Abstract
Patients with end stage renal disease (ESRD) on hemodialysis experience a high incidence of cardiovascular mortality, and sudden cardiac death (SCD) accounts for approximately 25% of all deaths in this patient population. Despite this high risk of SCD, many non-invasive SCD risk stratification tools that are frequently applied to other patient populations (such as those with prior myocardial infarction and reduced left ventricular systolic function) may be less useful markers of increased SCD risk in ESRD. Improved SCD risk stratification tools for use specifically in patients on hemodialysis are therefore necessary to optimally target use of primary prevention interventions aimed at decreasing SCD incidence. Electrocardiography is an effective, non-invasive SCD risk stratification tool in hemodialysis patients. This article reviews data supporting the association between various ECG parameters (QT interval, spatial QRS-T angle, signal averaged ECG, heart rate variability, and T-wave alternans) and mortality/SCD in the dialysis population. Despite the association between abnormal ECG parameters and SCD, it remains unclear if these abnormal parameters (such as prolonged QT interval) are mechanistically related to SCD and/or ventricular arrhythmias, or if they are simply markers for more severe cardiac disease, such as left ventricular hypertrophy, that may independently predispose to SCD. Current obstacles that impair widespread implementation of ECG risk stratification in the hemodialysis population are also discussed..
Sudden cardiac death (SCD) accounts for approximately 25% of deaths in patients with end stage renal disease (ESRD), with an incidence of approximately 50 events per 1,000 person-years [1]. Although chronic kidney disease is an independent risk factor for cardiovascular disease, factors beyond coronary atherosclerosis contribute to this disproportionately elevated SCD risk, as treatment of cardiovascular risk factors and coronary revascularization in ESRD patients have not resulted in reduced rates of cardiovascular mortality or SCD [2,3]. The majority of dialysis patients have preserved left ventricular systolic function [4], and left ventricular ejection fraction (LVEF), which is the foundation of SCD risk stratification in patients with prior myocardial infarction or non-ischemic cardiomyopathy, has poor sensitivity for SCD in ESRD patients. Chronic uremia in ESRD patients results in diffuse myocardial fibrosis, coronary calcification, left ventricular hypertrophy (LVH), endothelial dysfunction [5], and autonomic dysfunction [6], all of which increase the risk of ventricular arrhythmia independent of LVEF.
SCD is frequently the result of lethal ventricular arrhythmias secondary to abnormalities in ventricular conduction or repolarization. The electrocardiogram (ECG), which can identify some of these arrhythmogenic electrical abnormalities, is therefore a potentially useful SCD risk stratification tool [7]. This review will summarize data supporting use of the ECG as a SCD risk stratification tool in patients with ESRD on hemodialysis, and will discuss future challenges related to widespread deployment of ECG risk stratification in this population.
QT Interval
The QT interval (or heart rate corrected QT interval (QTc)) reflects the total duration of ventricular depolarization and repolarization. Since the duration of ventricular depolarization (as reflected by QRS duration) is usually relatively fixed, short-term variations in QT interval therefore primarily reflect variations in the duration of ventricular repolarization. The QT interval is dynamic and highly variable; electrolyte shifts (especially potassium, calcium, and magnesium) [8], acidosis, various medications, co-existing cardiac disease (especially left ventricular hypertrophy [9]), changes in autonomic tone, and genetics all can influence QT interval. Women also tend to have slightly longer QT intervals than men. A prolonged QTc interval has been associated with SCD and ventricular arrhythmias in various populations [10]. The QT/QTc interval is readily available and easy to assess, and therefore is an attractive SCD risk stratification parameter.
Many patients with ESRD have a prolonged QT/QTc interval, and studies investigating changes in QTc associated with a hemodialysis session have revealed mixed results. Over one third of patients on hemodialysis will have a prolonged QTc interval prior to a dialysis session [11,12]. The dialysis session itself, however, has a heterogeneous effect on QTc interval; some studies have demonstrated that hemodialysis is associated with QTc prolongation [12,13], while others have found no such association [11,14]. These disparate results are likely the result of variations in patient characteristics, coexisting cardiac comorbidities, and dialysis bath electrolyte concentrations among studies [15]. Prolonged QTc interval has been associated with increased ventricular ectopy in ESRD patients, but the direct association between ectopy and SCD is not clear, as patients with longer QT intervals also tend to have more severe LVH [16].
