Abstract
Radiation recall reaction is an acute inflammatory response evident in previously irradiated fields, induced by chemotherapy administration. Sixteen-year-old female with relapsed nasopharyngeal carcinoma was treated with gemcitabine and oxaliplatin. Disease remission was observed after four cycles. After the seventh cycle, patient developed acute pain and swelling involving the neck muscles. The affected muscles were within the previous irradiation field. Her symptoms improved with corticosteroid treatment. In contrast to other chemotherapy agents, gemcitabine can induce recall reaction involving deeper tissues. Gemcitabine therapy should be discontinued in the event of a radiation recall, as subsequent exposure will likely exacerbate symptoms.
Keywords: Radiation Recall, Myositis, Gemcitabine, Nasopharyngeal Carcinoma
Introduction
Combined modality therapy with surgery, irradiation, and chemotherapy is the standard of care for many pediatric solid tumors. While the adverse effects of an individual modality is well known, the adverse events resulting from interaction between the modalities is not well understood. The toxicity of radiation therapy is influenced by multiple factors including the tissue volume irradiated, daily and cumulative dose, fraction size and schedule, treatment techniques, patient age, physiologic reserve, co-morbidities, and genetics. Radiation therapy toxicity may also depend on concomitant chemotherapy; a quintessential example is the phenomenon of ‘radiation recall’ reaction secondary to subsequent systemic chemotherapy.1
D’Angio initially described a radiation recall reaction as an acute inflammatory response evident in previously irradiated fields that is induced when chemotherapy is administered after radiotherapy.1 Chemotherapeutic agents reported to cause a radiation recall reaction include dactinomycin, anthracyclines (doxorubicin), taxanes (docetaxel), and antimetabolites (gemcitabine).2–4 It is unclear whether the association of a radiation recall reaction with these agents is representative of a class effect, dosing regimen, or simply resultant of their prevalent application.2 The skin is the most common site of a radiation recall reaction, comprising about two-thirds of reported cases.1–2 The remaining third arise in other sites such as muscle or internal organs.1,5,6 Previous reports describing gemcitabine-induced radiation recall reactions have predominantly been in non-cutaneous sites with a majority involving soft tissues and internal organs, occurring weeks to months after radiation therapy.1,2,4,6,–8 Here, we report a pediatric patient with radiation recall myositis secondary to gemcitabine administration.9
Case Presentation
A 16 year old previously, healthy female presented with a five month history of bilateral neck swelling. Computed tomography (CT) and magnetic resonance imaging (MRI) of the head and neck revealed a nasopharyngeal mass and bilateral cervical lymphadenopathy. Whole body positron emission tomography (PET) scan was negative for distant metastasis. Left neck nodal biopsy revealed World Health Organization (WHO) Grade 3 undifferentiated nasopharyngeal carcinoma. The patient was diagnosed with a clinical Stage III (T2N2M0) nasopharyngeal carcinoma. She was treated with induction chemotherapy with cisplatin and 5-fluorouracil followed by concurrent cisplatin and radiation therapy to the head and neck to a total dose of 70.2 Gy using intensity modulated radiation therapy (IMRT).
At treatment culmination, the patient experienced acute right hip pain. Positron emission tomography (PET) revealed multiple osseous lesions along the axial skeleton and a paravertebral mass at the level of the eighth to tenth thoracic vertebrae concerning for disease progression. The patient was started on chemotherapy with gemcitabine (1000 mg/m2) and oxaliplatin (100 mg/m2) every two weeks. After four cycles, she had complete radiographic response of her metastatic lesions. At the end of the seventh cycle, the patient experienced acute bilateral neck swelling and pain. On examination, she had bilateral, firm, indurated, poorly defined swelling in the neck and restriction of neck movements due to pain. MRI of the head and neck revealed diffuse soft tissue edema of the muscles in the bilateral cervical neck region, consistent with myositis. We considered a potential para-neoplastic syndrome with dermatomyositis/polymyositis in the setting of nasopharyngeal carcinoma.10 Additionally, in our prime differential diagnosis, we considered autoimmune/inflammatory etiologies (polymyositis, dermatomyositis, or vasculitis), endocrinopathies (hypothyroidism), electrolyte abnormalities (hypokalemia or hypocalcemia) and infection (Epstein Barr Virus). Serum Creatinine kinase level was within normal limits and Epstein Barr Virus PCR was negative. In addition, we consulted the rheumatologist for a potential comprehensive rheumatologic work up with serologies including anti-nuclear antibodies, antibodies against nuclear antigens such as (anti-Ro/SSA, anti-La/SSB, anti-Sm, and anti-RNP), and more specific myositis antigens such as anti-histidy-tRNA (anti-Jo-1) as well as potential muscle biopsy. Based on the patient’s clinical picture, rheumatologist advised against such a work-up. Her symptoms resolved without intervention. After the eighth cycle of gemcitabine, the patient’s neck symptomatology recurred. The patient’s clinical presentation was ultimately concerning for myositis associated with a radiation recall reaction induced by gemcitabine given the correlation with radiation treatment volume and radiographic evidence of myositis (Figures 1 and 2). The timeframe from the final treatment of radiation to the development of myositis was 6 months. The patient was started on oral dexamethasone with immediate improvement, but the symptoms returned when the steroids were weaned. A low dose maintenance prednisone was started, and she has been asymptomatic for the past four months.
