure, particularly of short duration, is enough to trigger long-term sequelae in young children. While some retrospective studies identified an increased risk for developmental or behavioral disorder diagnosis even after a single short general anesthetic event,13,14 other studies produced ambiguous results15 or observed no increased risk.16 In fact, one recent study suggests no risk at all, not even after multiple anesthetic exposures.17
Most studies, both preclinical and clinical, have focused exclusively on cognitive function and learning disabilities as the primary outcome after anesthesia exposure at a young age. Very few have examined other functional outcomes, such as early motor development or socio-emotional development. Since anesthesia exposure early in life has neural consequences similar to fetal alcohol exposure, which is known to be associated with problems with social function (including anxiety level, curiosity and motor skills; e.g., 18), it is theorized that anesthesia exposure might have similar consequences.
We examined the effect of isoflurane exposure utilizing a nonhuman primate (NHP) model. Brain development of rhesus macaques, corresponds closely to humans (neurodevelopment of rhesus monkeys at birth corresponds to that of a 6-month-old human 14,19), thus they are a highly relevant model for social functioning, complex behavior, and emotional state. So far, only three other studies have explored behavioral and/or cognitive outcomes after anesthesia exposure in young NHPs. 20–22 In our study, we examined multiple behavioral outcomes after both single and multiple anesthesia exposures. Our primary interest was whether isoflurane exposure affected socio-emotional behavior as measured by standardized tests (in which the subject is presented with novel and/or potentially threatening stimuli) and behavior in the home cage. We hypothesized that exposure to isoflurane would cause an increase in the display of anxious/inhibited behavior in response to some provoked tests, and alteration in behavior in the home environment compared to controls. In addition, because anxiety can be difficult to reliably measure during the first month of life, we examined whether anesthesia affected reflexes and general reactivity during this time. The study compares single 5-hour isoflurane exposure with 3 consecutive isoflurane exposures over the course of the first 2 weeks of life to model the clinically relevant human condition of single (one extensive surgery) versus repeated anesthesia exposures of human infants. This allowed us to specifically test whether multiple exposures to anesthesia results in behavioral deficits in the NHP compared to single exposure.
Methods
Animals and overall study design
The subjects for this study were 24 male and female rhesus macaques (Macaca mulatta) reared at the Oregon National Primate Research Center (ONPRC). Infants were born in breeding groups containing approximately 30–50 individuals. They were housed in one of 11, 130 m2 outdoor enclosures. Along with their dams, the infants were temporarily removed from the group on three separate occasions during the first 12 months of life for experimental reasons (see Table 1). In each case, the pair was removed the day before testing and housed in a standard monkey cage overnight. When the subjects were approximately one year of age (mean= 358.7 +/− 12.6 days), they were weaned from their dam and moved into one of four indoor pens with 5–6 study subjects (see Table 2). The pens were approximately 3.7 m x 2.1 m x 2.1 m and were in rooms containing up to 32 cage-housed monkeys. Each pen was located in a different room.
Table 1.
Timeline of procedures for subjects.
| Infant Age | Procedure |
|---|---|
| Day 5 | Removed from group with dam |
| Day 6 | First ISO or sham exposure |
| Day 9 | Second ISO or sham exposure |
| Day 12 | Third ISO or sham exposure |
| Day 14 | IBAS test Release back to social group |
| Day 30 | IBAS test (removed from social group with dam day before) Release back to social group |
| 3 months | Free play/Human Intruder/Novel object tests (removed from social group with dam day before) Released back to social group |
| 12 months | Weaned from natal group; placed in new social group Home cage observations (started approximately 1–2 days after moved to new group) Human Intruder /Novel object test (approximately 1–2 weeks after end of focal observations) |
Abbreviations:
IBAS: Infant Behavior Assessment Scale
ISO: Isoflurane
Table 2.
Sex, treatment (e.g., exposed to isoflurane 0 (Control), 1 (ISO-1) or 3 (ISO-3) times), social group after weaning, age moved to new social group and year born for experimental subjects.
| Infant ID | Sex | Treatment | Social Group | Age moved to new social group | Year born | Comments |
|---|---|---|---|---|---|---|
| 1 | Male | ISO-3 | 1 | 384 | 2013 | |
| 2 | Female | Control | 1 | 371 | 2013 | |
| 3 | Male | ISO-1 | 1 | 370 | 2013 | |
| 4 | Male | Control | 1 | 357 | 2013 | |
| 5 | Female | ISO-3 | 1 | 340 | 2013 | |
| 6 | Male | ISO-1 | 2 | 373 | 2014 | |
| 7 | Male | Control | 2 | 360 | 2014 | |
| 8 | Female | Control | 2 | 359 | 2014 | |
| 9 | Female | ISO-1 | 2 | 359 | 2014 | |
| 10 | Female | ISO-1 | 2 | 342 | 2014 | |
| 11 | Female | ISO-3 | 2 | 342 | 2014 | |
| 12 | Male | Control | 3 | 375 | 2014 | |
| 13 | Female | ISO-1 | 3 | 371 | 2014 | |
| 14 | Male | ISO-1 | 3 | 369 | 2014 | |
| 15 | Female | Control | 3 | 365 | 2014 | |
| 16 | Female | ISO-3 | N/A | N/A | 2014 | A |
| 17 | Female | ISO-1 | 3 | 346 | 2014 | B |
| 18 | Male | ISO-1 | 3 | 345 | 2014 | C |
| 19 | Male | ISO-3 | 4 | 365 | 2014 | |
| 20 | Male | Control | 4 | 361 | 2014 | D |
| 21 | Male | ISO-3 | 4 | 358 | 2014 | |
| 22 | Male | Control | 4 | 349 | 2014 | |
| 23 | Female | ISO-1 | 4 | 347 | 2014 | |
| 24 | Male | ISO-3 | 4 | 343 | 2014 |
Infant became ill and was removed from social group at day 158. She stayed in the clinic with her dam for 26 d and then paired with Infant 20. The infants were paired with a surrogate dam at day 219. Infant remained in this housing until Infant 20 was moved to new social group, and was then removed from study due to illness.
