Abstract
Pediatric liver transplant recipients arguably have the most to gain and the most to lose from discontinuing immunosuppression (IS). While IS undoubtedly exerts a cumulative toll, there is concern that insufficient or no IS may contribute to allograft deterioration. Twelve pediatric recipients of parental living donor liver grafts, identified as operationally tolerant through complete IS withdrawal (WISP-R; NCT00320606) were followed for a total of five years (one year of IS withdrawal and four years off IS) with serial liver tests, auto- and allo-antibody assessments. Liver biopsies were performed two and four years off IS and, at these time points, immunoglobulin G (IgG) subclass and C1q binding activity for donor specific antibodies (DSAs) were determined. There were no cases of chronic rejection, graft loss, or death. Allografts did not exhibit progressive increase in inflammation or fibrosis. Smooth muscle actin (SMA) expression by stellate cells and CD34 expression by liver sinusoidal endothelial cells (LSECs) remained stable, consistent with the absence of progressive graft injury. Three subjects never exhibited DSA. However, three subjects showed intermittent de novo Class I DSA, four subjects showed persistent de novo Class II DSA and five subjects showed persistent pre-existing Class II DSA. Class II DSA was predominantly against donor DQ antigens, often of high mean fluorescence intensity (MFI), rarely of the IgG3 subclass, and often capable of binding C1q.
Conclusion
Operationally tolerant pediatric liver transplant recipients maintain generally stable allograft histology in spite of apparently active humoral allo-immune responses. The absence of increased inflammation or progressive fibrosis suggests that a subset of liver allografts seem resistant to the chronic injury that is characteristic of antibody-mediated damage.
Keywords: Immunosuppression withdrawal, Tolerance, Liver transplantation, Donor specific antibody, Allograft fibrosis
INTRODUCTION
Operational tolerance – the maintenance of stable allograft function and histology in the complete absence of immunosuppression (IS) – has now been demonstrated through clinical trials of IS withdrawal conducted for both adult and pediatric liver transplant recipients (1). These trials have typically enrolled stable, long-term liver transplant recipients and gradually reduced IS dosing in a structured manner under close supervision. With the framework of a clinical trial, IS withdrawal can be attempted safely. The episodes of acute rejection that occurred, with prompt diagnosis and treatment, were readily reversed and thus, did not appear to exert a negative impact beyond the transient exposure to increased IS. Treatment has typically consisted of increased doses of IS, occasionally bolus corticosteroids, and rarely administration of an antibody preparation.
Although there is now general acceptance that reducing IS can be safely attempted with close monitoring, the long-term impact of IS minimization or discontinuation on allograft health remains controversial. Within the IS withdrawal trials, assessment of tolerance typically occurs one year after the last dose of IS and is based on biochemical profile with or without histological assessment. For adult liver transplant recipients, there has been only a single publication delineating the histological status of eight tolerant allografts for a mean (range) of 78 (57 – 109) months after IS discontinuation (2). This experience, however, has limited generalizability because all subjects were adults with hepatitis C infection.
The concern for long-term allograft health is of particular concern for pediatric liver transplant recipients who require optimal graft longevity. It is now widely recognized that children maintained on standard of care IS experience clinically silent deterioration of liver histology over time. Multiple cross-sectional, single center studies have consistently shown that liver allografts in children exhibit a higher prevalence of inflammation/hepatitis and fibrosis with increased time after transplantation (3–8). Moreover, a cohort of operationally tolerant pediatric living donor liver transplant recipients, compared to a cohort maintained on IS, exhibited significantly higher fibrosis stages, although the cohorts differed in several demographic parameters such as age at and time after transplantation (9). Risk factors for fibrosis identified by more than one study include deceased donor grafts, prolonged cold ischemia time, and presence of autoantibodies. The early reports of children maintained on standard of care IS have not correlated history of rejection and the nature of the IS regimen, including the use of corticosteroids, with the development of fibrosis. In more recent reports, some of which include children who have undergone IS minimization, detection of DSAs and positive staining for C4d has been associated with fibrosis, implicating a role for humoral allo-immune responses (5, 10–12) Finally, the reinstitution of IS for those who have undergone withdrawal or the intensification of IS for those maintained on standard IS each have been reported to stabilize and even reverse fibrosis, implicating insufficient IS as a potential mechanism driving chronic allograft damage (6, 9, 13).