QTc prolongation has been associated with increased rates of total mortality and SCD in hemodialysis patients. QTc interval was evaluated in 122 patients on chronic hemodialysis (median age 71 years, 65% male, 38% with coronary artery disease (CAD), and median LVEF 60%) [11]. Importantly, 28% of this study population was on amiodarone. Prolonged QTc (found in 36% of patients) was associated with increased total mortality (adjusted HR 2.16, p=0.011), and an even higher risk of SCD (adjusted HR 8.33, p=0.009). After multivariable adjustment, QTc and left ventricular mass were the only variables which remained independently associated with SCD. Notably, among a subset of 44 patients with very prolonged QT intervals ≥480 ms, 29.5% of patients died, and 46% of those deaths were attributed to SCD [11].
Similarly, in a study of 280 patients with ESRD undergoing coronary angiography as part of a pre-transplant workup (mean age 53 years, 62% male, 63% with CAD, 47% with LVH, and mean LVEF 47%), a longer QTc was associated with increased total mortality (HR 1.008 per 1 ms QTc increase, p=0.016) even after adjusting for LVH, LVEF and CAD severity [17]. Increased QT dispersion, a measure of the difference between the longest and shortest QT interval measured on a 12-lead ECG, has also been associated with increased total and cardiovascular mortality [18], although a clear association between QT dispersion and SCD in the ESRD population has not yet been observed.
Table 1 summarizes the results of studies evaluating the association between QT interval, total/cardiovascular mortality, and SCD. Although there appears to be an association between QT interval prolongation and SCD in the hemodialysis population, it remains unclear if a prolonged QT interval is mechanistically related to SCD and/or ventricular arrhythmias, or if it is simply a marker for more severe cardiac disease such as left ventricular hypertrophy that may independently predispose to SCD.
Table 1.
Select studies investigating association between ECG parameters and cardiovascular outcomes among persons on hemodialysis
| Author (Year) |
Patients (n) |
ECG Parameter |
Time on HD | Mean Age (yrs) |
% Male | % CAD | % LVH | Mean LVEF (%) |
Study Findings |
|---|---|---|---|---|---|---|---|---|---|
| Genovesi (2013) [11] |
122 | QTc | 3 yrs* | 71* | 65 | 38 | -- | 60* | QTc >450 ms in men and >460 ms in women associated with total mortality (adjusted HR 2.16, p=0.011) and SCD (adjusted HR 8.33, p=0.009). |
| Hage (2010) [17] |
280 | QTc | 19 mo | 53 | 62 | 63 | 47 | 47 | QTc associated with total mortality (adjusted HR 1.008 per 1 ms increase in QTc, p=0.016). SCD not assessed. |
| Beaubien (2002) [18] |
147 (49 on HD) |
QT dispersion |
≥ 3 mo | 59 | 62 | 35 | -- | 69% with LVEF >60% |
Corrected QT dispersion ≥74 ms associated with total mortality (adjusted HR 1.53 per 50 ms increase, p=0.001) and cardiovascular mortality (adjusted HR 1.57 per 50 ms increase, p=0.028), but not SCD/fatal arrhythmia (p=0.061) |
| Nakamura (2005) [51] |
48 (all with cardiac symptoms) |
QT dispersion |
3.9 yrs | 61 | 83 | 54 | -- | -- | Post-HD corrected QT dispersion associated with cardiovascular mortality (adjusted HR 1.02 per 1 ms increase, p<0.05), but not with total mortality (p=0.66). SCD not assessed. |
| Guney (2014) [52] |
72 | QT dispersion |
> 6 mo | 44 | 46 | -- | -- | -- | Corrected QT dispersion associated with total mortality (adjusted HR 1.03 per 1 ms increase, p=0.01). SCD not assessed. |
| de Bie (2013) [21] |
277 | Spatial QRS-T angle |
139 d | 56 | 62 | 25 | 20 | -- | Spatial QRS-T angle as ≥130° in men and ≥116° in women associated with total mortality (adjusted HR 2.33, p<0.01) and SCD (adjusted HR 2.99, p=0.04). |
| Tereshchenko (2016) [22] |
358 | Spatial QRS-T angle |
< 6 mo | 55 | 59 | 37 | 75 | 66 | Spatial QRS-T angle ≥75° associated with all-cause mortality (adjusted HR 2.38, p=0.001), cardiovascular mortality (adjusted HR 2.99, p=0.01), and SCD (adjusted HR 4.52, p=0.03). |
| Roithinger (1992) [26] |
50 | SAECG | 33 mo | 55 | 58 | 10 | 78 | -- | Abnormal SAECG not associated with cardiovascular or arrhythmic mortality |
| Tereshchenko (2016) [22] |
358 | SAECG | < 6 mo | 55 | 59 | 37 | 75 | 66 | Abnormal SAECG not associated with all-cause mortality, CV mortality, or SCD. |
| Hayano (1994) [35] |
31 | HRV: TI | 9 yrs | 56 | 74 | 45 | 71 | 60 | TI <22 associated with total mortality (adjusted HR 8.1, p<0.05), and SCD (adjusted HR 12.6, p<0.05). TI strongest predictor of SCD (better than CAD, LVEF, ventricular tachycardia). SDNN not associated with SCD after multivariable adjustment. |