Infant became ill and was removed from social group with her dam at 148 days. After treatment, the infant and her dam were housed next to Infant 18 and dam such that the infants had access to each other.
Infant became ill and was removed from social group with dam at 149 days. After treatment, the infant and his dam were housed next to Infant 17 and dam such that the infants had access to each other.
Infant became ill and was removed from social group at 139 days. He stayed in clinic with his dam for 8 days and was then paired with infant 16 at 151 days. Both infants were paired with a surrogate dam at day 151.
Subjects were chosen based on the dominance status of their mothers. Because they needed to be removed from and returned to the group at various time points, we chose mid-ranking mothers with at least one other surviving offspring. The dams had not received any isoflurane during pregnancy, although most received low doses of sedative for physical exams (Ketamine HCl 5-10 mg/kg; 1-2 times) as part of the normal husbandry practice at the ONPRC.
Monkeys were fed commercial monkey chow (Purina, St. Louis, MO) and were given supplemental enrichment such as grain or produce daily. Water was provided ad libitum. The light cycle in the indoor pens was 12:12 (lights on at 7 am).
This study was approved by the ONPRC Institutional Animal Care and Use Committee (Beaverton, OR, USA), and was in compliance with all federal regulations as well as guidelines set forth in the Guide for the Care and Use of Laboratory Animals.23 The ONPRC is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC International).
Exposure to general anesthesia
At approximately five postnatal days of age (P5), dams and their neonate were transferred from group housing to an adequately sized single cage next to the surgical facilities. Animals were typically removed within 15 minutes with minimal distress to the group. On the morning of the experiment, the dam was mildly sedated and the neonate was transferred to the operating room.
After taking baseline measurements (e.g., heart rate, respiration, temperature, blood pressure, weight, blood gas + metabolism), the neonate was gently hand restrained while the anesthetic agent (isoflurane) was administered via face-mask and spontaneous ventilation. This previously-described induction technique models the method that is routinely used for human infants.24–26 After the animal had reached a state of tolerance, the trachea was intubated. The lungs were mechanically ventilated, and general anesthesia was maintained for 5 hours (endtidal isoflurane = 0.7–1.5 Vol%). The rationale for choosing 5 hours of isoflurane exposure was twofold: this clinically relevant anesthesia duration is often needed for more extensive surgical operations (there are no studies specifically addressing this issue, however data from our institution indicate that over 30% of all infant anesthesia exposures are longer than 3 hours; unpublished data from medical records; November 2015, H. Gries, M.D., Ph.D., Department of Anesthesiology and Perioperative Medicine, Oregon Health and Science University, Portland, OR, USA), and we have a substantial database documenting the pattern and extent of neuropathology and glial pathology that occurs in the perinatal rhesus brain following this exposure. Isoflurane was continuously titrated to maintain a surgical plane of anesthesia and verified every 30 min as ‘no movement and not more than a 10% increase in heart rate or blood pressure in response to a profound mosquito-clamp pinch at hand and foot’. General anesthesia was provided using the same setup as in a human operating room environment and comparable equipment and methods as previously reported.24–27 In brief, each animal was monitored and controlled using full physiologic monitoring (see 24–27 for details). At the end of the 5 hours, isoflurane was stopped and animals were extubated once recovered (after about 10 minutes) in all cases. Animals were then kept for 1–2 hours in a NHP incubator and formula-fed as tolerated. Once fully recovered from anesthesia, neonates were returned to their dams and kept in a caged environment until their first behavioral assessment on P14.
Animals randomized to receive multiple episodes of ISO, the ISO-3 group (n=8), underwent the same procedures as described above (Exposure to general anesthesia, first two paragraphs) on 3 separate postnatal days (approximately, P6, P9 and P12) with 48–96 hours of recovery between each episode. Animals in the control group (no isoflurane, n=8) underwent a “sham exposure”, which consisted of a similar procedure on the same postnatal days (P6, P9, P12) including removal from the dam, an IV cannula, physiologic measurements and a period of handling to simulate the environment that the other animals experienced before and after general isoflurane anesthesia. Animals in the ISO-1 group (1 exposure to isoflurane; n=8), received one general anesthesia as described above (Exposure to general anesthesia, first two paragraphs) on P6 followed by 2 sham exposures on P9 and P12. The sex distribution was balanced in all three groups (4 male; 4 female). This sample size was determined to be the minimum necessary to detect differences in cognitive abilities across groups, based on previous experience.
Reflex testing
Subjects were assessed at P14 and P30 with a modification of the Infant Behavior Assessment Scale (IBAS), a widely utilized test battery designed to assess the development of early reflexes and general reactivity in infants.28 The IBAS is modeled after the Neonatal Behavioral Assessment Scale,29 and has been validated in NHPs.
On the day of testing, the mother was lightly sedated with Ketamine HCl (5-10 mg/kg) to allow removal of the infant, which was transferred in a small transport box to the testing room. Upon arrival, the infant was removed from the box, swaddled in a towel and held by the observer. The observer then rated the infant on several behavioral categories known to have specific developmental milestones, including basic motor reflexes (e.g., grasping objects, labyrinthine righting, placing hands and/or feet on countertop), sensory reflexes (e.g., orientation to objects and sounds), and survival reflexes (e.g., startle response and suckling responses). See figure, Supplemental Digital Content 1 for specific details about the IBAS protocol. At the end of this testing battery, which took approximately 20 min, the infant was transferred to another room for a visual acuity test (not reported here) and was then returned to its dam. The infant-dam pair was returned to the social group later that day, once the dam had fully recovered from the ketamine sedation.
Subjects were given a score of “1” for each measure of the protocol in which they performed the behavior at the highest expected level of development and a score of “0” if they were below this level. For example, it is expected that infants will have a strong grasp reflex in each limb by age P14. Subjects that scored as “strong” on this part of the test received a 1, while subjects with a weak grasping reflex received a 0. Measures related to each category were grouped and totaled so that each animal received a single score for each category. Thus, these scores were directly correlated to the number of areas in which the subject reached the defined criterion.