We have conducted and reported a prospective pilot trial of IS withdrawal for pediatric recipients of living donor liver allografts (WISP-R; NCT00320606) (14). Among the twenty subjects enrolled at three centers, 12 were operationally tolerant, seven experienced acute rejection, and one was withdrawn from the study secondary to a violation of inclusion/exclusion criteria. We now report on the five year follow-up of the 12 tolerant children. Serial allograft biopsies demonstrate architectural preservation without increased inflammation or progressive fibrosis. However, longitudinal testing shows frequent DSA in the majority of tolerant subjects. Juxtaposition of the histological and the alloantibody data raises the intriguing possibility that the liver, compared to other organs, may possess intrinsic mechanisms that can resist allograft deterioration by an ongoing or active allo-immune response.
METHODS
Subjects and Assessments
WISP-R (NCT00320606) was a prospective trial of ISW conducted at three pediatric liver transplant centers in the United States. Written informed assent (as appropriate) and/or consent were obtained from all subjects and/or their legal guardian, respectively. The clinical trial protocol was reviewed and approved by the institutional review board of participating centers. None of the participating transplant centers utilize organs procured from executed prisoners.
WISP-R identified 12 operationally tolerant pediatric liver transplant recipients who maintained stable liver test profiles [alanine aminotransferase (ALT), aspartate aminotransferase (AST), total and direct bilirubin, gamma-glutamyl transpeptidase (GGT), and alkaline phosphatase] for 12 months after complete IS discontinuation. From the time of primary endpoint assessment and the determination of operational tolerance (Year 2; Figure 1), the primary trial extended for three years. A single participant withdrew consent 33.3 months after achieving the primary endpoint.
Figure 1. WISP-R timeline for transplant center visits, antibody assessments, and liver biopsies.
Schematic showing timeline of IS withdrawal, assessment of primary endpoint, and long-term follow-up, including timing and frequency of transplant center visit and liver biopsies
After determination of operational tolerance, liver tests were performed monthly with visits to the transplant center bi-annually for one year. Participants then transitioned to liver tests every two months with annual clinic visits. Two protocol biopsies were required, two and four years after the last IS dose (Yr 3 and Yr 5, respectively, in Figure 1). Alloantibodies were monitored bi-annually for one year and then annually. Auto-antibodies and quantitative IgG were monitored quarterly for one year, then biannually for two years, and then annually thereafter.
Routine and Specialized Histopathology Studies
High resolution 40X whole slide images of formalin-fixed, paraffin-embedded, and hematoxylin-eosin-stained 4mm tissue sections were prospectively scored for 42 histopathologic criteria (Supplementary Table 1). Glass slides were also reviewed at the year 0 and year 5 points. Portal, lobular, and peri-venular inflammation along with portal/peri-portal, Disse, and peri-venular fibrosis were graded and staged, respectively, [0=none; 1=mild (detectable, above baseline); 2=moderate, and 3=severe] with a total score range of 0 to 9 for both.
C4d deposition on snap frozen tissue was evaluated blindly using both single immunofluorescence (mouse monoclonal, A213, Quidel, San Diego, CA) and multiplex quantum dot immunostaining (rabbit polyclonal BI-RC4d, Alpco Diagnostics Salem, NH; 1:30), CD31 (mouse monoclonal JC/70A, ThermoFisher, Pittsburgh, PA; 1:25), and major histocompatibility class complex (MHC) class II (mouse monoclonal CR3/43; DAKO (MO775, Carpinteria, CA). Four vascular endothelial compartments (portal capillary and vein, sinusoidal, and central vein) were separately scored (0=none; 1=minimal; 2=focal; 3=diffuse) and summed for total C4d and MHC II scores (range of 0 to 12).