| Fukuta (2003) [50] |
120 | HRV: TI and ULF Power |
50 mo | 61 | 51 | 6 | -- | -- | TI associated with cardiac mortality (adjusted HR 3.28 per 1 standard deviation decrease, p<0.05). Log transformed ULF power associated with cardiac mortality (adjusted HR 1.92 per 1 standard deviation decrease, p<0.05). LF/HF power ratio and SDNN not associated with cardiac mortality. |
| Oikawa (2009) [33] |
383 | HRV: SDNN | 5 y | 57 | 57 | -- | -- | 65 | SDNN <75 ms associated with total mortality (adjusted HR 2.18, p<0.001) and cardiovascular mortality (adjusted HR 2.11, p=0.01). SDNN also associated with total mortality and cardiovascular death as continuous variable. |
| Nishimura (2010) [34] |
196 | HRV: LF/HF Power ratio |
91 mo | 65 | 58 | -- | 100 | 56 | Increased LF/HF power ratio associated with SCD (adjusted HR 1.42, p<0.001). SCD-free survival at 5 years: 98.1% if LF/HF power ratio <1.9 and 29.4% if LF/HF power ratio ≥1.9. |
| Green (2012) [39] |
48 | TWA | 2.7 yrs | 63 | 53 | 16 | -- | 63 | No association between TWA and total mortality or combined endpoint of major cardiovascular events or death. SCD not assessed. |
Median value
Abbreviations: LVEF-left ventricular ejection fraction; LVH-left ventricular hypertrophy; CAD-coronary artery disease; HD-hemodialysis; ULF-ultra-low frequency; TI-triangular index; SDNN-standard deviation of RR intervals; HR-hazard ratio; TWA-T wave alternans; QTc-corrected QT interval; HRV-heart rate variability; LF-low frequency; HF-high frequency
Spatial QRS-T Angle
The spatial QRS-T angle is a vectorcardiographic parameter that represents the 3-dimensional angle between the spatial QRS vector and spatial T vector. A wide QRS-T angle has been associated with abnormal depolarization/repolarization, increased global electrical heterogeneity, total mortality, and SCD in various populations [19]. In 1 small study, hemodialysis resulted in an increase in spatial QRS-T angle which was correlated with changes in serum potassium.
Spatial QRS-T angle was measured in 277 patients on chronic hemodialysis (mean age 56 years, 62% male, 25% with CAD, and 20% with LVH), and an abnormal QRS-T angle was defined as ≥130° in men and ≥116° in women. Importantly, the “normal” values were derived from a study of healthy individuals [20] and may not be directly applicable to the ESRD population. An abnormal QRS-T angle was present in 35% of study participants and was more frequently present in older patients and those with CAD, diabetes, LVH, reduced LVEF, and longer QTc intervals. An abnormal QRS-T angle was associated with significant increases in the risks of both all-cause mortality (HR 2.33, p<0.01), and SCD (HR 2.99, p=0.04) [21].
Recently, the spatial QRS-T angle was assessed in a prospsective cohort of 358 hemodialysis patients (mean age 55 years, 59% male, 37% with CAD, 75% with LVH, and mean LVEF 66%), and after multivariable adjustment, spatial QRS-T angle ≥75° was associated with all-cause mortality (HR 2.38, p=0.001), cardiovascular mortality (HR 2.99, p=0.01), and SCD (HR 4.52, p=0.03) [22]. The optimal abnormal cut-off value of spatial QRS-T angle for use in the ESRD population will require further study, but this ECG parameter, which integrates abnormalities of both depolarization and repolarization, shows promise as a non-invasive SCD risk stratification tool.
Signal Averaged ECG
The signal averaged ECG (SAECG) utilizes filtering and signal averaging to remove noise and measure µV level signals that cannot be easily detected on a standard ECG. Orthogonal X-, Y-, and Z- ECG leads are recorded and the vector magnitude (; the “filtered QRS complex”) is calculated. The duration of the filtered QRS complex (normal ≤114 ms), root mean square voltage in the terminal 40 ms of the filtered QRS complex (normal ≥20 µV), and duration of low amplitude signals in the terminal part of the filtered QRS that are <40 µV in amplitude (normal ≤38 ms) are assessed [23]. The SAECG has been primarily used to detect “late potentials”, which are low amplitude signals near the end of the QRS complex that are related to slow and/or inhomogeneous ventricular conduction and which may correlate with underlying myocardial electrical substrate favorable for ventricular arrhythmias. Data support use of the SAECG as a risk stratification tool in patients with prior myocardial infarction [24] or non-ischemic cardiomyopathy [25].