Anxiety testing
We used two methods to assess anxiety in our study; provoked response tests (e.g., Freeplay, Human Intruder and Novel Object tests, described in next three subsections) and home environment assessments. Anxiety and related traits are multicausal, and can be expressed in many different contexts;30 animals can be anxious in response to a social stimulus, but have no anxiety in other situations. Thus, this kind of combined testing paradigm provides a more comprehensive picture of the emotional states of the animals than does a solitary assessment method. Provoked response tests assess unconditioned response to various threatening or potentially threatening stimuli, while home environment assessments examine response to everyday, naturalistic events such as interactions with conspecifics.30 We performed provoked response tests at both three months and one year of age. Home environment assessments were only performed on the animals after they had been weaned and moved into their new housing groups (see Table 1). In all tests, the observer was blind to the experimental treatment of the infants. The Observer’ software (Noldus Information Technology, Wageningen, the Netherlands) was used to score behavior (both live and from video; see Table 3 for ethogram of behavior).
Table 3.
Ethogram of behaviors used in various testing paradigms. Behaviors were either measured as a duration (i.e., percent of time) or frequency (e.g., number of events per minute). Asterisks indicate individual behaviors that make up behavior “reaction”.
| Test | Behavior | How measured | Definition |
|---|---|---|---|
| Free play | Passive | Duration | Inactive; includes sleeping, sitting on dam not looking around, nursing |
| Explore | Duration | Purposeful touching or manipulating of novel objects, including car seat | |
| Vocalization | Frequency | Includes coo, chirp, shriek, other vocalization | |
| Anxiety | Frequency | Includes scratch, body shake, agitation | |
| Leave dam | Latency | Infant moves so is no longer in touch with dam (i.e., all four limbs off of car seat) | |
| Human Intruder | Freeze | Duration | Tense body posture with no movement (other than eyes) and no vocalizations |
| Anxiety | Frequency | Includes scratch, body shake, agitation | |
| Threat * | Duration | Intense staring with eyes wide open and/or ears pulled back, lips parted in o shape directed towards intruder | |
| Fear grimace* | Duration | Lips pulled back baring teeth | |
| Teeth grind* | Duration | Audible grinding of teeth; jaw moves in sideways motion | |
| Lip-smack* | Duration | Quick movement of jaw pressing lips together in up/down movement | |
| Vigilance | Duration | Looking directly at intruder | |
| Vocalization | Frequency | Includes coo, chirp, shriek, other vocalization | |
| Home cage | Social behavior | Duration | Includes groom, touch, huddle, play |
| Aggressive behavior | Frequency | Includes threat, chase, bite, slap | |
| Anxiety behavior | Frequency | Includes scratch, body shake | |
| Fear grimace (submissive behavior) | Frequency | Lips pulled back baring teeth | |
| Lip-smack (appeasement behavior) | Frequency | Quick movement of jaw pressing lips together in up/down movement | |
| Yawn | Frequency | Mouth open wide, usually showing canine teeth |
Free Play test
This test was designed to assess the infant’s propensity to explore an unfamiliar environment and intrinsic level of activity. A similar test is performed on children (e.g.,31,32) and non-primate animals (open field tests; e.g.,33). Prior to testing, the mother was lightly sedated with Ketamine HCl (5–10 mg/kg), after which both mother and infant (who always clung to the mother) were brought to the testing room (2.4 m x 3.0 m), which contained a novel climbing structure and toys. A one-way window allowed observation and videotaping. The dam and infant were placed in an infant car seat located in the corner of the room, and the dam was given a second dose of Ketamine. The mother was present to avoid the confounding factor of separation anxiety, but sedated to prevent her from interfering with her infant’s behavior. The infant was videotaped for 5-minutes to provide a measure of initial reaction to the new situation, and again at 25–30 min, to assess acclimation to the testing condition.
Human Intruder Test
The Human Intruder test34,35 is one of the most commonly used tests to assess anxiety and the related temperamental trait of behavioral inhibition in young macaques.30 This test was used to measure behavioral responses in three situations: being alone in a novel cage, being in the presence of a human stranger whose gaze was diverted (a potentially threatening stimulus), and being in the presence of a human stranger making direct eye contact (an overt threat).
The Human Intruder test (HIT) began with a 12-minute acclimation period in a novel monkey cage with no human present (Alone 1). After, a human intruder, who had never been in contact with the monkey, entered the room and stood approximately 0.3 meters from the cage for two min. The intruder was always female, to avoid the confounding factor of gender, and always wore the same protective clothing including a mask, transparent plastic face shield, and gloves. She stood with her facial profile presented to the monkey, taking care not to make eye contact with the infant (Profile).
The infant was again alone for the third two min time period (Alone 2). In the fourth period (Stare), the human intruder re-entered the room, and made continuous direct eye contact with the infant for two min. The intruder then left for another two min period (Alone 3) after which the Novel Object test commenced.
Novel Object Test
The novel object test was designed to test the infant’s inclination to explore ethologically relevant novel objects (i.e., pieces of unfamiliar fruit and novel items) that were both nonthreatening and potentially threatening. After the Alone 3 period of the HIT, the intruder re-entered the room and put various novel objects in the cage, each for 5 min. Except for the novel food, all items were removed before new objects were introduced. The novel objects, in order, were: a piece of kiwi (novel food), brightly colored bird toy, Mr. Potato Head (only eyes and feet attached), and a rubber snake with a piece of apple (highly desirable item) on top. Mr. Potato Head was chosen as a potentially threatening stimulus because of the large eyes. For the 1 year time point, we also included a black box hung on the outside of the cage. The box was a piece of PVC tubing with a cover at one end, so that the subject could not see what was in the box. At the end of the test, the infant was either returned to its mother and transferred back to the social group later in the day (3 month) or returned directly to its social group (1 year). Behaviors coded from this test included the latency to inspect (approached within 3 cm), touch (intentional contact) and manipulate each item.
