Abstract
Peroxisomes are ubiquitous eukaryotic organelles with the primary role of breaking down very long- and branched-chain fatty acids for subsequent β-oxidation in the mitochondrion. Like mitochondria, peroxisomes are major sites for oxygen utilization and potential contributors to cellular oxidative stress. The accumulation of oxidatively damaged proteins, which often develop into inclusion bodies (of oxidized, aggregated, and cross-linked proteins) within both mitochondria and peroxisomes, results in loss of organelle function that may contribute to the aging process. Both organelles possess an isoform of the Lon protease that is responsible for degrading proteins damaged by oxidation. While the importance of mitochondrial Lon (LonP1) in relation to oxidative stress and aging has been established, little is known regarding the role of LonP2 and aging-related changes in the peroxisome. Recently, peroxisome dysfunction has been associated with aging-related diseases indicating that peroxisome maintenance is a critical component of ‘healthy aging’. Although mitochondria and peroxisomes are both needed for fatty acid metabolism, little work has focused on understanding the relationship between these two organelles including how age-dependent changes in one organelle may be detrimental for the other. Herein, we summarize findings that establish proteolytic degradation of damaged proteins by the Lon protease as a vital mechanism to maintain protein homeostasis within the peroxisome. Due to the metabolic coordination between peroxisomes and mitochondria, understanding the role of Lon in the aging peroxisome may help to elucidate cellular causes for both peroxisome and mitochondrial dysfunction.
Keywords: Lon protease, mitochondria, peroxisome, aging, mitochondrial DNA, hormesis, reactive oxygen species, oxidative stress, chaperone
I. INTRODUCTION
Peroxisomes are highly dynamic organelles that can modulate their size and number depending on the cell’s metabolic demands (Lopez–Huertas et al., 2000). While both mitochondria and peroxisomes are equally important in the β-oxidation pathway, much greater emphasis has been placed upon understanding the role of the mitochondria rather than peroxisomes. Thus, it is well established that mitochondria are critical for a number of cellular functions that include acting as metabolic signalling centres and contributing to cellular stress responses such as autophagy, redox signalling, oxidative stress and apoptosis (Lenaz et al., 2002). Mitochondrial dysfunction has been shown to contribute to a number of diseases, including neurodegenerative and metabolic disorders (Cassarino & Bennett, 1999; Wallace, 2005).
Due to mitochondria being major sites for oxygen consumption, mitochondrial maintenance relies heavily on the proteolytic activity of Lon, the major protease in the mitochondrial matrix. The mitochondrial Lon protease (LonP1) degrades proteins damaged by reactive oxygen species generated by electron leakage from the mitochondrial respiratory chain, and also maintains the integrity of mitochondrial DNA (Bota & Davies, 2002; Matsushima, Goto & Kaguni, 2010). In the cytoplasm, nucleus and endoplasmic reticulum, the proteasome is responsible for selectively degrading oxidatively damaged proteins (Ullrich et al., 1999; Davies, 2001; Pickering et al., 2010, 2012) but in mitochondria, which have no proteasome, this task is accomplished by LonP1 (Bota & Davies, 2002). Thus mitochondrial LonP1, as might be expected of such an important, multi-functional enzyme, is highly conserved from prokaryotes to eukaryotes (Wang et al., 1993). In addition to the mitochondrial isoform of Lon (LonP1), there also exists a peroxisome-specific isoform known as LonP2. While mitochondrial Lon has been associated with adaptation to acute oxidative stress and has been shown to decline with age (Ngo & Davies, 2009; Ngo et al., 2011), both of which are attributes of the proteasome in other parts of the cell (Pickering et al., 2010, 2012), the role of the peroxisomal isoform of Lon in adaptation is not as well understood. This limited insight into Lon’s role within the peroxisome may be further restricted by an incomplete understanding of peroxisomal physiology, beyond its well-defined role in fatty acid metabolism.
The peroxisome is an abundant organelle, present in the majority of eukaryotic cells. Peroxisomes are self-replicating organelles that can also arise by budding off the endoplasmic reticulum (ER), and that are in dynamic flux to accommodate the metabolic needs of the cell. Unlike mitochondria, which have a double membrane, peroxisomes possess a single membrane with a crystalline core composed of urate oxidase (Lazarow, 1995). One of the primary functions of peroxisomes is their role in fatty acid metabolism, which also results in the synthesis of hydrogen peroxide (H2O2) as a by-product (Lazarow, 1995). Fortunately, peroxisomes contain enzymes, such as catalase and glutathione peroxidase, that can reduce H2O2 back to O2 and H2O (2H2O2 → 2H2O + O2), because uncontrolled levels of H2O2 could instigate damage to various cellular constituents (Nordgren & Fransen, 2014). Additionally, LonP2 can degrade proteins damaged by H2O2 that escape reduction. Thus, the peroxisome-specific Lon protease plays a major role in maintaining peroxisomal protein homeostasis, and provides a critical mechanism to avoid the accumulation of oxidative damage (Friguet, 2006).
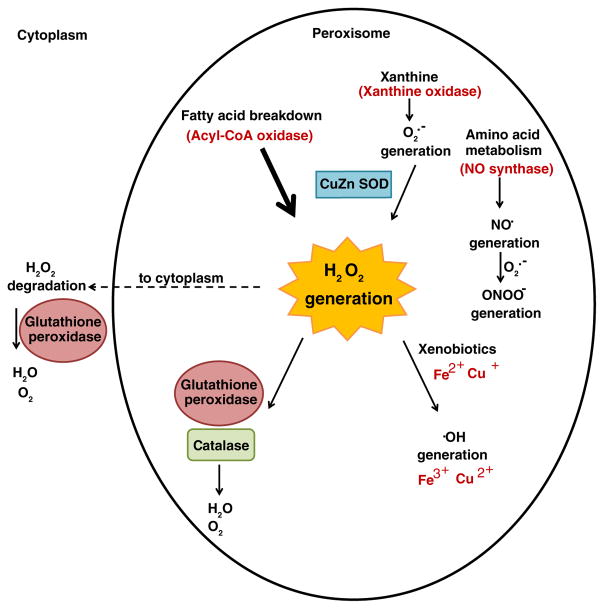
Despite the widespread perception of H2O2 and other reactive oxygen species as merely toxic by-products of aerobic metabolism, increasing evidence indicates that peroxisome-generated H2O2 may act as a signalling molecule involved in the stimulation of various pathways, such as cell proliferation, differentiation, migration, and apoptosis (Veal, Day & Morgan, 2007; Titorenko & Terlecky, 2011). At low concentrations, H2O2 can function as a non-toxic signal due to its ability to stimulate stress-response pathways by triggering the increased expression of various repair proteins and chaperones that can mitigate against oxidative damage (Jamieson, 1998; Narasimhan, Yen & Tissenbaum, 2009). This type of signalling pathway has already been well documented in mitochondria, which can release a sub-lethal dose of H2O2 resulting in activation of oxidant-sensitive pathways (Finley & Haigis, 2009). Because peroxisomes have been shown to dissipate some 20–60% of the H2O2 they generate into the cytoplasm (Boveris, Oshino & Chance, 1972), they may potentially play a similar role as mitochondria in redox cell signalling. This hypothesis is further supported by the high abundance of peroxisomal enzymes, such as catalase and gluthathione peroxidase, that control the degradation of H2O2 and may help maintain cellular redox homeostasis (Fig. 1) (Titorenko & Terlecky, 2011). Moreover, peroxisome dysfunction has been found to be associated not only with genetically based peroxisome disorders, but also with many chronic aging-related diseases such as Type II diabetes and cancer (Nordgren & Fransen, 2014), as well as aging itself (Perichon et al., 1998). Since an imbalance between the production and removal of reactive oxygen is a hallmark of aging and aging-related diseases, increased understanding of peroxisome dysfunction and peroxisomal Lon may also shed light on the aging-related decline of peroxisome function.
Fig. 1.
Sources of superoxide, hydrogen peroxide, nitric oxide radical, peroxynitrite, and hydroxyl radical production in the peroxisome. Hydrogen peroxide (H2O2) is the main metabolic by-product of fatty acid metabolism in peroxisomes. The primary oxidase to carry out the direct generation of H2O2 is acyl CoA oxidase (Foerster et al., 1981). In addition, superoxide (O2• −), a by-product of the breakdown of xanthine, is scavenged by copper-zinc superoxide dismutase (CuZn SOD) and converted into H2O2 and O2 (Foerster et al., 1981). The majority of H2O2 produced in the peroxisome is degraded by catalase and glutathione peroxidase. The remainder may undergo Fenton chemistry, resulting in the formation of the hydroxyl radical (•OH). In addition, a portion of H2O2 is leaked into the cytoplasm, where it is reduced by glutathione peroxidase (Halliwell & Gutteridge, 1989). Breakdown of amino acids may generate the nitric oxide radical (NO•) which reacts rapidly with O2• − to generate peroxynitrite (ONOO−).
II. THE ORIGIN OF PEROXISOMES
Organelles may be inherited from parental cells or synthesized de novo from the ER (Misteli, 2001). Neither mitochondria nor the ER can be generated by cells, therefore failure in their inheritance by daughter cells leads to apoptosis (Hoepfner et al., 2005). When peroxisomes were discovered, they were originally suggested to be descendants of the ER (Novikoff & Shin, 1964) and it was hypothesized that they were inherited organelles (De Duve, 1996), in part because peroxisomes were considered to be precursors of the more efficient mitochondria, which were thought to act as both detoxifying agents and energy powerhouses (De Duve, 1996). This viewpoint, however, fails to explain the regenerative, ‘self-replicating’ capabilities of peroxisomes (Shio & Lazarow, 1981; Lazarow & Fujiki, 1985). Experiments in yeast and human fibroblasts lacking peroxisomes demonstrate the adeptness of these cells to regenerate peroxisomes (Hoepfner et al., 2005). Cells lacking peroxisomes, due to the mutation of peroxisome genes, undergo de novo synthesis upon complementation of wild-type peroxisome genes, even after multiple passages of cells lacking peroxisomes (Subramani, 1998). Furthermore, electron microscopy has shown the physical connection between peroxisomes and ER membranes (Subramani, 1998).