Between 8–25% of hemodialysis patients will have an abnormal SAECG [22,26,27], and various small studies have found that a hemodialysis session can worsen or improve the SAECG [27,28]. Increases in filtered QRS duration after hemodialysis have been associated with decreases in serum potassium [27]. The dynamic appearance/disappearance of SAECG detected “late potentials” in hemodialysis patients therefore likely reflects functional variations in ventricular conduction due to electrolyte changes in the setting of existing myocardial fibrosis and/or scar [29]. Dynamic changes in the presence of late potentials might also be related to changes in QRS complex amplitude and duration associated with hemodialysis and fluid removal [30,31]. Despite the high frequency of abnormal SAECGs in hemodialysis patients, there are no currently available data supporting an association between SAECG abnormalities and total mortality, cardiovascular mortality, or SCD in this population [22,26].
Heart Rate Variability
Abnormalities in autonomic tone have been associated with SCD. Heart rate variability (HRV), which assesses the variation in beat-to-beat RR intervals via various time-domain and frequency-domain measurements, is one method of integrating multiple cardiac autonomic inputs that has potential as a non-invasive SCD risk stratification tool. In general, depressed HRV, which is classically thought to be secondary to low parasympathetic and/or high sympathetic tone, has been associated with an increased risk of SCD [32].
A limited number of small studies have investigated the associations between various HRV parameters and cardiovascular outcomes in hemodialysis patients (Table 1). In a study of 383 patients on hemodialysis (mean age 57 years, mean LVEF 65%) followed with 24-hour Holter monitoring, a standard deviation of RR intervals < 75 ms was associated with all-cause mortality (adjusted HR 2.18, p<0.001) and cardiovascular mortality (adjusted HR 2.11, p=0.01), although SCD was not assessed [33]. In a study of 196 dialysis patients with LVH, a low-frequency to high-frequency (LF/HF) ratio of ≥1.9 was associated with a dramatic increase in SCD risk; at 5 years an impressive 98.1% of patients with LF/HF ratio <1.9 remained free of SCD, while only 29.4% of patients with LF/HF ratio ≥1.9 remained free of SCD [34]. In another small study of 31 dialysis patients followed with 24 hour HRV measurement, a reduced triangular index (the number of intervals in the HRV frequency histogram divided by the height of the frequency histogram) was associated with all-cause mortality (HR 8.1, p<0.05), and SCD (HR 12.6, p<0.05). Among all HRV parameters assessed in this study, the triangular index had the strongest associations with all-cause mortality and SCD, and performed significantly better than did LVEF in identifying high risk patients [35].
In general, although a number of abnormal HRV parameters have been associated with cardiovascular mortality and SCD, further study is required to determine the optimal HRV parameters for use as mortality/SCD risk stratification tools in the hemodialysis population.
T-Wave Alternans
T-wave alternans (TWA) reflects repolarization heterogeneity and intracellular calcium signaling abnormalities, which result in beat-to-beat alteration in action potential duration and manifest as alternating changes in T-wave morphology/shape. Microvolt changes in T-wave morphology can be detected via signal processing/averaging techniques [36], and have been associated with increased arrhythmic risk in various populations [37].
When assessed with continuous Holter monitoring, up to >90% of dialysis patients will have abnormal TWA over a 24-hour period [38], and a dialysis session can result in changes in TWA [39,40]. Unfortunately, the associations between TWA and mortality and SCD in dialysis patients have not been well studied. In one small study of 48 dialysis patients, TWA increased during a dialysis session and then returned to baseline but had no correlation with electrolytes, LVEF, LV mass, major adverse cardiovascular events, or death [39]. There are currently no data regarding the association between TWA and SCD or ventricular arrhythmias specifically in the ESRD population.
Conclusions and Future Directions
Although small studies are promising, there are currently only limited data to support use of the ECG for risk stratification of cardiovascular mortality and SCD specifically in persons on hemodialysis. It remains unclear if ECG abnormalities in ESRD are mechanistically related to SCD, or if they are simply electrophysiological markers of more advanced cardiovascular disease such as severe fibrosis or LVH. Large, prospective studies with carefully adjudicated SCD [41] are needed to determine associations between ECG parameters and SCD, and to determine the optimal time to measure these parameters (pre-dialysis, during dialysis, or after dialysis) as a hemodialysis session itself can have significant effects on the ECG; fluid loss and resulting increases in total body impedance [31,42] usually result in increased amplitude of the entire P-QRS-T complex [14,30].