Figure 1.
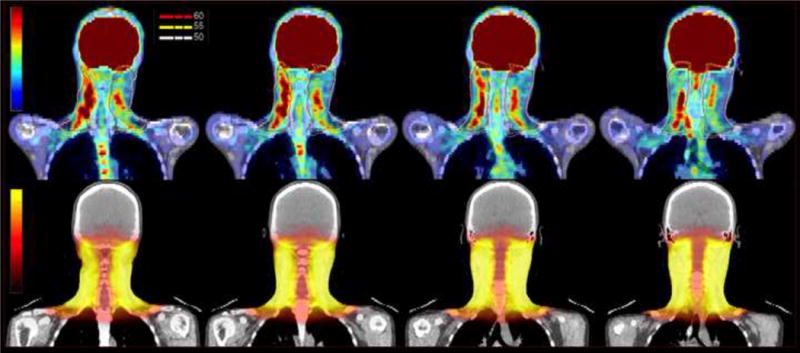
PET CT scan of the neck. Top row – coronal sections showing increased uptake in areas of myositis with superimposed 50 Gy, 55 Gy and 60 Gy isodose lines. Bottom row – corresponding coronal images from radiation treatment planning CT with dose color wash.
Figure 2.
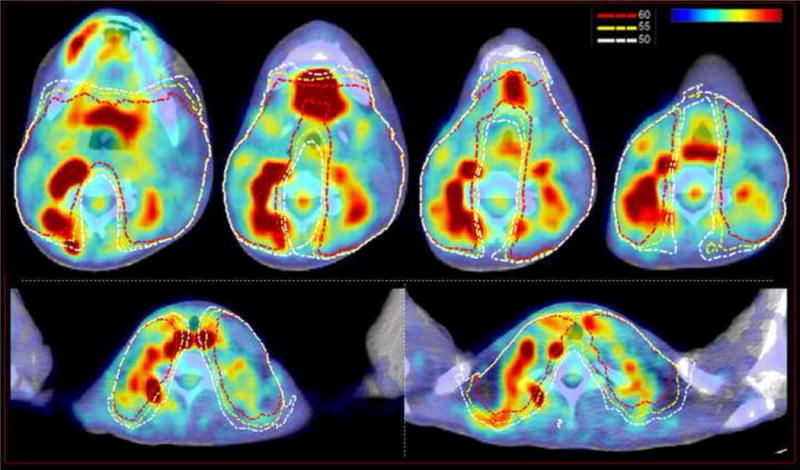
PET CT scan of the neck. Axial images showing increased uptake in areas of myositis with superimposed 50 Gy, 55 Gy and 60 Gy isodose lines.
Discussion
The temporality and true incidence of radiation recall reaction is not well defined. In general, “radio-sensitization reaction” occurs within a week of radiation exposure.2,5 Since a radiation recall reaction may occur weeks, months, or years after irradiation, its mechanisms are likely different from those of radio-sensitization.5,6 It is suggested that radiation recall reactions occur more commonly in radiation treatment plans with high energy photons (>6 MV), higher treatment doses and shorter time intervals between radiation therapy and subsequent chemotherapy, but there are no well-defined thresholds or parameters.2,11–13 A radiation recall reaction is deemed apparent after a comprehensive evaluation has ruled out any other causes of inflammation.2,14 Additionally, “re-challenge” with the inciting chemotherapy agent does not always accentuate the initial radiation recall reaction, making it more difficult to understand its inherent nature and causative factors.1,2,14 The actuarial risk of occurrence is difficult to measure given the paucity of literature, but observational studies have reported anywhere between 1–10%.2,5
Gemcitabine induced radiation recall is characterized by involvement of muscle and internal organs in addition to cutaneous involvement. There are more than 40 cases of gemcitabine induced radiation recall described including two in children.9,15 In the first report, a pediatric patient with refractory Hodgkin disease and history of chest irradiation developed a pericardial effusion after four cycles of gemcitabine and vinorelbine.15 In the second pediatric report, a 14-year-old female with right forearm synovial sarcoma developed severe swelling of her right forearm after 2 cycles of gemcitabine and docetaxel administered after 48 Gy irradiation.9. Similar to our patient, she had to continue low dose steroids for several months. Several mechanisms for radiation-induced myositis have been postulated including endothelial proliferation, cytokine mediated injury, and low-level chronic inflammatory response related to irradiation accentuated by subsequent chemotherapy injury.5 The timing of radiation recall reaction after radiation therapy ranges from a few months to even several years. Therefore, any patient with previous radiation exposure should be considered at risk for gemcitabine induced radiation recall. Similar to the current patient, radiation recall may not become apparent until after several cycles of gemcitabine administration. Therefore patients need continuous monitoring for development of radiation recall. Treatment of a radiation recall reaction depends on the site and severity. Many cases resolve spontaneously after optimal symptom management and supportive care.1,2,14 Systemic corticosteroids are effective for immediate symptom control but some patients may need a prolonged maintenance regimen. Gemcitabine therapy should be discontinued in the event of a radiation recall as subsequent exposure will likely exacerbate symptoms. Reactions may resolve in days, weeks, or months after halting the inciting chemotherapy regimen.1,2,14 At present, it is impossible to predict which factors predispose someone to develop radiation recall myositis.16
Conclusion
Herein, we report a case of radiation recall myositis subsequent to gemcitabine in a pediatric patient with nasopharyngeal carcinoma. As the utilization of gemcitabine containing regimens in pediatrics increases, awareness of the radiation recall phenomenon secondary to gemcitabine is paramount. Concentrated efforts must be applied to better define the individual patient-specific contributing factors (genetic, molecular, and biological), the specific drug factors, and the environmental factors that may contribute to a radiation recall reaction.