The process of peroxisome formation from the ER, and the demonstration of cellular de novo synthesis of these organelles, was not fully accepted until the work of Hoepfner et al. (2005). Utilizing two mutant Saccharomyces cerevisiae strains (PEX3 and PEX19) they demonstrated the formation of peroxisomes from a specialized compartment within the ER. Peroxin 3 (Pex3) and Peroxin 19 (Pex19) are generated in the cytoplasm and first targeted to the ER before appearing in the peroxisome (Hoepfner et al., 2005). Results from fluorescent imaging showed the initial interaction between Pex3 and Sec63, both critical membrane proteins, during peroxisome membrane formation within the ER. Budding of this membrane indicated that Pex3 becomes concentrated into structures that form adjacent to the ER, and that there is a subsequent migration of Pex19 to these structures (Hoepfner et al., 2005). The physical interaction of Pex3 and Pex19 results in the import of proteins into the peroxisomal membrane (Hoepfner et al., 2005). The subsequent incorporation of peroxisomal membrane proteins (PMPs) and the budding of the maturing peroxisome from the ER leads to the import of peroxisome matrix proteins, through Pex5 and Pex7 (Gould & Valle, 2000).
Recently, this foundational work in peroxisome biogenesis was challenged by Knoops et al. (2014). Their findings report that after reintroduction of Pex3 in a Pex3-deficient Hansenula polymorpha strain, it is not incorporated into the ER, but is instead collected in pre-peroxisomal structures that mature into normal peroxisomes. Interestingly, these pre-peroxisomal structures are present even upon the deletion of Pex3 (Knoops et al., 2014). Therefore, there is currently evidence that peroxisomes use two mechanisms of proliferation: fission from pre-existing peroxisomes and budding from the ER, and that different organisms may display preference for one pathway over the other.
Recognition of peroxisomal enzymes is accomplished via two targeting sequences: peroxisome targeting signal 1 (PTS1) and peroxisome targeting signal 2 (PTS2). PTS1 is comprised of three amino acids (serine, lysine/arginine, leucine), and is the predominant targeting signal for most peroxisome proteins (Brocard & Hartig, 2006). Unlike other imported organelle proteins, the PTS1 signal is located at the C-terminus of target proteins and is recognized by Pex5 (Gould & Valle, 2000). This differs from other targeting signals located on the N-terminus of most imported proteins, which are typically removed prior to, or during, transport into the targeted organelle (Brocard & Hartig, 2006). As a result, proteins destined for the peroxisome must be fully synthesized, and proteolytically matured in the cytoplasm, before being imported into the peroxisome matrix (Brocard & Hartig, 2006). This mechanism may provide a protein quality-control measure, as peroxisome-targeted proteins can assume their mature and active states prior to import. This protein preassembly obviates the need for peroxisomes to import and maintain their own assortment of molecular chaperones to assist in the correct folding and assembly of peroxisome matrix proteins (Brocard & Hartig, 2006). However, the conjecture that imported proteins must be in a native conformational state prior to import was challenged by the import of denatured human serum albumin (HSA) incorporated with a PTS1 sequence, demonstrating that the predominant determinant for protein import is the presence of the PTS1 localization signal, rather than the state of protein folding (Brocard et al., 2003).
The second peroxisome targeting signal, PTS2, is a less common localization sequence comprised of nine amino acids located on the N-terminus of proteins, which is recognized by Pex7 (Gould & Valle, 2000). When Pex7 and Pex5 are attached to a substrate, they are recognized and bound by Pex13 and Pex14 to generate a pore within the peroxisome membrane, resulting in the subsequent release and import of the peroxisome-targeted protein (Gould & Valle, 2000).
III. THE DISCOVERY OF PEROXISOME-SPECIFIC LON
Peroxisomes were discovered in the mid-1950s and were initially characterized as ‘microbodies’ (Rhodin, 1954). Their actual metabolic functions were not uncovered until a decade later, at which time it was also found that peroxisomal enzymes generate H2O2 as a metabolic by-product of fatty-acid metabolism (Cooper & Beevers, 1969; Lazarow & De Duve, 1976), leading to the coining of the name ‘peroxisomes’ (De Duve & Baudhuin, 1966). The identification of which enzymes are actually targeted to the peroxisome was limited until the advent of mass spectrometry. Thus, the discovery of the peroxisomal isoform of Lon did not occur until 2004 (Kikuchi et al., 2004). In order to identify organelle proteins, the organelles must first be purified from other cellular components. Due to their functional similarity, it is critical to ensure the complete removal of contaminating mitochondria when isolating peroxisomes. The method outlined by Ghosh & Hajra (1986) is still the standard approach for peroxisome isolation from rat liver, which relies upon the Nycodenz density gradient to remove the majority of other cellular components, with an additional purification step suggested by Kikuchi et al. (2004) that utilizes immunoprecipitation by targeting a peroxisomal membrane protein (PMP70).
Localization of Lon to the peroxisome was demonstrated in both rat and human tissue through the use of an antibody generated against the LonP2 C-terminal sequence found in mice (Kikuchi et al., 2004). Further studies, employing ultracentrifugation, indicated that LonP2 is not only an abundant enzyme in the peroxisome, but concentrates specifically within the dense crystalline core (Kikuchi et al., 2004). Of note, the crystalline core found in rat liver peroxisomes is primarily comprised of urate oxidase and is nearly devoid of catalase and other oxidases, which may highlight a possible explanation for the concentration of LonP2 within the peroxisomal core (Hayashi et al., 1976). As H2O2 cannot be degraded in this peroxisomal sub-compartment, it likely contributes to the rapid oxidation of proximal urate oxidase that may contribute to protein damage. Hence, it is logical that a proteolytic enzyme, such as LonP2, would also be in close proximity so as to prevent protein aggregation. Furthermore, the relative abundance of Lon within the highly oxidizing environment of the peroxisome’s core highlights a similarity shared with LonP1, the mitochondrial isoform of Lon. Both enzymes are in high concentration and close proximity to proteins that are at risk of oxidative damage and have the need for rapid degradation.
In mammals, it has since been found that peroxisomal Lon is ubiquitous throughout the body, with particularly high expression patterns in the pancreas, kidney, and liver (Omi et al., 2008). In addition, although a peroxisome-specific isoform of Lon has been identified in many widely diverse organisms (rat, mouse, Caenorhabditis elegans and human), LonP2 may not be as highly conserved as the mitochondrial isoform, since it has not been physically detected in Drosophila melanogaster (Bartoszewska et al., 2012).
IV. CHARACTERIZATION OF THE MITOCHONDRIAL (LONP1) AND PEROXISOMAL (LONP2) ISOFORMS OF LON
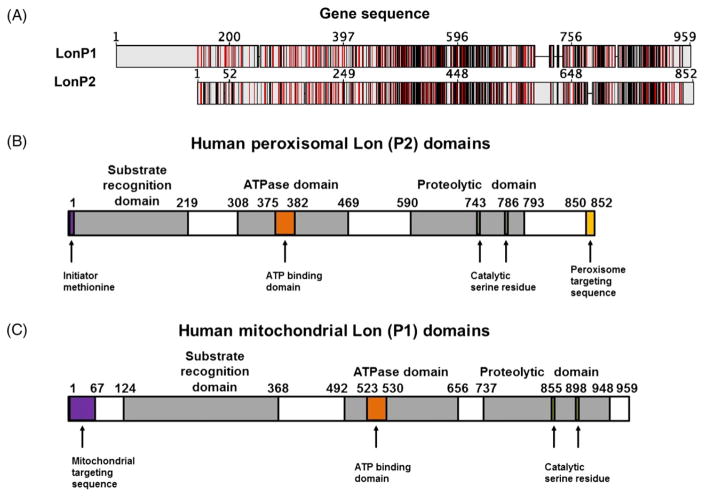
Both isoforms of Lon contain conserved domains that catalyse proteolytic activity within the peroxisome and the mitochondrion. Peroxisome-specific Lon, LonP2, is comprised of an 852 amino acid sequence belonging to the Lon A family, the primary form of Lon found in prokaryotes and eukaryotes (Altschul et al., 1997; Bartoszewska et al., 2012), and has a molecular weight of 95 kDa (Kikuchi et al., 2004). In humans, LonP2 is encoded within the nucleus from chromosome 16, assembled in the cytoplasm, and targeted to the peroxisome matrix by the PTS1 targeting signal located on its C-terminus (Gould et al., 1989). Prior to import, it is hypothesized that LonP2 folds into its catalytically active conformation consisting of three domains: the Lon N (‘substrate recognition’) domain, the ATPase domain, and the proteolytic domain (Fig. 2B). Substrates of LonP2 are recognized by a non-catalytically active serine residue within the Lon N domain that facilitates binding with substrates. LonP2 is an ATPase, which expedites degradation of oxidized proteins through the hydrolysis of ATP. The ATPase domain contains an AAA region consisting of the Walker A and B motifs, which are conserved three-dimensional protein structures characteristic of ATPases (Altschul et al., 1997; Dougan et al., 2002). Lastly, the proteolytic domain contains a catalytically active serine–lysine dyad for enzymatic substrate degradation (Stahlberg et al., 1999; Bartoszewska et al., 2012).
Fig. 2.
Peroxisomal and mitochondrial Lon protease. (A) The sequence alignment between the mitochondrial (LonP1) and peroxisomal (LonP2) forms of Lon. Identical sequences are shown in red and similar sequences are shown in grey. (B, C) Conserved domains of the P2 peroxisome-specific (B) and P1 mitochondrion-specific (C) isoforms of Lon. Both LonP1 and LonP2 contain a substrate recognition, ‘N’ domain to bind oxidized proteins. Both isoforms possess a classical ATPase domain containing Walker (A) and (B) motifs that have a characteristic tertiary structure commonly found in ATP-binding proteins. Lastly, both contain a proteolytic domain that relies upon a catalytically active serine residue for substrate degradation. However, unlike LonP1, which has a mitochondrial targeting sequence on the N-terminus, LonP2 relies upon the peroxisomal targeting sequence, which consists of a conserved three amino acid SRL motif.