As no single ECG parameter is likely to be highly sensitive or specific for SCD there will likely be a benefit to assessing multiple ECG parameters simultaneously. There may also be a role for combining ECG parameters with clinical characteristics/comorbidities, cardiac imaging, and new biomarkers of renal function such as cystatin C [43] and neutrophil gelatinase-associated lipocalin (NGAL).
Regardless of our ability to perform accurate SCD risk stratification in the hemodialysis population, it remains unclear how to manage the patients who are found to be at especially elevated SCD risk. Treatment of cardiovascular risk factors and cardiac revascularization do not appear to significantly reduce the risk of SCD in the ESRD population [2,3], and there are no high-quality, randomized controlled trial data to support the benefit of implantable cardioverter-defibrillators (ICDs) in hemodialysis patients. Dialysis patients were excluded from the major primary prevention ICD trials, and retrospective data have suggested that severe renal dysfunction significantly reduces the benefit to ICD implantation because of high rates of competing non-arrhythmic mortality [44,45] and increased rates of ICD-related complications such as bleeding and infection [46]. Studies specifically designed to determine if ICDs reduce mortality in the dialysis population are ongoing [47].
Further study of the relative contribution of bradyarrhythmias/heart block, ventricular tachyarrhythmias, and non-arrhythmic events to SCD in the hemodialysis population is also critical to improve SCD risk stratification and reduce SCD incidence [48]. Small studies utilizing implantable loop recorders have demonstrated that severe bradyarrhythmias might be the mechanism of SCD in many hemodialysis patients [49], and the proportion of SCD events without any associated arrhythmia is currently unknown. Until the mechanisms of SCD in ESRD/hemodialysis patients are better understood, efforts to reduce SCD incidence will certainly have reduced efficacy.
Despite these limitations, however, use of the ECG to refine SCD risk in ESRD patients remains an important goal, as data do support an association between ECG abnormalities and SCD in this population. Understanding the mechanisms leading to SCD in ESRD will be valuable in planning SCD mitigation strategies. The ECG is inexpensive, readily available, has minimal risk, and it is easily interpretable.. Therefore, the ECG represents an ideal risk stratification tool which might be sensitive to electrophysiological manifestations associated with SCD in the ESRD population.
Figure 1.
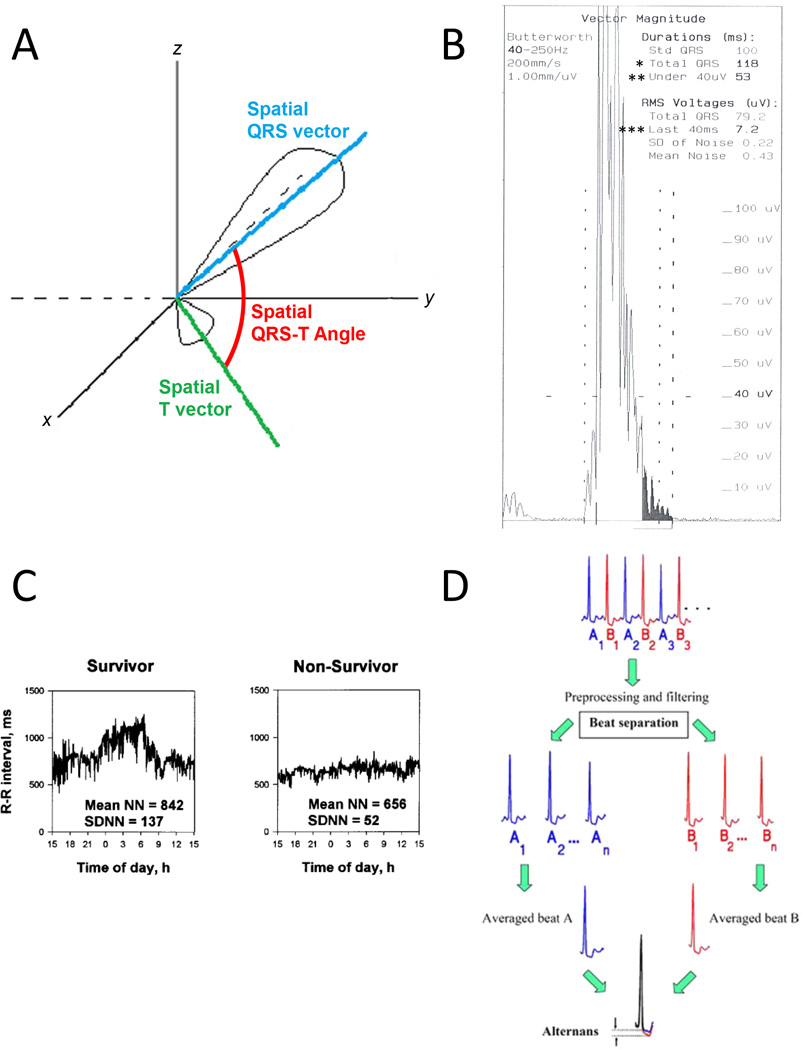
Selected ECG parameters associated with total mortality and sudden death in hemodialysis patients. A: Spatial QRS-T angle is the 3-dimensional angle between the spatial QRS vector and the spatial T vector. Adapted with permission from Oehler et al. [19]. B: Example of an abnormal signal averaged ECG. The filtered QRS complex, which is the vector magnitude of orthogonally recorded X-, Y-, and Z- ECG leads after signal averaging over many cardiac cycles and filtering, is displayed. The duration of the filtered QRS complex (denoted by *) is abnormally prolonged at 118 ms (normal ≤114 ms). The duration of low amplitude signals in the terminal part of the filtered QRS complex that are less than 40 µV (denoted by **) is abnormally long at 53 ms (normal ≤38 ms). The root mean square (RMS) voltage in the terminal 40 ms of the filtered QRS complex (denoted by ***) is abnormally low at 7.2 µV (normal ≥20 µV). C: Heart rate variability: standard deviation of RR intervals (SDNN). The graphs represent plots of RR intervals over a 24 hour period in 2 separate patients. SDNN is also displayed. The survivor has a larger SDNN (137 ms) than the patient who died of SCD (52 ms). Reproduced with permission from Fukuta et al. [50]. D: T wave alternans: signal averaging and filtering techniques are used on alternating QRS complexes (denoted by A beats and B beats) over many cardiac cycles. The average A beat and average B beat are then compared. T wave alternans is calculated as the difference between the ST-T segments in the average A beat and average B beat. Reproduced with permission from Verrier et al. [37].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.United States Renal Data System 2015 Reference Tables. http://www.usrds.org/reference.aspx. [Google Scholar]