Footnotes
Conflicts of Interest Notification: No conflicts of interest or disclosures to report for all authors.
References
- 1.D’Angio GJ, Farber S, Maddock CL. Potentiation of x-ray effects by actinomycin D. Radiology. 1959;73:175–77. doi: 10.1148/73.2.175. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227–37. doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodym E, Kalinska R, Ehringfeld C, Sterbik-Lamina A, Kodym R, Hohenberg G. Frequency of radiation recall dermatitis in adult cancer patients. Onkologie. 2005;28:18–21. doi: 10.1159/000082175. [DOI] [PubMed] [Google Scholar]
- 4.Smith KJ, Germain M, Skelton H. Histopathologic features seen with radiation recall or enhancement eruptions. J Cutan Med Surg. 2002;6:535–40. doi: 10.1007/s10227-001-0156-0. [DOI] [PubMed] [Google Scholar]
- 5.Delavan JA, Chino JP, Vinson EN. Gemcitabine-induced radiation recall myositis. Skeletal Radiol. 2015;44:451–5. doi: 10.1007/s00256-014-1996-1. [DOI] [PubMed] [Google Scholar]
- 6.Graf SW, Limave VS, Cleland LG. Gemcitabine-induced radiation recall myositis in a patient with dermatomyositis. Int J Rheum Dis. 2014;17:696–7. doi: 10.1111/1756-185X.12233. [DOI] [PubMed] [Google Scholar]
- 7.Welsh JS, Torre TG, DeWeese TL, O’Reilly S. Radiation myositis. Ann Oncol. 1999;10:1105–08. doi: 10.1023/a:1008365221440. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander PA, Bansal R, Schwartz L, Wagman R, Posner J, Kemeny N. Gemcitabine-related radiation recall preferentially involves internal tissue and organs. Cancer. 2004;100:1793–99. doi: 10.1002/cncr.20229. [DOI] [PubMed] [Google Scholar]
- 9.Eckhardt MA, Bean A, Selch MT, Federman N. A child with gemcitabine-induced severe radiation recall myositis resulting in a compartment syndrome. J Pediatr Hematol Oncol. 2013;35:156–61. doi: 10.1097/MPH.0b013e31827e4c28. [DOI] [PubMed] [Google Scholar]
- 10.Chen DY, Chen YM, Lan JL, Chen HH, Hsieh CW, Wey SJ, Lu JJ. Polymyositis/dermatomyositis and nasopharyngeal carcinoma: the Epstein-Barr virus connection? J Clin Virol. 2010;49:290–95. doi: 10.1016/j.jcv.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Mizumoto M, Harada H, Asakura H, Zenda S, Fuji H, Murayama S, Nishimura T. Frequency and characteristics of docetaxel-induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys. 2006;66:1187–91. doi: 10.1016/j.ijrobp.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 12.Yeo W, Leung SF, Johnson PJ. Radiation-recall dermatitis with docetaxel establishment of a requisite radiation threshold. Eur J Cancer. 1997;33:698–9. doi: 10.1016/s0959-8049(96)00461-3. [DOI] [PubMed] [Google Scholar]
- 13.Stelzer KJ, Griffin TW, Koh WJ. Radiation recall skin toxicity with bleomycin in a patient with Kaposi sarcoma related to acquired immune deficiency syndrome. Cancer. 1993;71:1322–5. doi: 10.1002/1097-0142(19930215)71:4<1322::aid-cncr2820710425>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Azria D, Magne N, Zouhair A, Castadot P, Culine S, Ychou M, Stupp R, Van Houtte P, Dubois JB, Ozsahin M. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31:555–70. doi: 10.1016/j.ctrv.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Cole PD, Schwarz CL, Drachtman RA, de Alarcon PA, Chen L, Trippett TM. Phase II study of weekly gemcitabine and vinorelbine for children with recurrent or refractory Hodgkin’s disease: a children’s oncology group report. J Clin Oncol. 2009;27:1456–61. doi: 10.1200/JCO.2008.20.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pentsova E, Liu A, Rosenblum M, O’ Reilly E, Chen X, Hormigo A. Gemcitabine induced myositis in patients with pancreatic cancer: case reports and topic review. J Neurooncol. 2012;106:15–21. doi: 10.1007/s11060-011-0672-8. [DOI] [PubMed] [Google Scholar]