Similarly, mitochondrion-specific Lon, LonP1, is 959 amino acids in length with a molecular weight of 100 kDa and also contains the three characteristic domains of the Lon A family: a substrate-recognition ‘N’ region, an ATP-binding and hydrolysis region, and a serine-proteolytic site (Fig. 2C) (Ngo & Davies, 2007). In addition, human lonP1 is a nuclear-encoded gene from chromosome 19 that is transported into mitochondria via a mitochondrial targeting sequence located at the N-terminus (Wang et al., 1994). As LonP1 is imported into the mitochondrion, the mitochondrial targeting sequence is removed and it is folded into a catalytically active state, becoming either a six-or seven-membered, monomeric ring complex. Therefore, both the peroxisomal and mitochondrial isoforms of Lon demonstrate conserved domain similarity, but work is still needed to determine if these proteins evolved in parallel or from the same ancestral lineage.
The relationship between structure and function was further explored by statistical modelling (Bartoszewska et al., 2012) using the yeast species Penicilium chysogenum. Peroxisomal Lon assumes a heptameric ring structure consisting of seven monomers, which is consistent with the mitochondrial Lon homologue observed in S. cerevisiae (Stahlberg et al., 1999). The similarity between the tertiary structures of yeast LonP1 and LonP2 is consistent with the overall structural conservation between the two isoforms, especially within the C-terminal region containing the ATPase domain (Dougan et al., 2002). This conserved structural similarity, as well as the localization within the matrix of mitochondria or peroxisomes, further suggests the conserved role of peroxisomal Lon as a protease. Sequence comparison of the mitochondrial and peroxisomal isoforms of Lon revealed 39.6% of the amino acid sequence to be conserved (Fig. 2A) (Geneious Pro 5.1.7). This overall sequence similarity, although not high, sheds light upon key regions that contain critical domains that are highly conserved. The regions that show the greatest overlap are within the conserved ATPase domain and the proteolytic domain. In addition, there is some similarity between the substrate recognition domains. The high amount of overlap within the functional domains of the two proteases suggests that peroxisomal Lon may have a similar function to mitochondrial Lon.
V. THE FUNCTIONAL ROLE OF PEROXISOMAL LON
Peroxisomes play a central role in cellular lipid metabolism and in the metabolism of oxidation by-products, evident from the variety of enzymes capable of generating or degrading H2O2 and superoxide (Nordgren & Fransen, 2014). However, scavenging of H2O2 and superoxide is not perfect, and some proteins do undergo oxidative modification. Because a degree of protein oxidation is an inevitable outcome of peroxisomal metabolism, the ability to degrade proximal proteins damaged through oxidation is critical for peroxisome homeostasis (Till et al., 2012), as excessive protein oxidation can result in protein aggregation and eventual loss in peroxisome function (Terlecky, Koepke & Walton, 2006).
Early studies that focused on determining the functional role of Lon in the peroxisome utilized the yeast strain, H. polymorpha. To understand if peroxisomal Lon played a role in degrading misfolded proteins, investigators created a mutant dihydrofolate reductase (DHFR) incapable of correctly folding, which, upon transport into the peroxisome, was subsequently degraded. Yeast strains lacking LonP2 showed stabilization of the mutated form of DHFR. In addition, a mutant form of alcohol oxidase, which is misfolded upon peroxisome import indicated that in the absence of LonP2, the misassembled protein was not degraded (Aksam et al., 2007). Together, these findings support the role of perixosomal Lon as a critical protease to degrade peroxisome matrix proteins (Aksam et al., 2007).
Further studies have examined the role of Lon in the quality control of proteins in the peroxisome. Similar to its mitochondrial isoform, peroxisome-specific Lon is an ATP-stimulated enzyme (Bartoszewska et al., 2012). The ATP-stimulated activity of LonP2 was discovered from studies using the fungus, P. chrysogenum. In in vitro assays, isolated LonP2 rapidly degraded misfolded α- or β-casein in the presence of ATP (Bartoszewska et al., 2012). In addition, quantitative measurement of the proteolytic degradation of resorufin-labelled β-casein demonstrated high proteolytic activity of LonP2 only upon the addition of ATP (Bartoszewska et al., 2012). As proof of principle, a mutation at the catalytically active serine residue rendered the enzyme non-functional and in the presence of either α-or β-casein and ATP, the substrate could not be degraded (Bartoszewska et al., 2012). We suggest that future studies should delve further into understanding the specific role of ATP in the enzymatic activity of LonP2. This is important because LonP1 utilizes ATP hydrolysis not for substrate degradation, but rather for a conformational change in its proteolytic site to allow for proteolysis (Patterson-Ward, Huang & Lee, 2007). The inclusion of other ATP variants, specifically non-hydrolysable ATP analogs, would inform whether LonP2 utilizes ATP in a similar or different manner from that of LonP1. We feel that such initial in-vitro studies will be important in determining whether peroxisomal Lon can function as an ATP-stimulated protease, mirroring the role of mitochondrial Lon.
Peroxisome-specific Lon has also been demonstrated to be necessary for cell viability. Preliminary studies, conducted in yeast, assessed cell survival by deleting the homolog for peroxisomal Lon, pln. In addition, a critical yeast signalling molecule for autophagy, atg1, was also deleted, forming pln.atg1 double mutants (Aksam et al., 2007). Singular deletion of peroxisomal Lon had little effect on cell viability, but did contribute to the elevation of reactive oxygen species, whereas the loss of atg1 significantly decreased cell survival. In the double mutant, loss of both pln and atg1 showed a synergistic effect, which further decreased cell survival (Aksam et al., 2007). This suggests that the proteolytic role of Lon is important for general protein maintenance of the peroxisome. However, the accumulation of high levels of protein damage may exceed the enzymatic degradation rate of Lon, making autophagy of the peroxisome the cell’s most efficient mechanism to control protein damage. The cumulative loss of both pln and atg1 greatly mitigated cell survival due to the increase in protein damage and inability to degrade the peroxisome when the damage became too extensive (Aksam et al., 2007).
In addition, pln and pln.atg1 deletion mutants had an increase in the accumulation of protein aggregates, as well as increased peroxisome number and size in P. chrysogenum (Aksam et al., 2007; Bartoszewska et al., 2012). The protein aggregates were hypothesized to be substrates for peroxisomal Lon, suggesting that LonP2 is necessary to prevent the accumulation of misfolded proteins in the peroxisome matrix. This downstream effect resulting from pln deletion is reminiscent of the protein aggregates that arise in C. elegans mutants lacking the mitochondrial Lon homologue (PIM1) (Erjavec et al., 2013). Protein aggregates are also a prevalent marker in senescent cells lacking mitochondrial Lon (Bota, Ngo & Davies, 2005). Together, this indicates that loss of peroxisomal Lon, coupled with the cell’s inability to degrade damaged proteins via pexophagy, results in electron-dense aggregates (Bartoszewska et al., 2012) which presumably accelerate cellular dysfunction.
Optimal protein quality control is crucial to counteract the continual damage resulting from reactive oxygen species generation, a trait common to both peroxisomes and mitochondria (Bulteau, Szweda & Friguet, 2006; Bonekamp et al., 2009). In P. chrysogenum, mutants lacking LonP2 showed increased DNA damage compared to their wild-type counterparts (Bartoszewska et al., 2012). DNA damage was further exacerbated when these mutants were grown in an environment that restricted access to a carbon source, through the breakdown of oleic acid via the β-oxidation pathway (Poirier et al., 2006). This restrictive approach greatly increased peroxisome metabolic activity and accelerated the generation of reactive oxygen species and subsequent oxidative damage (Bartoszewska et al., 2012). A similar trend was detected in the yeast strain, H. polymorpha where pln.atg1 double mutants exhibited a significant increase in intracellular levels of reactive oxygen species (Aksam et al., 2007). This demonstrates the connection between the metabolic state of peroxisomes, a major generator of H2O2, and the consequences of uncontrolled peroxisome protein maintenance.
Due to LonP2’s role in maintaining the peroxisome proteome during normal conditions, it is important to determine if its expression is stress dependent. This is plausible because mitochondrial-specific LonP1 expression is rapidly elevated following multiple types of stressors including heat shock, serum starvation and H2O2 exposure (Ngo & Davies, 2009). In addition, ER stress has been implicated in causing increased transcription of the mitochondrial Lon protease (Hori et al., 2002). Interestingly, peroxisomes proliferate in response to various forms of oxidative stress such as hypoxia/reoxygenation (also known as reperfusion injury) ultraviolet (UV) irradiation and H2O2 exposure, with increased expression of peroxisome Pex family import machinery proteins (Lopez–Huertas et al., 2000). Interestingly, in a glucose-rich environment these stressors do not appear to increase peroxisome-specific Lon protein levels in yeast (Aksam et al., 2007). However, for methylotrophic yeast strains cultured in methanol-enriched media, which is a documented trigger for peroxisome biogenesis, peroxisomal Lon expression was found to increase (Aksam et al., 2007). This may occur because methanol, as the sole carbon source, can only be broken down in peroxisomes, thereby resulting in peroxisome biogenesis and increased H2O2 production (van der Klei et al., 2006). Therefore it is difficult to distinguish if LonP2 levels increase simply because there are more peroxisomes or if peroxisomal LonP2 is induced to a higher extent than other peroxisomal enzymes. More work is needed to understand if peroxisomal Lon has a similar stress-response role to that exhibited by mitochondrial Lon.