Changes in LSEC (15) and stellate cell phenotype (16) before and after IS withdrawal was studied by comparing similarly-sized portal tracts, central veins, and sinusoids in the pre-weaning versus Yr 5, formalin-fixed, paraffin-embedded biopsy multiplex-stained for CD34 (mouse monoclonal, QBE-10, DAKO) and SMA (mouse monoclonal, 1A4; DAKO).
Human Leukocyte Antigen (HLA) Typing, Allo-antibody Detection, IgG Subclass Determination, and C1q Assay
HLA typing was performed by automated DNA sequencing (University of California San Francisco; San Francisco, CA). HLA antibody screening and specificity determination were performed using FlowPRA Screening™ and LabScreen® Single Antigen™ assays (One Lambda Inc., Canoga Park, CA) (Emory University, Atlanta, GA). A MFI threshold of 2,000 was used to identify a DSA. Class II DSA subtypes were determined using phycoerythrin-conjugated, IgG subclass–specific, anti-human IgG. C1q binding assays were performed and data was acquired and analyzed as previously described (17). All IgG subtype and C1q binding activity assays were batched.
RESULTS
Clinical Status, Laboratory Profiles, and Adverse Events (AEs) of Operationally Tolerant Pediatric Liver Transplant Recipients
Twelve pediatric recipients (8 male; 9 biliary atresia; Table 1) of parental living donor liver allografts were identified as operationally tolerant through gradual reduction and ultimate discontinuation of IS (14). One participant (Subject 7) withdrew from the trial 45.3 months after their last dose of IS; the remaining 11 completed the five year study. ALT and GGT profiles for all 12 operationally tolerant subjects are shown in Figure 2A–C.
Table 1.
Characteristics of 12 Operationally Tolerant Participants from WISP-R
| Characteristic | N = 12 | |
|---|---|---|
| Age* | At Transplant | 0.6 (0.3, 2.4) |
| At Study Entry | 9.0 (5.2, 12.1) | |
| Gender^ | Male | 8 |
| Liver Disease^ | Biliary atresia | 9 |
| A-1 anti-trypsin | 1 | |
| Familial cholestasis (Byler Disease) | 1 | |
| Neonatal sclerosing cholangitis | 1 | |
| Calcineurin inhibitor at study entry^ | Tacrolimus | 7 |
| Cyclosporine | 5 | |
| Alanine transaminase (U/L)* | 31 (18, 48) | |
| γ-glutamyl transpeptidase (U/L) * | 27 (12, 88) | |
| Presence of donor-specific antibody at study entry^ | Class I | 0 |
| Class II | 4 | |
| DQ alone | 1 | |
| DR alone | 1 | |
| DQ+DR | 2 | |
Median (range)
N
Figure 2. ALT and GGT Profiles for Operationally Tolerant WISP-R Subjects.
The 12 operationally tolerant subjects are divided into three groups as previously described (14).
A. Six subjects exhibiting generally stable profile throughout study follow-up
B. Three subjects with discrete spikes in ALT and GGT, reflecting the diagnosis of biliary obstruction made during the study
C. Three subjects exhibiting persistent and/or recurrent elevation of predominantly GGT during the study
No instances of death, graft loss, or chronic rejection occurred. During the five year follow-up, one subject experienced one study-related AE: an episode of cholangitis precipitated by a protocol biopsy (Subject 1; Supplementary Table 2). Four subjects experienced a total of eight study-unrelated serious adverse events (SAEs): five SAEs were related to biliary stricture/obstruction in three subjects [previously reported in (14)], including one caused by an incarcerated diaphragmatic hernia (Subject 2); one SAE was secondary to portal vein stenosis [Subject 17; also previously reported in (14)].