Home cage behavioral assessments
Home cage behavioral assessments were performed to examine how the subjects adapted to the stress of being weaned from their mothers and put into a new social group. A highly trained and experienced observer (NDR) took 10 min continuous focal observations36 on the monkeys 2–3 times/week for 2–3 weeks (for a total of 60 min of observations per individual) beginning 1–2 days after the subjects were introduced into their new housing. The observer, who was familiar to the monkeys, entered the room and stood next to the pen for 10 min to allow the animals to acclimate to her presence. Monkeys are used to having people in their rooms and typically ignored her after a few minutes. She then began to record behavior of each individual directly onto a laptop computer for 10 min each. To limit time of day effects, observations were taken between 12:00–3:00 pm. Behaviors coded (Table 3) focused on social behavior, aggression, and aspects of emotionality such as anxiety behaviors and appeasement/submissive behavior.
Statistics
We used Analysis of Variance (ANOVA) to examine differences among our three groups. For all variables, assumptions of normality and homoscedacity were tested. Data were normalized with arcsine square root or other transformations. When no transformations normalized the data, nonparametric analyses (Kruskal-Wallis test) were utilized. Chi square analyses were used for categorical data. Alpha values (two tailed) were set at 0.05.
We did not detect sex differences in any of the variables. To address the multiple hypothesis testing, the false discovery rate (expected proportion of Type 1 errors among all significant findings) was controlled using the Benjamini-Hochberg procedure.37 Using an ordered list of p-values generated from the individual tests, the Benjamini-Hochberg procedure determines which tests should be rejected in order to maintain a predetermined false discovery rate. We set the false discovery rate at 5%, and report the raw p-values from the ANOVA or Kruskal-Wallis test. For raw p-values less than 0.05, we report the Benjamini-Hochberg adjusted p value (q) as well. Because an individual’s response to one stimulus does not necessarily predict how it will respond to other stimuli30, we utilized the Benjamini-Hochberg within each testing paradigm (i.e., IBAS testing, response to provocative tests, home cage testing). Post hoc comparisons (using the Tukey or its non-parametric equivalent, the Nemenyi test), were carried out on significant findings.
SYSTAT 11 software was used for all analyses. Benjamini-Hochberg adjusted p values and Nemenyi Q values were calculated by hand. 37 Data are presented as mean +/- standard deviation unless otherwise stated.
Results
Subjects were either exposed to isoflurane one time (ISO-1), three times (ISO-3) or were not exposed (controls). Infants tolerated the isoflurane exposure and remained physiologically stable throughout each 5-hour general anesthesia sessions and post-anesthesia recovery. All infants appeared in good health and had vital signs and metabolic values within normal limits during the second or third procedure. Animals that were not exposed to isoflurane on a given experimental day were also separated from their mothers and underwent IV catheterization and some handling following the same schedule and time of day as exposed infants. At the end of each procedure, study subjects were returned to their dams and, at P14 (after IBAS testing), were subsequently released back into their natal group.
Four infants across all interventional groups became ill (e.g., prolonged diarrhea) while in their natal groups, and were moved indoors for clinical treatment. After the treatment was finished, two were housed in indoor cages with their dams until they were weaned at approximately 12 months. The cages were connected with a stainless steel tunnel, allowing the infants to have access to each other to simulate group peer interactions. The other two were housed together with a surrogate dam for 5 months. All 7 of these animals (the four subjects, two dams and surrogate dam) were housed in the same room, with visual contact to one another. One subject was refractory to treatment and was not able to complete the study, and thus 23 juvenile monkeys were housed in small groups indoors after weaning (Table 1).
Behavioral assessments during first month of life
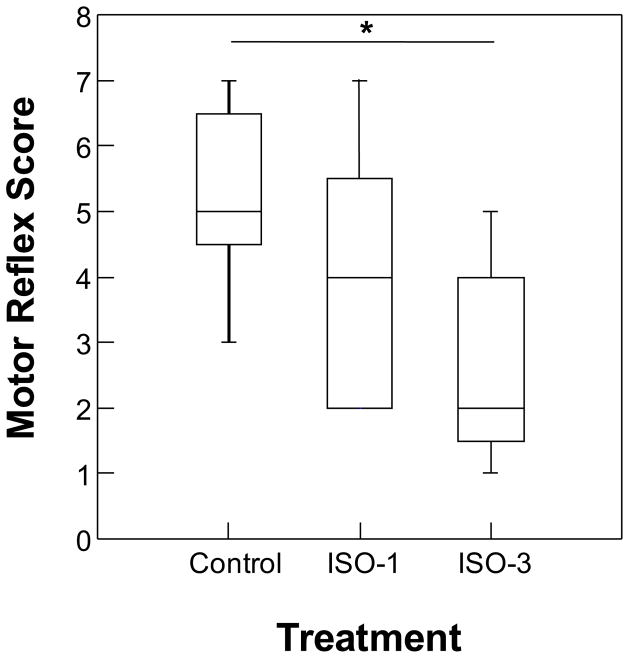
We saw no group difference in behavior on Infant Behavior Assessment Scale (IBAS) when tested at P14 (see table, Supplemental Digital Content 1, which provides statistics for all tests). In contrast, treatment modality did influence IBAS responses when infants were tested at P30; basic motor reflexes differed across groups at this age (H=7.89, p=0.015, q= 0.045; Figure 1). Infants in the control group scored significantly higher in basic motor reflex compared to those in the ISO-3 group at this age (post hoc analysis: Nemenyi test comparison Q (ISO-3 vs control) = 2.81, p<0.005). Their scores for this test were approximately two times those of ISO-3 monkeys. While not significantly different than controls, ISO-1 infants had intermediate scores (i.e., in between the ISO-3 and controls; Nemenyi test comparison Q (ISO-1 vs control) = 1.35, p>0.50, Figure 1). No other group differences were observed at this time point (see table, Supplemental Digital Content 1).
Figure 1.
Boxplots of motor reflex score from the Infant Behavior Assessment Scale (IBAS) test measured at 30 days of age for infants exposed to isoflurane 0 (Control), 1 (ISO-1) or 3 (ISO-3) times. Middle line in box represents the median, box outline represents interquartile range and bars represent range. Asterisk represents p<0.05.