During periods of decreased peroxisome content, removal of peroxisome proliferation signals causes Lon to degrade β-oxidation enzymes, a potential consequence of the decline in demand for peroxisome fatty-acid metabolism (Yokota, Haraguchi & Oda, 2008). Phthalate and adipate ester plasticizers, such as di(2-ethylhexyl)phthalate (DEHP) and di(2-ethylhexyl)adipate, are used to chemically induce peroxisome proliferation (Lock, Mitchell & Elcombe, 1989). Rats treated with DEHP for a period of 2 weeks exhibited an increase in peroxisome proliferation markers, specifically β-oxidation enzymes and acyl-CoA oxidase, in hepatic tissue. Lon levels were also increased during the 2-week drug regimen and reached maximal expression upon discontinuation of DEHP (Yokota et al., 2008). By contrast, the β-oxidation enzymes acyl-CoA oxidase and thiolase showed immediate decline following removal of DEHP, whereas Lon levels did not subside until 6 days following the termination of treatment (Yokota et al., 2008). Therefore, due to the lag in the reduction of Lon levels following discontinuation of DEHP and the concurrent immediate decline of β-oxidation enzymes, it is likely that peroxisome-specific Lon is necessary for the degradation of β-oxidation enzymes (Yokota et al., 2008). These results further support Lon’s integral role in maintaining overall peroxisome homeostasis, in addition to its role as a protease to degrade damaged proteins.
VI. LONP1’S ROLE IN MITOCHONDRIAL DNA INTEGRITY
LonP1 has been shown to interact directly with mitochondrial DNA (mtDNA) (Fu, Smith & Markovitz, 1997). High binding affinity of the prokaryotic Lon found in Escherichia coli showed binding to sequences similar to those found in the small non-coding region of the human mitochondrial displacement loop (D-loop) (Fu & Markovitz, 1998). Additional studies of E. coli Lon (La) found that during periods of stress, La blocks DNA replication through degradation of the transcriptional initiator, DnaA, halting division and allowing for repair (Jonas et al., 2013). Point-mutation within the ATPase-domain of La blocks protein degradation, but not DNA binding (Amerik et al., 1991; Fischer & Glockshuber, 1994). In a conserved manner, human LonP1 preferentially binds only to single-stranded mitochondrial DNA within the D-loop (Fu & Markovitz, 1998; Lu et al., 2003). The fact that LonP1 has no preference for binding to double-stranded DNA could suggest a potential role as a transcriptional regulator. This was confirmed by LonP1’s proteolytic regulation of mitochondrial transcription factor A (TFAM), which binds directly to the D-loop and is required for mtDNA integrity (Matsushima et al., 2010). Phosphorylation of TFAM prevents its binding to mtDNA and its subsequent degradation by LonP1 (Lu et al., 2013). Conversely, loss of LonP1 leads to the accumulation of TFAM. LonP1 has also been shown to regulate mitochondrial transcription factor B2 (mtTFB2), as loss of Lon leads to the accumulation of mtTFB2, a critical regulator of mitochondrial DNA transcription (Matsushima et al., 2010). In addition, loss of Lon has been linked to decreased respiration and lesions to mtDNA (van Dyck, Pearce & Sherman, 1994; van Dyck, Neupert & Langer, 1998; Guha et al., 2011). While the predominant role of Lon is the proteolytic degradation of oxidized proteins, it is clear that LonP1 may also contribute, albeit indirectly, to transcriptional regulation within the mitochondria.
VII. BIOLOGICAL SUBSTRATES FOR PEROXISOMAL LON
The electron-dense inclusion bodies that developed in the P. chrysogenum mutant with peroxisomes lacking Lon were primarily composed of normal Lon substrates that were unable to be degraded and, thus, accumulated and aggregated (Bartoszewska et al., 2012). Mass spectrometry revealed that a catalase-peroxidase was the major component of these peroxisome protein aggregates (Bartoszewska et al., 2012). Catalase-peroxidase is the most abundant H2O2 decomposing enzyme of the peroxisome. It is estimated that for every one molecule of H2O2-producing enzyme there is one molecule of catalase to counteract it, demonstrating the importance of catalase as a detoxifying enzyme in the peroxisome (Antonenkov et al., 2010). To investigate the proteolytic relationship between catalase-peroxidase and Lon, purified catalase was subjected to increasing concentrations of H2O2 until loss of protein conformation led to loss of enzymatic activity, and then was subsequently exposed to peroxisome-specific Lon. Without H2O2 pre-treatment, catalase-peroxidase was resistant to proteolysis by Lon. However, following exposure to low concentrations of H2O2, significant catalase degradation by LonP2 was observed (Bartoszewska et al., 2012). This finding demonstrates that oxidatively misfolded catalase-peroxidase, but not its native counterpart, is a good substrate for peroxisome-specific Lon. In a subsequent study, HEK293 cells stably overexpressing LonP2 caused the mislocalization of catalase and its accumulation in the cytoplasm. Moreover, catalase activity was greatly decreased, highlighting the additional role that peroxisomal Lon may play in catalase import (Omi et al., 2008).
The necessity to degrade nonfunctioning catalase is critical for peroxisome function. As catalase is a major peroxisomal enzyme, any loss of function can dramatically disregulate the balance between H2O2 generation and degradation (Antonenkov et al., 2010). This is important due to the large amount of H2O2 generated by peroxisomes. Studies using rat liver homogenates found the overall rate of H2O2 production to be 38 nmol min−1 g−1 of liver protein (Boveris et al., 1972). However, isolated peroxisomes are estimated to generate approximately 44–172 nmol min−1 g−1 protein. Fortunately, only some 30% of peroxisome-generated H2O2 actually appears to diffuse into the cytoplasm (approximately 13.2–51.6 nmol min−1 g−1 protein), where it is primarily broken down by glutathione peroxidase, and the remainder is degraded by catalase (Boveris et al., 1972). The relationship of H2O2 breakdown between glutathione peroxidase and catalase appears to be sigmoidal (Jones et al., 1981). At low homeostatic concentrations, catalase is the dominant catabolic enzyme, whereas at higher concentrations, glutathione peroxidase becomes the predominant means of breaking down H2O2 (Jones et al., 1981). This relationship demonstrates that at physiologically normal concentrations, the majority of H2O2 degradation is contained within the peroxisome. However, excess levels of H2O2 generation may result in greater cytoplasmic H2O2 leakage and the necessity of cytoplasmic glutathione peroxidase activity as a secondary defence.
It is important to note that even at low concentrations of H2O2 (100 μM), catalase can undergo approximately 40% loss of enzymatic function (Bartoszewska et al., 2012). Higher levels of H2O2 exacerbate the loss of catalase function and the accumulation of nonfunctioning proteins. To mitigate loss, the peroxisome may compensate by maintaining a high concentration of catalase and its reliance upon LonP2 to degrade dysfunctional catalase efficiently.
The delicate balance between protein damage and protein degradation in peroxisomes may well parallel mitochondrial protein homeostasis. Mitochondrial Lon plays a critical role in preventing the accumulation of oxidized proteins that will diminish mitochondrial function. However, if the protein damage is too extensive, mitochondrial Lon can no longer maintain protein homeostasis, resulting in increased mitochondrial damage and cellular apoptosis (Bota & Davies, 2002). Although this has not yet been shown to occur in peroxisomes, this mechanism of oxidative dysfunction may also arise when LonP2 proteolytic capacity is overwhelmed in the presence of increased damage to proximal peroxisomal proteins.
Recently, LonP2 has been implicated in the degradation of the serine protease, trypsin domain-containing 1 (Tysnd1). Similar to Lon, Tysnd1 has been shown to degrade multiple PTS1-containing proteins and remove the PTS2 targeting sequences from protein precursors (Okumoto, Kametani & Fujiki, 2011). Loss of PTS1 results in the accumulation of immature β-oxidation enzymes and in diminished processing of long-chain fatty acids. Upon self-cleavage of Tysnd1, the inactive subunits are further degraded by peroxisomal Lon. The proteolysis of Tysnd1 by LonP2 may not be due to a specific relationship between these enzymes, but simply the affinity of Lon to degrade damaged proteins marked by the exposure of hydrophobic residues (Gur & Sauer, 2008). Therefore, the capability of the peroxisome to remove unnecessary and nonfunctioning proteins from its matrix is critical to ensure continued function.
VIII. THE CHAPERONE-LIKE ACTIVITY OF PEROXISOMAL LON
Protein chaperones are an important feature of the mitochondrial protein quality-control system. They are critical in helping to ensure correct protein folding during periods of cellular stress. Various synergistic partnerships are formed between multiple ATP-dependent and independent chaperones to promote native folding of proteins (Fink, 1999). In C. elegans, the coordinated biochemical roles of heat shock protein 78 (Hsp78), a chaperone-like enzyme, and PIM1, a mitochondria-specific yeast Lon homolog, are important in preventing the accumulation of protein aggregates (Leidhold et al., 2006; Voos, 2009). However, unlike mitochondria, peroxisomes do not have protein-folding machinery such as heat shock response proteins, potentially because proteins are imported into peroxisomes as fully folded entities (Gould et al., 1989). Despite the fact that peroxisomes appear not to have a dedicated protein machinery, it has been suggested that LonP2 has chaperone-like activities in addition to its role as a protease. To explore the potential role of Lon as a chaperone protein, purified peroxisome-specific Lon was incubated with denatured citrate synthase to determine if the enzyme could prevent protein aggregation. In the presence of Lon, independent of ATP, protein aggregation was decreased by 40% in comparison to control samples (Bartoszewska et al., 2012). Complete inhibition of protein aggregation occurred with increasing amounts of Lon and enzymatic activity of the denatured protein was re-established with the addition of ATP, thus indicating that prevention of aggregation was not through a proteolytic pathway (Bartoszewska et al., 2012). Thus, these findings add to the known multifunctional characteristics of peroxisomal Lon.