- 2.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA, Strief JW, Collins AJ, Gilbertson DT. Cause-specific mortality of dialysis patients after coronary revascularization: why don't dialysis patients have better survival after coronary intervention? Nephrol Dial Transplant. 2008;23:2629–2633. doi: 10.1093/ndt/gfn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parfrey PS, Foley RN, Harnett JD, et al. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11:1277–1285. [PubMed] [Google Scholar]
- 5.Edwards NC, Moody WE, Chue CD, et al. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2014;7:703–714. doi: 10.1016/j.jcmg.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Robinson TG, Carr SJ. Cardiovascular autonomic dysfunction in uremia. Kidney Int. 2002;62:1921–1932. doi: 10.1046/j.1523-1755.2002.00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Wellens HJ, Schwartz PJ, Lindemans FW, et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35:1642–1651. doi: 10.1093/eurheartj/ehu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozos I. Laboratory markers of ventricular arrhythmia risk in renal failure. Biomed Res Int. 2014;2014:509204. doi: 10.1155/2014/509204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh JP, Johnston J, Sleight P, et al. Left ventricular hypertrophy in hypertensive patients is associated with abnormal rate adaptation of QT interval. J Am Coll Cardiol. 1997;29:778–784. doi: 10.1016/s0735-1097(96)00576-1. [DOI] [PubMed] [Google Scholar]
- 10.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 11.Genovesi S, Rossi E, Nava M, et al. A case series of chronic haemodialysis patients: mortality, sudden death, and QT interval. Europace. 2013;15:1025–1033. doi: 10.1093/europace/eus412. [DOI] [PubMed] [Google Scholar]
- 12.Covic A, Diaconita M, Gusbeth-Tatomir P, et al. Haemodialysis increases QT(c) interval but not QT(c) dispersion in ESRD patients without manifest cardiac disease. Nephrol Dial Transplant. 2002;17:2170–2177. doi: 10.1093/ndt/17.12.2170. [DOI] [PubMed] [Google Scholar]
- 13.Genovesi S, Rivera R, Fabbrini P, et al. Dynamic QT interval analysis in uraemic patients receiving chronic haemodialysis. J Hypertens. 2003;21:1921–1926. doi: 10.1097/00004872-200310000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Drighil A, Madias JE, Benjelloun M, et al. Changes in the QT intervals, QT dispersion, and amplitude of T waves after hemodialysis. Ann Noninvasive Electrocardiol. 2007;12:137–144. doi: 10.1111/j.1542-474X.2007.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genovesi S, Dossi C, Vigano MR, et al. Electrolyte concentration during haemodialysis and QT interval prolongation in uraemic patients. Europace. 2008;10:771–777. doi: 10.1093/europace/eun028. [DOI] [PubMed] [Google Scholar]
- 16.Stewart GA, Gansevoort RT, Mark PB, et al. Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int. 2005;67:217–226. doi: 10.1111/j.1523-1755.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 17.Hage FG, de Mattos AM, Khamash H, et al. QT prolongation is an independent predictor of mortality in end-stage renal disease. Clin Cardiol. 2010;33:361–366. doi: 10.1002/clc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaubien ER, Pylypchuk GB, Akhtar J, Biem HJ. Value of corrected QT interval dispersion in identifying patients initiating dialysis at increased risk of total and cardiovascular mortality. Am J Kidney Dis. 2002;39:834–842. doi: 10.1053/ajkd.2002.32005. [DOI] [PubMed] [Google Scholar]