Behavioral assessment at three months of life
Free play test
Only 7 infants moved from their mothers during this 30 min test. There were no treatment differences with respect to this latency or in any of the behaviors we measured during either the first or last five minutes of the test (see table, Supplemental Digital Content 1). However, there was a difference in the change of behavior across the test. We compared the amount of time infants spent passive (i.e., sleeping or remaining completely inactive) during the two time points, to determine how infants acclimated to the 30 min time period (i.e., whether animals became more or less active during the test). While control monkeys tended to show the same amount of passive behavior (within 5%) in both time points, infants in the ISO-1 treatment group showed more passive behavior at the end of the test (i.e., reduced activity over time), and ISO-3 infants showed more passive behavior during the first 5 minutes (i.e., increased activity over time; χ2= 16.3, df=4, p=0.003, q=0.03; Table 4).
Table 4.
Number of individuals exposed to isoflurane 0 (Control), 1 (ISO-1) or 3 (ISO-3) times) that were more passive in the first 5 minutes of the Free Play test than the last five minutes (i.e., increased activity over the course of the test), more passive in the last 5 minutes than the first (i.e., decreased activity over the test), or showed the same amount (i.e., within 5%) of time in this behavior (i.e., had no change in activity over the test).
| Treatment | More passive during first 5 minutes | More passive during last 5 minutes | |
|---|---|---|---|
| Control | 2 | 1 | 5 |
| ISO-1 | 1 | 6 | 1 |
| ISO-3 | 6 | 2 | 0 |
Human Intruder test
Contrary to our expectations, we did not see any group differences with respect to behaviors indicative of anxiety or behavioral inhibition in either the Profile or Stare periods (see table, Supplemental Digital Content 1, for statistics). Only 2 animals displayed anxiety behaviors (e.g., scratching, shaking) during this stimulation. There was a difference in the amount of time it took the infants to respond to the direct eye contact (i.e., ‘latency to respond’; H=7.24, p=0.03; q=0.13), although this difference was not significant (NS) after Benjamini-Hochberg correction for multiple testing. Only 15 subjects (5 in each of the three treatment groups) showed aggressive behavior towards the intruder. Examining only these 15 individuals, there was a difference between treatment groups in the amount of time subjects were aggressive towards the intruder (H= 7.62, p=0.02, q=0.13, NS), yet this difference did not reach statistical significance after correcting for multiple testing. Further, the frequency of grunting, a specific kind of vocalization, in the period immediately following the ‘Stare’ threat (Alone 3) was different between groups, although not statistically significant after correcting for multiple testing. While none of the control animals grunted during this time period, four of the ISO-3 animals and five of the ISO-1 group continued to react to the intruder by grunting (χ2= 7.47, df=2, p=0.02, q=0.13, NS) even though there was no longer any threat present.
Novel Object test
There were no differences across groups with respect to this test when tested at P90 (see table, Supplemental Digital Content 1).
Behavioral assessments at twelve months of life
Because one female study subject was removed from the study, sample sizes for the analyses at the 12-months time point were n=8 for Control, n=8 for ISO-1 and n=7 (4 males, 3 females) for ISO-3 subjects.
Home cage assessments
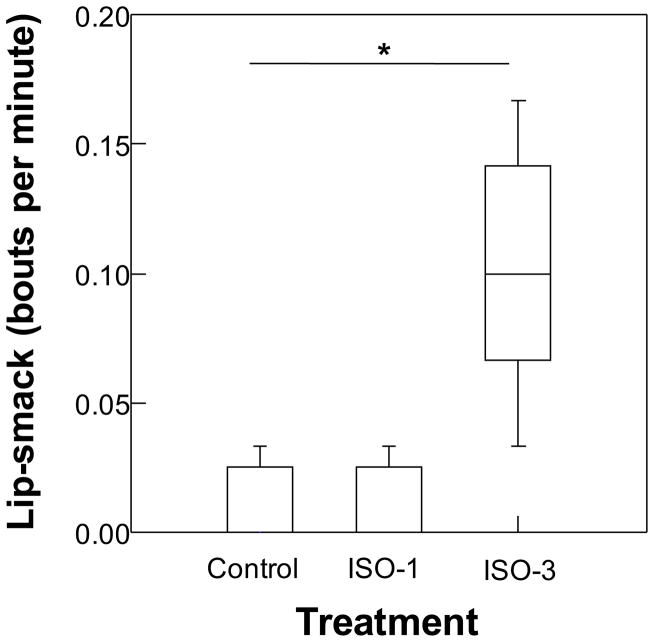
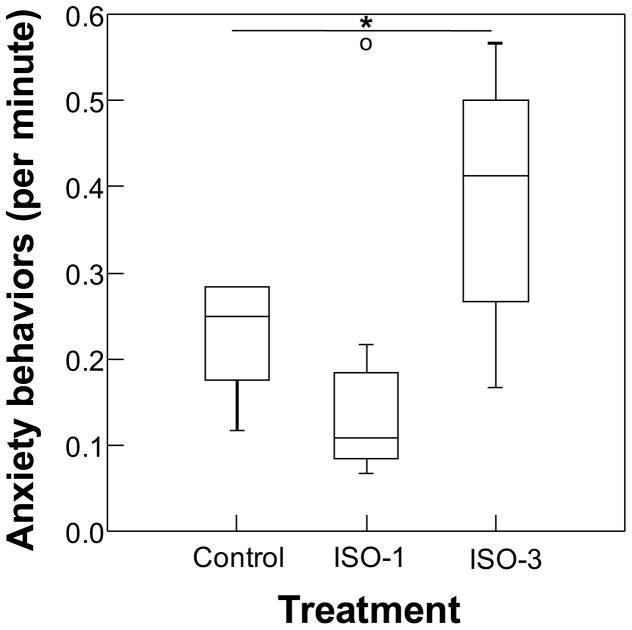
In general, subjects exhibited low levels of submissive fear grimaces (0.02 +/- 0.03 SD bouts/minute), aggressive (0.1 +/− 0.08 bouts per min), or anxious (0.3 +/− 0.2 per min) behaviors in their home group. There were group differences in lip-smack (H=13.53, p=0.001, q= 0.006; Figure 2) and anxiety (scratch and shake; H=7.78, p=0.015, q=0.045; Figure 3). Interestingly, ISO-3 treated subjects were five times as likely to engage in lip-smack, a behavior which is often used in appeasement or affiliation, than control monkeys, and 10 times more likely to lip-smack compared to ISO-1 treated monkeys. They also displayed the highest levels of anxiety behaviors, exhibiting this behavior more than 1.5 times as often as control monkeys. ISO-3 animals were more likely to yawn more than the others, although the difference did not reach statistically significance (χ2=5.0, df=2, p=0.08, NS). Only a total of six animals yawned during the focal observations; four of these were in the ISO-3 treatment group. There were no other differences across treatments (see table, Supplemental Digital Content 1, for statistics).