Based on the current studies focused on the role of Lon in the peroxisome, LonP2 has consistently been shown to be a protease that mirrors the biological function of the mitochondrial isoform, LonP1. The conserved structure and domains between peroxisomal and mitochondrial Lon emphasize how configuration facilitates their function as serine proteases. However, only peroxisome-specific Lon has been directly shown to have chaperone-like activity. LonP1’s role as a chaperone has only been hypothesized, but further work in a newly discovered class of Lon-like proteases (LonC), which lack the canonical ATPase domain, has hinted that they act as chaperones (Li et al., 2013). In addition, unlike mitochondria, which possess an additional chaperone system, peroxisomes do not contain heat shock response proteins to assist in proper protein folding. Instead, peroxisomal Lon appears to have evolved into a multifaceted protein that is capable of acting as both an ATP-dependent protease and a chaperone. The relationship between these two activities was shown to be modulated depending on cellular requirements. During peroxisome biogenesis, Lon’s chaperone-like activity may assist in the correct folding and normal assembly of imported proteins. During periods of decreased metabolic needs, the proteolytic activity of Lon hinders its chaperone-like activity, which allows for the removal of unnecessary peroxisome proteins through a combination of proteolysis and activation of autophagy pathways (Goto-Yamada et al., 2014). Thus, the dynamic role of peroxisomal Lon is dependent upon the biological needs of the peroxisome.
IX. PEROXISOMES, LONP2, AGING AND DISEASE
Similar to other organelles, peroxisomes are impacted by aging, as identified through changes in metabolism and biogenesis (Titorenko & Terlecky, 2011). One such characteristic is the overall decline in peroxisome protein import, with accumulation of ‘older’ peroxisomal proteins, as demonstrated through immunocytochemical analysis of middle- to late-passage human fibroblasts (Terlecky et al., 2006). In response to the natural decline in peroxisome import, the senescent cell compensates by inducing peroxisome biogenesis, resulting in a twofold increase in peroxisome volume compared to early- and middle-passage cells (Terlecky et al., 2006). However, an increase in peroxisome number does not equate to an overall increase in peroxisome activity. This is partly due to the primary mechanism of peroxisome proliferation, which arises from cellular fission and division. Peroxisome fission is an asymmetric process, resulting in the nascent portion receiving the majority of newly synthesized matrix proteins (Delille et al., 2010). This results in the formation of a seemingly healthier peroxisome at the expense of a more highly damaged one. Consequently, the division of an already damaged organelle may temporarily alleviate cellular stress, but eventually the damaged peroxisomes, if not cleared, will outweigh the formation of healthy ones (Manivannan et al., 2012).
As previously described, one of the main H2O2 detoxifying enzymes of the peroxisome is catalase. Without this enzyme, the capacity to degrade major toxic by-products of peroxisome metabolism would be significantly diminished resulting in the accumulation of damage. Importantly, one hallmark of cellular senescence is a marked decline in the peroxisomal import of catalase (Terlecky et al., 2006). This results in the accumulation of catalase as a nonfunctioning entity within the cytoplasm, paralleled by the peroxisome’s diminished capacity to degrade H2O2 (Legakis et al., 2002). This aging-dependent decline has been hypothesized to result from the low affinity between catalase’s targeting sequence, which contains a divergent form of the PTS1 signal (lysine–alanine–asparagine–leucine), and the ability of Pex5 to recognize and target catalase to the peroxisome, even in low-passage cells (Legakis et al., 2002). With age, the import efficiency of catalase further declines because of an increased ‘sticking’ of Pex5 to the peroxisome membrane, thereby reducing the cytoplasmic pool of available Pex5 that can be used to import peroxisomal proteins (Legakis et al., 2002). Loss of Pex5 import efficiency may be partially explained by its high redox sensitivity, resulting in loss of functionality (Apanasets et al., 2014), which further contributes to the cytoplasmic retention of catalase in aged cells.
The lack of catalase import results in a redox imbalance within the peroxisome which can no longer properly compensate for H2O2-generating reactions, transitioning the organelle into a major producer of H2O2 (Walton & Pizzitelli, 2012). Furthermore, the process of catalase mislocalization has been found to begin in as early as middle-passage cells and contributes to the gradual increase in reactive oxygen species, which is a hallmark of cellular senescence (Terlecky et al., 2006). This progressive decline in activity and mislocalization of catalase was substantiated by the comparison of liver tissue from young and old rats (Beier, Völkl & Fahimi, 1993). In aged rats, there was a substantial decrease in catalase expression and a 30–40% loss of enzymatic activity. In addition, the decline in catalase levels was coupled with an increase in peroxisome size and heterogeneity within the organelle’s population (Beier et al., 1993). This may be partly due to the asymmetric fission pattern of peroxisomes. The asymmetrical division causes the formation of two pools of peroxisomes: slightly enlarged and potentially dysfunctional peroxisomes and smaller, enzymatically active peroxisomes. Thus, peroxisome heterogeneity may be the cell’s attempt at restoring declining peroxisome activity (Huber et al., 2012). Further research is needed to determine whether loss of catalase leads to peroxisome dysfunction or is an inherent characteristic of these oxidant-producing organelles.
Interestingly, no work of which we are aware has focused on the relationship between mammalian LonP2 and aging. As catalase has been identified as the primary peroxisome-specific protein for which loss is the most detrimental to organelle function, it is important to understand the relationship between the aging-dependent decline of catalase and peroxisomal Lon. We can hypothesize, because the proteolytic activity between LonP1 and LonP2 appears to be conserved, that loss of LonP2 activity may lead to an accumulation of nonfunctioning catalase, which may accelerate loss of overall peroxisome function.
In an initial study focused on the age-related changes of mitochondrial Lon, a fourfold decrease in lonP1 mRNA expression was found in the skeletal muscle of aged mice compared to young control mice (Lee et al., 1999). Subsequently, it was shown that muscle LonP1 protein and enzymatic activity exhibit a similar decline with age, and that this is exacerbated in manganese superoxide dismutase heterozygotes which suffer a lifelong oxidative stress (Bota, Van Remmen & Davies, 2002). However, the impact of an age-associated decrease of mitochondrial Lon was not fully understood until studies focused on the differences in young versus senescent human fibroblasts (Bota et al., 2005; Ngo et al., 2011). These studies demonstrated the consequences of mitigated LonP1 expression, which results in the accumulation of oxidized proteins, inability to adapt to oxidative stress, decreased mitochondrial membrane potential, and increased mitochondrial mass (Ngo et al., 2011). Furthermore, the adaptability of Lon is greatly diminished in senescent cells compared to their younger counterparts (Ngo et al., 2011). As Lon is a stress-response protein, the necessity to respond rapidly to oxidative demands is crucial in maintaining mitochondrial function. In a similar manner, peroxisomal Lon expression is dependent on peroxisome biogenesis. The rapid import of LonP2 into peroxisomes is necessary to ensure the proper folding and removal of nonfunctional enzymes in order to maintain peroxisome function.
In addition, aconitase, an iron–sulfur protein that plays a critical role in energy production via the tricarboxylic acid (TCA) cycle, is a target substrate for mitochondrial Lon (Bota & Davies, 2002) and has been shown to have decreased activity with age. This decline in enzymatic function has been associated with a reduction in lifespan in Drosophila melanogaster (Yan, Levine & Sohal, 1997). Furthermore, aconitase was identified as one of the predominant proteins subject to oxidative damage as a result of aging (Yan et al., 1997). Mice lacking the mitochondrial form of superoxide dismutase (SOD2) were more susceptible to oxidative stress due to the consequent decrease in the rate of superoxide dismutation to H2O2 (Bota et al., 2002). In consequence, these animals provided a good model to understand Lon’s role in protein removal and accumulation of damaged proteins with age. Young animals were found to have higher basal levels of Lon in contrast to either the wild-type older animals or their aged SOD2−/+ counterparts. Furthermore, though aconitase protein levels did not change, its activity substantially decreased in aged animals (Bota et al., 2002). Thus the link between mitochondrial Lon and its substrate, aconitase, may lend to speculation that a similar relationship exists between peroxisomal Lon and catalase.
LonP1 plays an important role in maintaining mitochondrial integrity during aging and aging-related diseases. A study focused on identifying DNA variants associated with mitochondrial disorders uncovered multiple small nucleotide polymorphisms (SNPs) in the lonP1 gene (Wang et al., 2010). While these SNPs were benign, a later study demonstrated a much more deleterious impact of SNP variation in the lonP1 gene. Specifically, four mutations in lonP1 were found directly to cause the development of CODAS syndrome, a rare multi-congenital disorder characterized by cerebral, ocular, dental, auricular, and skeletal abnormalities. All four mutations were found to be clustered within LonP1’s ATPase domain, thus altering Lon’s ability to utilize ATP efficiently (Strauss Kevin et al., 2015). Although CODAS is a very rare genetic disease, this finding highlights that loss of Lon function can be detrimental to organismal well-being. In a similar study conducted in mice, researchers discovered that the homozygous loss of Lon results in embryonic lethality (Quirós Pedro et al., 2014). Interestingly, heterozygous mice developed normally, further demonstrating that a dose-dependent threshold of Lon is necessary for survival (Quirós Pedro et al., 2014).
While LonP1 is critical for mitochondrial protein maintenance, chronic upregulation of LonP1 has been associated with tumorigenesis. Various tumour cell lines, such as lymphoma cells and hepatocyte carcinomas, have been shown to have higher levels of Lon expression and activity compared to non-cancerous tissue (Bernstein et al., 2012; Kita, Suzuki & Ochi, 2012). Cervical cancer has been found to have a twofold higher Lon expression compared to healthy cervical tissue. Moreover, the proliferation of tumour cells was abrogated by knockdown of Lon, which lowered cellular respiration and further limited tumorigenesis (Nie et al., 2013). Similarly, silencing of Lon in colon cancer promotes cell death, alters mitochondrial proteome expression, and decreases mtDNA transcripts and oxidative phosphorylation complexes, thus contributing to lowered mitochondrial efficiency (Gibellini et al., 2014). Overall, dysregulation of LonP1 is highly detrimental to cellular homeostasis and is a potential contributing factor to various chronic and developmental diseases. Much less is known about the impact of peroxisomal Lon during disease states, indicating that further work is needed to explore how changes in LonP2 expression can impact chronic disease progression.