- 19.Oehler A, Feldman T, Henrikson CA, Tereshchenko LG. QRS-T angle: a review. Ann Noninvasive Electrocardiol. 2014;19:534–542. doi: 10.1111/anec.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherptong RW, Henkens IR, Man SC, et al. Normal limits of the spatial QRS-T angle and ventricular gradient in 12-lead electrocardiograms of young adults: dependence on sex and heart rate. J Electrocardiol. 2008;41:648–655. doi: 10.1016/j.jelectrocard.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 21.de Bie MK, Koopman MG, Gaasbeek A, et al. Incremental prognostic value of an abnormal baseline spatial QRS-T angle in chronic dialysis patients. Europace. 2013;15:290–296. doi: 10.1093/europace/eus306. [DOI] [PubMed] [Google Scholar]
- 22.Tereshchenko LG, Kim ED, Oehler A, et al. Electrophysiological Substrate and Risk of Mortality in Incident. Hemodialysis. 2016 doi: 10.1681/ASN.2015080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Signal-averaged electrocardiography. J Am Coll Cardiol. 1996;27:238–249. [PubMed] [Google Scholar]
- 24.Simson MB. Use of signals in the terminal QRS complex to identify patients with ventricular tachycardia after myocardial infarction. Circulation. 1981;64:235–242. doi: 10.1161/01.cir.64.2.235. [DOI] [PubMed] [Google Scholar]
- 25.Mancini DM, Wong KL, Simson MB. Prognostic value of an abnormal signal-averaged electrocardiogram in patients with nonischemic congestive cardiomyopathy. Circulation. 1993;87:1083–1092. doi: 10.1161/01.cir.87.4.1083. [DOI] [PubMed] [Google Scholar]
- 26.Roithinger FX, Punzengruber C, Rossoll M, et al. Ventricular late potentials in haemodialysis patients and the risk of sudden death. Nephrol Dial Transplant. 1992;7:1013–1018. [PubMed] [Google Scholar]
- 27.Berta E, Erdei A, Cseke B, et al. Evaluation of the metabolic changes during hemodialysis by signal averaged ECG. Pharmazie. 2012;67:380–383. [PubMed] [Google Scholar]
- 28.Girgis I, Contreras G, Chakko S, et al. Effect of hemodialysis on the signal-averaged electrocardiogram. Am J Kidney Dis. 1999;34:1105–1113. doi: 10.1016/S0272-6386(99)70017-X. [DOI] [PubMed] [Google Scholar]
- 29.Morales MA, Gremigni C, Dattolo P, et al. Signal-averaged ECG abnormalities in haemodialysis patients. Role of dialysis. Nephrol Dial Transplant. 1998;13:668–673. doi: 10.1093/ndt/13.3.668. [DOI] [PubMed] [Google Scholar]
- 30.Drighil A, Madias JE, Yazidi A, et al. P-wave and QRS complex measurements in patients undergoing hemodialysis. J Electrocardiol. 2008;41:60.e1–60.e7. doi: 10.1016/j.jelectrocard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Madias JE, Narayan V. Augmentation of the amplitude of electrocardiographic QRS complexes immediately after hemodialysis: a study of 26 hemodialysis sessions of a single patient, aided by measurements of resistance, reactance, and impedance. J Electrocardiol. 2003;36:263–271. doi: 10.1016/s0022-0736(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 32.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 33.Oikawa K, Ishihara R, Maeda T, et al. Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol. 2009;131:370–377. doi: 10.1016/j.ijcard.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura M, Tokoro T, Nishida M, et al. Sympathetic overactivity and sudden cardiac death among hemodialysis patients with left ventricular hypertrophy. Int J Cardiol. 2010;142:80–86. doi: 10.1016/j.ijcard.2008.12.104. [DOI] [PubMed] [Google Scholar]
- 35.Hayano J, Takahashi H, Toriyama T, et al. Prognostic value of heart rate variability during long-term follow-up in chronic haemodialysis patients with end-stage renal disease. Nephrol Dial Transplant. 1999;14:1480–1488. doi: 10.1093/ndt/14.6.1480. [DOI] [PubMed] [Google Scholar]