Figure 2.
Boxplots of bouts of lip-smacking behavior exhibited by infants exposed to isoflurane 0 (Control), 1 (ISO-1) or 3 (ISO-3) times during focal observations at 12 months of age. Middle line in box represents the median, box outline represents interquartile range and bars represent range. Asterisk represents p<0.05.
Figure 3.
Boxplots of anxiety behavior (e.g., scratch, shake) exhibited by infants exposed to isoflurane 0 (Control), 1 (ISO-1) or 3 (ISO-3) times during focal observations at 12 months of age. Middle line in box represents the median, box outline represents interquartile range, bars represent range, and open circle represents outlier. Asterisk represents p<0.05.
Human Intruder test
One-year old subjects spent the majority of time (61.3 +/- 25.7 percent) of the Profile period freezing, and spent little time in other behaviors. There were no treatment differences (see table, Supplemental Digital Content 1, for statistics).
Subjects showed more of a behavioral response to the intruder in the Stare period than in the Profile period, although there were no differences across treatment groups (see table, Supplemental Digital Content 1, for statistics). In contrast to the testing at 3 months, none of the subjects grunted in the Alone 3 period.
Novel Objects test
There were no treatment differences with respect to the novel objects (see table, Supplemental Digital Content 1, for statistics). It is worth noting that while none of the ISO-3 monkeys put their hand inside the black box, in contrast, 3 ISO-1 and 4 control animals did explore the box.
Discussion
This study demonstrates long-term behavioral consequences following repeated isoflurane anesthesia exposure in infant NHPs. We examined the behavioral and emotional consequences of such exposure during the first year of life. Specifically, ISO-3 treatment resulted in motor reflex deficits when animals were tested at 30 days of age. Animals in the ISO-1 group also had lower motor reflex scores than controls, although the difference did not reach statistical significance. These measures of early neuromotor activity are important, as they have been associated with later cognitive functioning. Schnieder et al.,38 found low neonatal motor scores correlated with poor performance on a nonmatching-to-sample cognitive test during adolescence in rhesus macaques. Furthermore, animals exposed to pre-natal alcohol showed motor deficits similar to those in our ISO-3 subjects.39 Our subjects underwent cognitive tests to determine the effect of isoflurane exposure on learning and memory (data currently being analyzed). Based on our findings of motor reflex deficits, we expect to see cognitive impairment in ISO-3 animals, while ISO-1 animals might be less impaired. Such results would be similar to recent retrospective studies in children showing that multiple, but not single, exposures of anesthesia early in life are associated with later learning disabilities.6,7
ISO-3 subjects were more likely than others to display anxiety behavior (e.g., scratch and/or shake) at one year of age, after they were weaned from their natal group. These monkeys engaged in more lip-smacking, a behavior that generally conveys affiliation or appeasement.40 Appeasement behaviors have been associated with social anxiety in humans.41 Taken together, these results suggest that ISO-3 animals were more anxious than others after being moved to their new group.
There was a difference in general activity during the Free Play test when infants were tested at 3 months. While there were no group differences with respect to amount of time animals spent sitting passively (i.e., sleeping or sitting very still, as opposed to being alert and attentive to their surroundings) at either the beginning (0–5 min) or end (25–30 min) of the test, there was a difference among groups with respect to change in this behavior over time. The control infants tended to remain relatively consistent across the time points; they spent about the same amount of time sitting passively at the beginning and end of the test. Interestingly, ISO-3 infants tended to become more active during the test, while ISO-1 infants tended to become more passive. The reasons for these changes are not clear. It is possible that the ISO-1 animals were initially in a heightened state of arousal, and later became drowsy and thus more passive. ISO-3 infants, on the other hand, may have become more aroused during the course of the test. It is important to note that there were no differences in amount of time spent inactive during either time point; the ISO-3 infants were not less active than the other groups at the first time point. Rather, they were more likely than other groups to show a delayed onset and subsequent increase in activity and attention to the environment over time.
These results suggest that multiple exposures to isoflurane early in life may lead to behavioral differences later in life. Our results are particularly striking given our relatively low sample size. It is possible we lacked sufficient statistical power to determine differences with respect to other tests. Alternatively, it is possible that infant exposure to isoflurane results in anxiety behaviors that manifests in a social, but not in a non-social context. Mice exposed neonatally to sevoflurane, an anesthetic similar to isoflurane, had deficits in social interaction tests but not in response to novelty when tested as adults.1 Similarly, Raper and colleages21 recently found that infant rhesus macaques exposed to sevoflurane within the first month of life displayed more anxiety behavior on the Human Intruder test when tested five months later compared to controls. That study did not examine anxiety in a non-social setting. We did not see statistically significant group differences with respect to the behavior on the Human Intruder test, which could be due to several methodological differences between Raper et al.,21 and our study. Still, both studies found that multiple early exposure to general anesthesia resulted in heightened levels of anxiety and emotionality 42,43 in rhesus macaques during their subsequent development, which underscores the relevance of these findings. Additional research is needed to examine (1) the morphological basis for this altered phenotype, (2) the potential for other anesthetics resulting in the same behavioral effects, (3) potential modulators that could protect against these effects, and (4) the relationship between the observed behavioral effects and learning and memory performance at later stages of development.