X. THE INTERACTION BETWEEN MITOCHONDRIA AND PEROXISOMES
Peroxisomes and mitochondria have an important role in redox metabolism. During aging, abnormalities in mitochondria can cause them greatly to increase their basal production of oxidants (Fig. 3). As a result, the damage that occurs within the mitochondrion is not isolated, but impacts the rest of the cell by increasing the overall amount of reactive oxygen species, protein oxidation, lipid peroxidation, and DNA damage (Balaban, Nemoto & Finkel, 2005). A similar relationship, involving the spread of oxidative damage, is believed to occur between peroxisomes and the cell (Manivannan et al., 2012).
Fig. 3.
The Lon protease and aging-related changes. (A, C) During periods of acute oxidative stress, peroxisomes and mitochondria quickly adapt to the oxidative insult by up-regulating LonP1 and LonP2. (A) In young mitochondria, the resulting increase in proteolytic activity of LonP1 ensures rapid degradation of damaged proteins and maintenance of homeostasis. (B) Young peroxisomes adapt to changes in metabolic demand by rapidly increasing the breakdown of odd-chain fatty acids for increased acetyl-CoA production, which consequently fuels mitochondrial energy production. To compensate for the excess generation of hydrogen peroxide, up-regulation of LonP2 counteracts the increased peroxisome-dependent protein damage. (B, D) Senescent cells that are exposed to chronic oxidative stress lose ability to degrade oxidized organelle proteins. (B) In aged mitochondria, the ability to rapidly up-regulate LonP1 declines, leading to the accumulation of protein aggregates, and eventual loss of mitochondrial function. (D) In peroxisomes, a similar phenomenon may occur, in which the decreased ability to elevate LonP2 levels rapidly could also lead to protein aggregation and the gradual loss of peroxisome function.
The ability of oxidative damage generated in one organelle to affect the whole cell emphasizes another similarity between mitochondria and peroxisomes. To investigate this potential relationship, a study was conducted using mice that lacked the peroxisome import protein, Pex5. This deletion resulted in animals with nonfunctional peroxisomes and an inability to provide mitochondria with fatty substrates for β-oxidation (Baumgart et al., 2001). This led to a decrease in the cellular availability of acetyl-CoA, the starting point for the first phase of mitochondrial TCA cycle energy conversion (Cohen et al., 2014). Interestingly, peroxisome dysfunction was not isolated to the peroxisome but also resulted in deleterious alterations in the mitochondrial electron transport chain. This region of the mitochondrion is considered one of the major areas of superoxide and H2O2 production. In addition, a parallel increase in the levels of manganese superoxide dismutase, an enzyme which converts superoxide into H2O2 and oxygen (2O2• − + 2H+ → H2O2 + O2), was observed in these animals (Baumgart et al., 2001). Further evidence for a decline in mitochondrial respiratory function included an increase in the population-wide heterogeneity of mitochondrial membrane potential, indicative of mitochondrial impairment (Chen, Chomyn & Chan, 2005). In a possibly looped signalling mechanism, damaged mitochondria cause cells to activate the mitochondrial retrograde signalling pathway (RTG) (Liu & Butow, 2006). Further evidence suggests that peroxisomal reactive oxygen species influence mitochondrial redox levels and may lead to apoptosis via the mitochondrial pathway (Ivashchenko et al., 2011; Wang et al., 2013). In this respect, peroxisomes may have a direct impact on the oxidation status of mitochondrial proteins, potentially tethering the role of LonP2 to mitochondrial metabolism.
In response to mitochondrial dysfunction, a series of stress-response transcription signals are activated, leading to an increase in peroxisome proliferation. Interestingly, chronic activation of the mitochondrial RTG, and the associated increase in peroxisome biogenesis, has been shown to be a predominant indicator for yeast longevity (Borghouts et al., 2004). The metabolic product, acetyl CoA is both an output molecule of peroxisome fatty acid metabolism, and an input molecule for the mitochondrial TCA cycle. The importance of acetyl CoA in mitochondrial respiration is an obvious point of coordination between the metabolic processes of peroxisomes and mitochondria. The first evidence of communication between mitochondria and peroxisomes was the discovery of mitochondria-derived vesicles (MDVs) expressing mitochondria-anchored protein ligase (MAPL), a mitochondrial outer-membrane protein, shown to fuse with a subset of peroxisomes (Neuspiel et al., 2008). In addition, peroxisomes have been shown to localize at the ER–mitochondria junction, rather than sporadically throughout the cell. This close proximity between mitochondria and peroxisomes may allow for improved synchronization between their respective metabolic processes (Cohen et al., 2014).
Overall, the ability of peroxisome dysfunction to cause mitochondrial changes, and vice versa, indicates a strong link between these organelles. However, this link may only become obvious in either disease states or in aging, when mitochondrial or peroxisome impairment is most pronounced. Multiple studies have examined how mitochondria and peroxisomes become susceptible to protein damage from reactive oxygen species, but little work has focused on the initial triggers of organelle abnormality.
Additionally, no work of which we are aware has examined the potential link between the proteostasis machinery of mitochondria (LonP1) and peroxisomes (LonP2), or the consequences of losing either on the other organelle. So far, it has been shown that mitochondrial Lon levels are mitigated with age, and that its inducibility upon oxidative stress also declines. A similar response may occur for peroxisome-specific Lon, resulting in the peroxisome’s inability to remove damaged proteins (i.e. catalase), thus tipping the balance towards the peroxisome becoming an overall oxidant producer. Lon has been viewed as a cellular defence protease to prevent the loss of organelle function due to oxidative damage, whether in the mitochondrion or the peroxisome. Therefore, it will be interesting to explore the aging-related decline of the LonP1 and LonP2 protease isoforms and their impact on these sister organelles.
XI. CONCLUSIONS
The peroxisome has an important role in metabolizing fatty acids in the cell at the expense of H2O2 generation. Therefore, peroxisomal proteins are at an increased risk of oxidative damage due to their close proximity to oxidant production.
LonP2 plays an important role in removing such oxidatively modified proteins, thus preventing their accumulation, and permitting continued normal peroxisomal function.
It is unclear whether Lon adaptation to oxidative stress is regulated transcriptionally or translationally, however it may be that the protein aggregates that accumulate during aging may actually sequester Lon, and inhibit proteolytic activity.
To our knowledge, little to no evidence currently exists on the role of peroxisomal Lon during aging, highlighting a significant gap in our understanding of the peroxisome quality-control system.
During aging, peroxisomes exhibit a gradual decline in metabolic function. This decline may be attributed, in part, to an aging-associated loss of peroxisomal Lon expression or activity. The age-dependent increase in damaged proteins and the presence of inclusion bodies of oxidized, aggregated, and cross-linked proteins within these organelles is indicative of dysfunctional protein homeostasis.
Interestingly, the similarities between mitochondria and peroxisomes, including the duality of the two Lon proteases, have led to new evidence indicating potential crosstalk between these redox-regulated organelles.
The protease activity of Lon plays a critical role in maintaining function in both mitochondria and peroxisomes.
Understanding the protein quality-control mechanisms in both these entities may help to highlight how a detrimental change in one can affect the other.
While it is clear that mitochondria are major mediators for cellular aging, further research is needed to incorporate peroxisomes into the cellular aging processes and to understand fully the dynamic relationship between Lon and the peroxisome.