- 36.Nearing BD, Verrier RL. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol (1985) 2002;92:541–549. doi: 10.1152/japplphysiol.00592.2001. [DOI] [PubMed] [Google Scholar]
- 37.Verrier RL, Malik M. Quantitative T-wave alternans analysis for guiding medical therapy: an underexploited opportunity. Trends Cardiovasc Med. 2015;25:201–213. doi: 10.1016/j.tcm.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Secemsky EA, Verrier RL, Cooke G, et al. High prevalence of cardiac autonomic dysfunction and T-wave alternans in dialysis patients. Heart Rhythm. 2011;8:592–598. doi: 10.1016/j.hrthm.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 39.Green D, Batchvarov V, Wijesekara C, Kalra PA, Camm AJ. Dialysis-dependent changes in ventricular repolarization. Pacing Clin Electrophysiol. 2012;35:703–710. doi: 10.1111/j.1540-8159.2012.03364.x. [DOI] [PubMed] [Google Scholar]
- 40.Friedman AN, Groh WJ, Das M. A pilot study in hemodialysis of an electrophysiological tool to measure sudden cardiac death risk. Clin Nephrol. 2007;68:159–164. doi: 10.5414/cnp68159. [DOI] [PubMed] [Google Scholar]
- 41.Parekh RS, Meoni LA, Jaar BG, et al. Rationale and design for the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study. BMC Nephrol. 2015;16:63. doi: 10.1186/s12882-015-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madias JE, Bazaz R, Agarwal H, Win M, Medepalli L. Anasarca-mediated attenuation of the amplitude of electrocardiogram complexes: a description of a heretofore unrecognized phenomenon. J Am Coll Cardiol. 2001;38:756–764. doi: 10.1016/s0735-1097(01)01429-2. [DOI] [PubMed] [Google Scholar]
- 43.Deo R, Sotoodehnia N, Katz R, et al. Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes. 2010;3:159–164. doi: 10.1161/CIRCOUTCOMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 45.Singh SM, Wang X, Austin PC, et al. Prophylactic defibrillators in patients with severe chronic kidney disease. JAMA Intern Med. 2014;174:995–996. doi: 10.1001/jamainternmed.2014.1208. [DOI] [PubMed] [Google Scholar]
- 46.Tompkins C, McLean R, Cheng A, et al. End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol. 2011;22:1099–1104. doi: 10.1111/j.1540-8167.2011.02066.x. [DOI] [PubMed] [Google Scholar]
- 47.de Bie MK, Lekkerkerker JC, van Dam B, et al. Prevention of sudden cardiac death: rationale and design of the Implantable Cardioverter Defibrillators in Dialysis patients (ICD2) Trial--a prospective pilot study. Curr Med Res Opin. 2008;24:2151–2157. doi: 10.1185/03007990802237343. [DOI] [PubMed] [Google Scholar]
- 48.Charytan DM, Foley R, McCullough PA, et al. Arrhythmia and Sudden Death in Hemodialysis Patients: Protocol and Baseline Characteristics of the Monitoring in Dialysis Study. Clin J Am Soc Nephrol. 2016;11:721–734. doi: 10.2215/CJN.09350915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong MC, Kalman JM, Pedagogos E, et al. Bradycardia and asystole is the predominant mechanism of sudden cardiac death in patients with chronic kidney disease. J Am Coll Cardiol. 2015;65:1263–1265. doi: 10.1016/j.jacc.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 50.Fukuta H, Hayano J, Ishihara S, et al. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:318–325. doi: 10.1093/ndt/18.2.318. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura S, Ogata C, Aihara N, et al. QTc dispersion in haemodialysis patients with cardiac complications. Nephrology (Carlton) 2005;10:113–118. doi: 10.1111/j.1440-1797.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 52.Guney M, Ozkok A, Caliskan Y, et al. QT dispersion predicts mortality and correlates with both coronary artery calcification and atherosclerosis in hemodialysis patients. Int Urol Nephrol. 2014;46:599–605. doi: 10.1007/s11255-013-0549-1. [DOI] [PubMed] [Google Scholar]