There were some differences between the ISO-1 and other animals, although they did not reach statistically significance after controlling for multiple comparisons. ISO-1 and, to a lesser extent ISO-3, animals continued to respond to the intruder making eye contact by grunting, even after the threat had left the room, something that the control animals did not do. ISO-1 animals that showed aggression to the intruder during the Stare period (n=5) did so at a higher frequency than others. These results might indicate an increased tendency towards aggression in animals exposed one time compared to controls, although studies are needed to explicitly examine this hypothesis. It is somewhat surprising that the data from the behavioral tests did not result in statistically significant differences between ISO-1 and control animals, given that ISO-1 exposure reproducibly causes a 10-14-fold increase in apoptotic cell death in gray and white matter of neonatal rhesus macaques as we showed previously.24,25 It is possible that the high degree of behavioral variability between individual study subjects, along with our relatively low sample size, led to a lack of statistical power to detect more moderate changes in behavioral phenotype between ISO-1 and control animals. While we did not see group differences in anxiety that reached statistical significance between ISO-1 and control animals, we do not conclude that a 5-hour exposure to volatile anesthetics is “safe” for children, as has been suggested recently based on a less rigorous exploration of 3- and 7-month old cynomolgus macaques after infant sevoflurane exposure.22 Rather, our data suggest a need for further systematic and well-designed work with larger sample sizes, longer than 12 months follow-up and additional tests to determine the effects of a single neonatal exposure of anesthesia on behavioral outcomes in nonhuman primates.
There were several limitations to this study. First, we had a relatively small sample size, which, along with the multiple tests we used, affected our ability to interpret the results. A larger sample size would have allowed us to better detect other, potentially subtle changes in behavior, particularly after single exposure, and, if there were differences, determine whether they were due to individual sensitivity or systematic effects of the total duration of the isoflurane exposure (one versus three times). Larger sample sizes would also have allowed us to determine whether there were sex-differences in sensitivity to infant isoflurane exposure as was reported recently in rodents.44 Second, we only tested isoflurane; it is uncertain whether exposure to other anesthetics known to be neurotoxic for the developing brain27,45 would result in similar behavioral abnormalities in this model. Third, we tested the effects of isoflurane anesthesia in the absence of surgery, while most anesthetic exposures in humans occur in conjunction with a surgical procedure. Such studies are urgently needed, since it is possible that the long-term effects of anesthesia and surgery combined may be worse than those of anesthesia alone. However, while such combined studies are important from a translational perspective, they have their own methodological limitations by introducing the specific pathophysiologic responses to surgery which can confound the results. In contrast, our current study design allowed testing the effects of infant isoflurane exposure alone on subsequent behavioral development in the primate, which is an important step towards understanding whether anesthetics themselves cause toxicity that result in long-term behavioral deficits.
Conclusions
Our findings suggest that infant exposure to isoflurane has long-term consequences affecting both motor and socio-emotional aspects of behavior in a nonhuman primate model. Our data suggest dose-dependent effects, with multiple exposures resulting in more profound changes in some behaviors, particularly long-term anxious behavior and heightened emotionality already appearing at 1 year of age. While these results do not prove a direct link between early anesthesia exposure, most often provided to tolerate surgery, and long-term behavioral consequences in humans, they suggest that emotional consequences should be analyzed in future clinical studies that aim to identify long-term outcomes of infant anesthesia. Most recent studies, including the Pediatric Anesthesia Neurodevelopment Assessment (PANDA) and General Anaesthesia compared to Spinal anesthesia (GAS) studies, focus on cognitive function and intellectual performance (language skills, IQ) as the primary outcome measures (e.g.,46–49); few have examined other functional outcomes, such as socioemotional development. Further, future experimental research in primates should aim to identify the mechanisms responsible for the behavioral consequences of infant anesthesia exposure in order to develop safe anesthesia regimes or protective strategies for young children requiring surgical or diagnostic interventions.
Supplementary Material
Acknowledgments
Funding: This work was supported by Frontiers in Anesthesia Research Award 2012 to AMB (awarded by the International Anesthesia Research Society (San Francisco, CA) and National Institutes of Health 8P51OD011092 to the Oregon National Primate Research Center, Beaverton, OR
We are grateful to the animal husbandry and clinical staff at the Oregon National Primate Research Center (ONPRC), Beaverton, OR USA, for their excellent care of the monkeys. We also thank the following for their help with the behavioral testing and/or manuscript preparation: Adriane Maier, MS (Division of Comparative Medicine, ONPRC, Beaverton, OR), Lisa A. Houser, BS (Division of Comparative Medicine, ONPRC, Beaverton, OR), Daniel H. Gottlieb, Ph.D. (Division of Comparative Medicine, ONPRC, Beaverton, OR), Michelle Bermudez (Division of Comparative Medicine, ONPRC, Beaverton, OR), Katie Menzel Ellis, MD, (Department of Anesthesiology and Perioperative Medicine, OHSU, Portland, OR)
Footnotes
Conflict of interest: None of the authors have any conflict of interest.
Contributor Information
Kristine Coleman, Divisions of Comparative Medicine and Neuroscience, Oregon National Primate Research Center, Beaverton, OR
Nicola D. Robertson, Division of Comparative Medicine Oregon National Primate Research Center, Beaverton, OR
Gregory A. Dissen, Division of Neuroscience Oregon National Primate Research Center, Beaverton, OR, Oregon National Primate Research Center
Martha D. Neuringer, Division of Neuroscience Oregon National Primate Research Center, Beaverton, OR, Oregon National Primate Research Center