XII. REFERENCES
- Aksam EB, Koek A, Kiel J, Jourdan S, Veenhuis M, van der Klei IJ. A peroxisomal lon protease and peroxisome degradation by autophagy play key roles in vitality of Hansenula polymorpha cells. Autophagy. 2007;3:96. doi: 10.4161/auto.3534. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerik AY, Antonov VK, Gorbalenya AE, Kotova SA, Rotanova TV, Shimbarevich EV. Site-directed mutagenesis of La protease: a catalytically active serine residue. FEBS Letters. 1991;287:211–214. doi: 10.1016/0014-5793(91)80053-6. [DOI] [PubMed] [Google Scholar]
- Antonenkov VD, Grunau S, Ohlmeier S, Hiltunen JK. Peroxisomes are oxidative organelles. Antioxidants & Redox Signaling. 2010;13:525–537. doi: 10.1089/ars.2009.2996. [DOI] [PubMed] [Google Scholar]
- Apanasets O, Grou CP, Van Veldhoven PP, Brees C, Wang B, Nordgren M, Dodt G, Azevedo JE, Fransen M. PEX5, the shuttling import receptor for peroxisomal matrix proteins, is a redox–sensitive protein. Traffic. 2014;15:94–103. doi: 10.1111/tra.12129. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bartoszewska M, Williams C, Kikhney A, Opaliński Ł, van Roermund CW, de Boer R, Veenhuis M, van der Klei IJ. Peroxisomal proteostasis involves a Lon family protein that functions as protease and chaperone. Journal of Biological Chemistry. 2012;287:27380–27395. doi: 10.1074/jbc.M112.381566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart E, Vanhorebeek I, Grabenbauer M, Borgers M, Declercq PE, Fahimi HD, Baes M. Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (< i > PEX5</i > Knockout Mouse) The American Journal of Pathology. 2001;159:1477–1494. doi: 10.1016/S0002-9440(10)62534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier K, Völkl A, Fahimi HD. The impact of aging on enzyme proteins of rat liver peroxisomes: quantitative analysis by immunoblotting and immunoelectron microscopy. Virchows Archiv B. 1993;63:139–146. doi: 10.1007/BF02899254. [DOI] [PubMed] [Google Scholar]
- Bernstein SH, Venkatesh S, Li M, Lee J, Lu B, Hilchey SP, Morse KM, Metcalfe HM, Skalska J, Andreeff M. The mitochondrial ATP-dependent Lon protease: a novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Blood. 2012;119:3321–3329. doi: 10.1182/blood-2011-02-340075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp NA, Völkl A, Fahimi HD, Schrader M. Reactive oxygen species and peroxisomes: struggling for balance. Biofactors. 2009;35:346–355. doi: 10.1002/biof.48. [DOI] [PubMed] [Google Scholar]
- Borghouts C, Benguria A, Wawryn J, Jazwinski SM. Rtg2 protein links metabolism and genome stability in yeast longevity. Genetics. 2004;166:765–777. doi: 10.1534/genetics.166.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nature Cell Biology. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radical Biology and Medicine. 2005;38:665–677. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Letters. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochemical Journal. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard C, Hartig A. Peroxisome targeting signal 1: is it really a simple tripeptide? Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2006;1763:1565–1573. doi: 10.1016/j.bbamcr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Brocard CB, Jedeszko C, Song HC, Terlecky SR, Walton PA. Protein structure and import into the peroxisomal matrix. Traffic. 2003;4:74–82. doi: 10.1034/j.1600-0854.2003.40203.x. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Experimental Gerontology. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Bennett JP., Jr An evaluation of the role of mitochondria in neurodegenerative diseases: mitochondrial mutations and oxidative pathology, protective nuclear responses, and cell death in neurodegeneration. Brain Research Reviews. 1999;29:1–25. doi: 10.1016/s0165-0173(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. Journal of Biological Chemistry. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Klug YA, Dimitrov L, Erez Z, Chuartzman SG, Elinger D, Yofe I, Soliman K, Gartner J, Thoms S, Schekman R, Elbaz-Alon Y, Zalckvar E, Schuldiner M. Peroxisomes are juxtaposed to strategic sites on mitochondria. Molecular BioSystems. 2014;10(7):1742–1748. doi: 10.1039/c4mb00001c. [DOI] [PubMed] [Google Scholar]
- Cooper T, Beevers H. β oxidation in glyoxysomes from castor bean endosperm. Journal of Biological Chemistry. 1969;244:3514–3520. [PubMed] [Google Scholar]
- Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- De Duve C. The birth of complex cells. Scientific American. 1996;274:50–57. doi: 10.1038/scientificamerican0496-50. [DOI] [PubMed] [Google Scholar]
- De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles) Physiological Reviews. 1966;46:323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Delille HK, Agricola B, Guimaraes SC, Borta H, Lüers GH, Fransen M, Schrader M. Pex11pβ-mediated growth and division of mammalian peroxisomes follows a maturation pathway. Journal of Cell Science. 2010;123:2750–2762. doi: 10.1242/jcs.062109. [DOI] [PubMed] [Google Scholar]
- Dougan DA, Mogk A, Zeth K, Turgay K, Bukau B. AAA+ proteins and substrate recognition, it all depends on their partner in crime. FEBS Letters. 2002;529:6–10. doi: 10.1016/s0014-5793(02)03179-4. [DOI] [PubMed] [Google Scholar]
- Erjavec N, Bayot A, Gareil M, Camougrand N, Nystrom T, Friguet B, Bulteau AL. Deletion of the mitochondrial Pim1/Lon protease in yeast results in accelerated aging and impairment of the proteasome. Free Radical Biology and Medicine. 2013;56:9–16. doi: 10.1016/j.freeradbiomed.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiological Reviews. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Finley LW, Haigis MC. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Research Reviews. 2009;8:173–188. doi: 10.1016/j.arr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Glockshuber R. A point mutation within the ATP-binding site inactivates both catalytic functions of the ATP-dependent protease La (Lon) from Escherichia coli. FEBS Letters. 1994;356:101–103. doi: 10.1016/0014-5793(94)01244-x. [DOI] [PubMed] [Google Scholar]
- Foerster EC, Fahrenkemper T, Rabe U, Graf P, Sies H. Peroxisomal fatty acid oxidation as detected by H2O2 production in intact perfused rat liver. Biochemical Journal. 1981;196:705–712. doi: 10.1042/bj1960705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Letters. 2006;580:2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Fu GK, Markovitz DM. The human LON protease binds to mitochondrial promoters in a single-stranded, site-specific, strand-specific manner. Biochemistry. 1998;37:1905–1909. doi: 10.1021/bi970928c. [DOI] [PubMed] [Google Scholar]
- Fu GK, Smith MJ, Markovitz DM. Bacterial protease Lon is a site-specific DNA-binding protein. Journal of Biological Chemistry. 1997;272:534–538. [PubMed] [Google Scholar]
- Ghosh MK, Hajra AK. A rapid method for the isolation of peroxisomes from rat liver. Analytical Biochemistry. 1986;159:169–174. doi: 10.1016/0003-2697(86)90323-4. [DOI] [PubMed] [Google Scholar]
- Gibellini L, Pinti M, Boraldi F, Giorgio V, Bernardi P, Bartolomeo R, Nasi M, De Biasi S, Missiroli S, Carnevale G, Losi L, Tesei A, Pinton P, Quaglino D, Cossarizza A. Silencing of mitochondrial Lon protease deeply impairs mitochondrial proteome and function in colon cancer cells. The FASEB Journal. 2014;28:5122–5135. doi: 10.1096/fj.14-255869. [DOI] [PubMed] [Google Scholar]
- Goto-Yamada S, Mano S, Nakamori C, Kondo M, Yamawaki R, Kato A, Nishimura M. Chaperone and protease functions of LON protease 2 modulate the peroxisomal transition and degradation with autophagy. Plant and Cell Physiology. 2014;55(3):482–496. doi: 10.1093/pcp/pcu017. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Keller GA, Hosken N, Wilkinson J, Subramani S. A conserved tripeptide sorts proteins to peroxisomes. The Journal of Cell Biology. 1989;108:1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Valle D. Peroxisome biogenesis disorders: genetics and cell biology. Trends in Genetics. 2000;16:340–345. doi: 10.1016/s0168-9525(00)02056-4. [DOI] [PubMed] [Google Scholar]
- Guha S, López-Maury L, Shaw M, Bähler J, Norbury CJ, Agashe VR. Transcriptional and cellular responses to defective mitochondrial proteolysis in fission yeast. Journal of Molecular Biology. 2011;408:222–237. doi: 10.1016/j.jmb.2011.02.044. [DOI] [PubMed] [Google Scholar]
- Gur E, Sauer RT. Recognition of misfolded proteins by Lon, a AAA+ protease. Genes & Development. 2008;22:2267–2277. doi: 10.1101/gad.1670908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. Protection against oxidants in biological systems: the superoxide theory of oxygen toxicity. In: Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. Clarendon Press; New York: 1989. p. 86. [Google Scholar]
- Hayashi H, Kohji T, Tetsuya S, Niinobe S. Studies on peroxisomes VI. Relationship between the peroxisomal core and urate oxidase. Journal of Biochemistry. 1976;79:1029–1034. doi: 10.1093/oxfordjournals.jbchem.a131143. [DOI] [PubMed] [Google Scholar]
- Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Hori O, Ichinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, Ron D. Transmission of cell stress from endoplasmic reticulum to mitochondria enhanced expression of Lon protease. The Journal of Cell Biology. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Koch J, Kragler F, Brocard C, Hartig A. A subtle interplay between three Pex11 proteins shapes de novo formation and fission of peroxisomes. Traffic. 2012;13:157–167. doi: 10.1111/j.1600-0854.2011.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashchenko O, Van Veldhoven PP, Brees C, Ho YS, Terlecky SR, Fransen M. Intraperoxisomal redox balance in mammalian cells: oxidative stress and interorganellar cross-talk. Molecular Biology of the Cell. 2011;22:1440–1451. doi: 10.1091/mbc.E10-11-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson DJ. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998;14:1511–1527. doi: 10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Jonas K, Liu J, Chien P, Laub MT. Proteotoxic stress induces a cell cycle arrest by stimulating Lon to degrade the replication initiator DnaA. Cell. 2013;154:623–636. doi: 10.1016/j.cell.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Eklöw L, Thor H, Orrenius S. Metabolism of hydrogen peroxide in isolated hepatocytes: relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2. Archives of Biochemistry and Biophysics. 1981;210:505–516. doi: 10.1016/0003-9861(81)90215-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Hatano N, Yokota S, Shimozawa N, Imanaka T, Taniguchi H. Proteomic analysis of rat liver peroxisome presence of peroxisome-specific isozyme of Lon protease. Journal of Biological Chemistry. 2004;279:421–428. doi: 10.1074/jbc.M305623200. [DOI] [PubMed] [Google Scholar]
- Kita K, Suzuki T, Ochi T. Diphenylarsinic acid promotes degradation of glutaminase c by mitochondrial Lon protease. Journal of Biological Chemistry. 2012;287:18163–18172. doi: 10.1074/jbc.M112.362699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops K, Manivannan S, Cepińska MN, Krikken AM, Kram AM, Veenhuis M, van der Klei IJ. Preperoxisomal vesicles can form in the absence of Pex3. The Journal of Cell Biology. 2014;204:659–668. doi: 10.1083/jcb.201310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow PB. Peroxisome structure, function, and biogenesis-human patients and yeast mutants show strikingly similar defects in peroxisome biogenesis. Journal of Neuropathology & Experimental Neurology. 1995;54:720–725. doi: 10.1097/00005072-199509000-00015. [DOI] [PubMed] [Google Scholar]
- Lazarow PB, De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P, Fujiki Y. Biogenesis of peroxisomes. Annual Review of Cell Biology. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Legakis JE, Koepke JI, Jedeszko C, Barlaskar F, Terlecky LJ, Edwards HJ, Walton PA, Terlecky SR. Peroxisome senescence in human fibroblasts. Molecular Biology of the Cell. 2002;13:4243–4255. doi: 10.1091/mbc.E02-06-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidhold C, Janowsky Bv, Becker D, Bender T, Voos W. Structure and function of Hsp78, the mitochondrial ClpB homolog. Journal of Structural Biology. 2006;156:149–164. doi: 10.1016/j.jsb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Bovina C, D’aurelio M, Fato R, Formiggini G, Genova ML, Giuliano G, Pich MM, Paolucci U, Castelli GP. Role of mitochondria in oxidative stress and aging. Annals of the New York Academy of Sciences. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Li JK, Liao JH, Li H, Kuo CI, Huang KF, Yang LW, Wu SH, Chang CI. The N-terminal substrate-recognition domain of a LonC protease exhibits structural and functional similarity to cytosolic chaperones. Acta Crystallographica Section D: Biological Crystallography. 2013;69:1789–1797. doi: 10.1107/S090744491301500X. [DOI] [PubMed] [Google Scholar]
- Liu Z, Butow RA. Mitochondrial retrograde signaling. Annual Review of Genetics. 2006;40:159–185. doi: 10.1146/annurev.genet.40.110405.090613. [DOI] [PubMed] [Google Scholar]
- Lock EA, Mitchell AM, Elcombe CR. Biochemical mechanisms of induction of hepatic peroxisome proliferation. Annual Review of Pharmacology and Toxicology. 1989;29:145–163. doi: 10.1146/annurev.pa.29.040189.001045. [DOI] [PubMed] [Google Scholar]
- Lopez–Huertas E, Charlton WL, Johnson B, Graham IA, Baker A. Stress induces peroxisome biogenesis genes. The EMBO Journal. 2000;19:6770–6777. doi: 10.1093/emboj/19.24.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Lee J, Nie X, Li M, Morozov YI, Venkatesh S, Bogenhagen DF, Temiakov D, Suzuki CK. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Molecular Cell. 2013;49:121–132. doi: 10.1016/j.molcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Liu T, Crosby JA, Thomas-Wohlever J, Lee I, Suzuki CK. The ATP-dependent Lon protease of Mus musculus is a DNA-binding protein that is functionally conserved between yeast and mammals. Gene. 2003;306:45–55. doi: 10.1016/s0378-1119(03)00403-7. [DOI] [PubMed] [Google Scholar]
- Manivannan S, Scheckhuber CQ, Veenhuis M, van der Klei IJ. The impact of peroxisomes on cellular aging and death. Frontiers in Oncology. 2012;2:50. doi: 10.3389/fonc.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima Y, Goto YI, Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM) Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18410–18415. doi: 10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T. The concept of self-organization in cellular architecture. The Journal of Cell Biology. 2001;155:181–186. doi: 10.1083/jcb.200108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Current Biology. 2009;19:R657–R666. doi: 10.1016/j.cub.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Current Biology. 2008;18:102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Ngo JK, Davies KJ. Importance of the lon protease in mitochondrial maintenance and the significance of declining lon in aging. Annals of the New York Academy of Sciences. 2007;1119:78–87. doi: 10.1196/annals.1404.015. [DOI] [PubMed] [Google Scholar]
- Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radical Biology and Medicine. 2009;46:1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo JK, Pomatto LC, Bota DA, Koop AL, Davies KJ. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:1178–1185. doi: 10.1093/gerona/glr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Li M, Lu B, Zhang Y, Lan L, Chen L, Lu J. Down-regulating overexpressed human Lon in cervical cancer suppresses cell proliferation and bioenergetics. PLoS ONE. 2013;8(11):e81084. doi: 10.1371/journal.pone.0081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren M, Fransen M. Peroxisomal metabolism and oxidative stress. Biochimie. 2014;98:56–62. doi: 10.1016/j.biochi.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Novikoff A, Shin WY. The endoplasmic reticulum in the Golgi zone and its relations to microbodies. Golgi apparatus and autophagic vacuoles in rat liver cells. Journal of Microscopy. 1964;3:187–206. [Google Scholar]
- Okumoto K, Kametani Y, Fujiki Y. Two proteases, trypsin domain-containing 1 (Tysnd1) and peroxisomal lon protease (PsLon), cooperatively regulate fatty acid β-oxidation in peroxisomal matrix. Journal of Biological Chemistry. 2011;286:44367–44379. doi: 10.1074/jbc.M111.285197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omi S, Nakata R, Okamura-Ikeda K, Konishi H, Taniguchi H. Contribution of peroxisome-specific isoform of Lon protease in sorting PTS1 proteins to peroxisomes. Journal of Biochemistry. 2008;143:649–660. doi: 10.1093/jb/mvn020. [DOI] [PubMed] [Google Scholar]
- Patterson-Ward J, Huang J, Lee I. Detection and characterization of two ATP-dependent conformational changes in proteolytically inactive Escherichia coli Lon mutants by stopped flow kinetic techniques. Biochemistry. 2007;46:13593–13605. doi: 10.1021/bi701649b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perichon R, Bourre J, Kelly J, Roth G. The role of peroxisomes in aging. Cellular and Molecular Life Sciences CMLS. 1998;54:641–652. doi: 10.1007/s000180050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A, Koop A, Teoh C, Ermak G, Grune T, Davies K. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochemical Journal. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. Journal of Biological Chemistry. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Antonenkov VD, Glumoff T, Hiltunen JK. Peroxisomal β-oxidation—a metabolic pathway with multiple functions. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2006;1763:1413–1426. doi: 10.1016/j.bbamcr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Quirós Pedro M, Español Y, Acín-Pérez R, Rodríguez F, Bárcena C, Watanabe K, Calvo E, Loureiro M, Fernández-García MS, Fueyo A, Vázquez J, Enríquez JA, López-Otín C. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Reports. 2014;8:542–556. doi: 10.1016/j.celrep.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Rhodin J. Doctor dissertation. Karolinska Institute; Stockholm. Nord. bokh: 1954. Correlation of Ultrastructural Organization and Function in Normal and Experimentally Changed Proximal Convoluted Tubule Cells Of The Mouse Kidney: an Electron Microscopic Study, Including an Experimental Analysis of the Conditions for Fixation of the Renal Tissue for High Resolution Electron Microscopy. [Google Scholar]
- Shio H, Lazarow PB. Relationship between peroxisomes and endoplasmic reticulum investigated by combined catalase and glucose-6-phosphatase cytochemistry. Journal of Histochemistry & Cytochemistry. 1981;29:1263–1272. doi: 10.1177/29.11.6274950. [DOI] [PubMed] [Google Scholar]
- Stahlberg H, Kutejová E, Suda K, Wolpensinger B, Lustig A, Schatz G, Engel A, Suzuki CK. Mitochondrial Lon of Saccharomyces cerevisiae is a ring-shaped protease with seven flexible subunits. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6787–6790. doi: 10.1073/pnas.96.12.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, Jinks RN, Puffenberger EG, Venkatesh S, Singh K, Cheng I, Mikita N, Thilagavathi J, Lee J, Sarafianos S, Benkert A, Koehler A, Zhu A, Trovillion V, McGlincy M, Morlet T, Deardorff M, Innes AM, Prasad C, Chudley AE, Lee INW, Suzuki CK. CODAS Syndrome Is Associated with Mutations of LONP1, Encoding mitochondrial AAA+ Lon protease. The American Journal of Human Genetics. 2015;96:121–135. doi: 10.1016/j.ajhg.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover, and movement. Physiological Reviews. 1998;78:171–188. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- Terlecky SR, Koepke JI, Walton PA. Peroxisomes and aging. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2006;1763:1749–1754. doi: 10.1016/j.bbamcr.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till A, Lakhani R, Burnett SF, Subramani S. Pexophagy: the selective degradation of peroxisomes. International Journal of Cell Biology. 2012;2012:18. doi: 10.1155/2012/512721. Article ID 512721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titorenko VI, Terlecky SR. Peroxisome metabolism and cellular aging. Traffic. 2011;12:252–259. doi: 10.1111/j.1600-0854.2010.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinheckel T, Sitte N, Hass R, Grune T, Davies KJ. Poly-ADP ribose polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6223–6228. doi: 10.1073/pnas.96.11.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Klei IJ, Yurimoto H, Sakai Y, Veenhuis M. The significance of peroxisomes in methanol metabolism in methylotrophic yeast. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2006;1763:1453–1462. doi: 10.1016/j.bbamcr.2006.07.016. [DOI] [PubMed] [Google Scholar]
- van Dyck L, Neupert W, Langer T. The ATP-dependent PIM1 protease is required for the expression of intron-containing genes in mitochondria. Genes & Development. 1998;12:1515–1524. doi: 10.1101/gad.12.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck L, Pearce DA, Sherman F. PIM1 encodes a mitochondrial ATP-dependent protease that is required for mitochondrial function in the yeast Saccharomyces cerevisiae. Journal of Biological Chemistry. 1994;269:238–242. [PubMed] [Google Scholar]
- Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Molecular Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Voos W. Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Research in Microbiology. 2009;160:718–725. doi: 10.1016/j.resmic.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annual Review of Genetics. 2005;39:359. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton PA, Pizzitelli M. Effects of peroxisomal catalase inhibition on mitochondrial function. Frontiers in Physiology. 2012;3:108. doi: 10.3389/fphys.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Gottesman S, Willingham MC, Gottesman MM, Maurizi MR. A human mitochondrial ATP-dependent protease that is highly homologous to bacterial Lon protease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11247–11251. doi: 10.1073/pnas.90.23.11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Maurizi MR, Emmert-Buck L, Gottesman MM. Synthesis, processing, and localization of human Lon protease. Journal of Biological Chemistry. 1994;269:29308–29313. [PubMed] [Google Scholar]
- Wang W, Shen P, Thiyagarajan S, Lin S, Palm C, Horvath R, Klopstock T, Cutler D, Pique L, Schrijver I, Davis RW, Mindrinos M, Speed TP, Scharfe C. Identification of rare DNA variants in mitochondrial disorders with improved array-based sequencing. Nucleic Acids Research. 2010;39(1):44–58. doi: 10.1093/nar/gkq750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Van Veldhoven PP, Brees C, Rubio N, Nordgren M, Apanasets O, Kunze M, Baes M, Agostinis P, Fransen M. Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells. Free Radical Biology and Medicine. 2013;65:882–894. doi: 10.1016/j.freeradbiomed.2013.08.173. [DOI] [PubMed] [Google Scholar]
- Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proceedings of the National Academy of Sciences. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Haraguchi CM, Oda T. Induction of peroxisomal Lon protease in rat liver after di-(2-ethylhexyl) phthalate treatment. Histochemistry and Cell Biology. 2008;129:73–83. doi: 10.1007/s00418-007-0328-0. [DOI] [PubMed] [Google Scholar]