L. Drew Martin, Division of Comparative Medicine Oregon National Primate Research Center, Beaverton, OR.
Verginia C. Cuzon Carlson, Division of Neuroscience Oregon National Primate Research Center, Beaverton, OR, Oregon National Primate Research Center
Christopher Kroenke, Division of Neuroscience Oregon National Primate Research Center, Beaverton, OR
Damien Fair, Department of Behavioral Neuroscience, Oregon Health and Science University, Portland, OR
Ansgar Brambrink, Department of Anesthesiology and Perioperative Medicine, Oregon Health and Science University, Portland, OR
References
- 1.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 2.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 3.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee BH, Hazarika OD, Quitoriano GR, Lin N, Leong J, Brosnan H, Chan JT, May LD, Yu D, Alkhamisi A, Stratmann G, Sall JW. Effect of combining anesthetics in neonates on long-term cognitive function. Int J Dev Neurosci. 2014;37:87–93. doi: 10.1016/j.ijdevneu.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–85. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 6.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bong CL, Allen JC, Kim JT. The effects of exposure to general anesthesia in infancy on academic performance at age 12. Anesth Analg. 2013;117:1419–28. doi: 10.1213/ANE.0b013e318299a7c2. [DOI] [PubMed] [Google Scholar]
- 9.Glatz P, Sandin RH, Pederson NL, Edstedt Bonamy A, Eriksson LI, Granath FN. Academic performance after anesthesia and surgery during childhood: A large-scale nation-wide study. Anest Analg. 2015;120:S-289. [Google Scholar]
- 10.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 11.Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–9. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, Christensen K. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–85. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 13.Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–85. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 14.Taghon TA, Masunga AN, Small RH, Kashou NH. A comparison of functional magnetic resonance imaging findings in children with and without a history of early exposure to general anesthesia. Paediatr Anaesth. 2015;25:239–46. doi: 10.1111/pan.12606. [DOI] [PubMed] [Google Scholar]
- 15.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136:e1–12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O'Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–38. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Jevtovic-Todorovic V, Absalom AR, Blomgren K, Brambrink A, Crosby G, Culley DJ, Fiskum G, Giffard RG, Herold KF, Loepke AW, Ma D, Orser BA, Planel E, Slikker W, Jr, Soriano SG, Stratmann G, Vutskits L, Xie Z, Hemmings HC., Jr Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–51. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–30. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123(5):1084–92. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, Wang Z, Zhou H, Liu T, Lu F, Wang S, Li J, Peng S, Zuo Z. Neonatal exposure to sevoflurane may not cause learning and memory deficits and behavioral abnormality in the childhood of Cynomolgus monkeys. Sci Rep. 2015;5:11145. doi: 10.1038/srep11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council. Guide for the Care and Use of Laboratory Animals. 8. Washington, D.C: National Academies Press; 2011. [Google Scholar]
- 24.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–41. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, Dissen GA, Creeley CE, Dikranian KT, Olney JW. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–35. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin LD, Dissen GA, McPike MJ, Brambrink AM. Effects of anesthesia with isoflurane, ketamine, or propofol on physiologic parameters in neonatal rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2014;53:290–300. [PMC free article] [PubMed] [Google Scholar]
- 27.Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110(Suppl 1):i29–38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): Developmental changes, behavioral stability and early experience. Infant Behavior and Development. 1992;15:155–77. [Google Scholar]
- 29.Als H, Tronick E, Lester BM, Brazelton TB. The Brazelton Neonatal Behavioral Assessment Scale (BNBAS) J Abnorm Child Psychol. 1977;5:215–31. doi: 10.1007/BF00913693. [DOI] [PubMed] [Google Scholar]
- 30.Coleman K, Pierre PJ. Assessing anxiety in nonhuman primates. ILAR J. 2014;55:333–46. doi: 10.1093/ilar/ilu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–71. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer M, Goldsmith HH, Davidson RJ, Rickman M. Continuity and change in inhibited and uninhibited children. Child Dev. 2002;73:1474–85. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- 33.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 34.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–21. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 35.Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–83. [PubMed] [Google Scholar]
- 36.Altmann J. Observational study of behavior: Sampling methods. Behaviour. 1974;49:227–67. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Statistical Soc. 1995;57:289–300. [Google Scholar]
- 38.Schneider ML, Moore CF, Becker EF. Timing of moderate alcohol exposure during pregnancy and neonatal outcome in rhesus monkeys (Macaca mulatta) Alcohol Clin Exp Res. 2001;25:1238–45. [PubMed] [Google Scholar]
- 39.Schneider ML, Moore CF, Kraemer GW. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25:1383–92. [PubMed] [Google Scholar]
- 40.Maestripieri D. Gestural communication in macaques: Usage and meaning of nonvocal signals. Evol Commun. 1997;1:193–222. [Google Scholar]
- 41.Price JS, Gardner R, Jr, Erickson M. Can depression, anxiety and somatization be understood as appeasement displays? J Affect Disord. 2004;79:1–11. doi: 10.1016/S0165-0327(02)00452-4. [DOI] [PubMed] [Google Scholar]
- 42.Capitanio JP. Individual differences in emotionality: Social temperament and health. Am J Primatol. 2011;73:507–15. doi: 10.1002/ajp.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coleman K, Dahl RE, Ryan ND, Cameron JL. Growth hormone response to growth hormone-releasing hormone and clonidine in young monkeys: correlation with behavioral characteristics. J Child Adolesc Psychopharmacol. 2003;13:227–41. doi: 10.1089/104454603322572561. [DOI] [PubMed] [Google Scholar]
- 44.Murphy KL, Baxter MG. Long-term effects of neonatal single or multiple isoflurane exposures on spatial memory in rats. Front Neurol. 2013;4:87. doi: 10.3389/fneur.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–84. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ing CH, DiMaggio CJ, Malacova E, Whitehouse AJ, Hegarty MK, Feng T, Brady JE, von Ungern-Sternberg BS, Davidson AJ, Wall MM, Wood AJ, Li G, Sun LS. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–32. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 47.Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern Sternberg BS, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gleich SJ, Flick R, Hu D, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, Hanson A, Buenvenida S, Wilder RT, Sprung J, Voigt RG, Paule MG, Chelonis JJ, Warner DO. Neurodevelopment of children exposed to anesthesia: Design of the Mayo Anesthesia Safety in Kids (MASK) study. Contemp Clin Trials. 2015;41:45–54. doi: 10.1016/j.cct.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR, DiMaggio CJ, Cooper TJ, Rauh V, Maxwell LG, Youn A, McGowan FX. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.