Assessment of Tolerant Allografts for Inflammation, Fibrosis, C4d Deposition, and Evidence of Subclinical Injury
Protocol liver biopsies two (Yr 3) and four years (Yr 5) after IS discontinuation were compared to the baseline biopsy performed for trial eligibility (Yr 0) (Figure 1). Figure 3 displays the sequential scores for inflammation (portal, lobular, and peri-venular) and fibrosis (portal, Disse, and peri-venular).
Figure 3. Inflammation and Fibrosis Scores and Class II DSA MFIs (Single Antigen Bead, IgG 1–4 Subclasses, and C1q) for Operationally Tolerant WISP-R Subjects.
For each operationally tolerant subject, data is shown at three time points: baseline (BL), prior to study entry and IS withdrawal; Yr 3, three years after study entry, corresponding to 2+ years after last dose of IS; and Yr 5, five years after study entry, corresponding to 4+ years after last dose of IS. The top two rows show inflammation and fibrosis scores (range 0 – 3) by compartment. The remaining rows show single antigen bead MFIs, IgG 1, 2, 3, and 4 MFIs, and C1q binding activity MFIs.
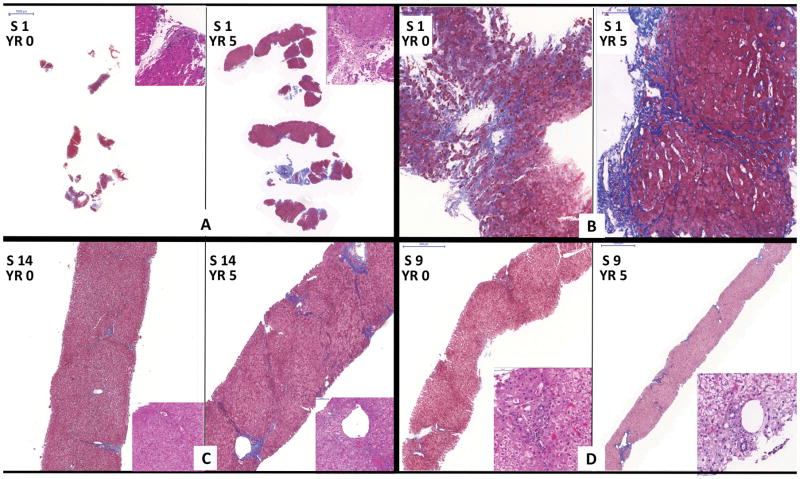
As a group, the baseline (Yr 0) biopsies were generally small [mean (range): 1.1 (0.4 – 3.4 cms)] sampling a mean (range) of 8 (3 – 24) portal tracts. All pre-withdrawal biopsies showed nodular regenerative hyperplasia (NRH) and portal venopathy of variable severity which resulted in gross fragmentation of three Yr 0 biopsies (Subjects 1, 2, and 4). For two participants (Subjects 2 and 4; Figure 4), fragmentation was likely exacerbated by use of a small (18G) biopsy needle since biopsies obtained later in the course of the trial using a larger (16G) biopsy needle later showed less fragmentation. However, for the remaining participant (Subject 1) who experienced recurrent biliary obstruction requiring multiple interventions during the trial [(14); Supplementary Table 2], the Yr 5 biopsy for continued to show fragmentation (Figure 5). NRH changes in the other subjects did not appreciably progress over time. More detailed accounting of the minor changes in inflammation, fibrosis, and overall architecture are detailed in Supplementary Table 3. There was no evidence for progressive increase in inflammation or fibrosis among the tolerant subjects without biliary issues during the follow-up period.
Figure 4. Biopsies from two representative WISP-R subjects with consistently normal liver test profiles as shown in Figure 2A.
Biopsies from two representative WISP-R participants (Subjects 4 and 5; liver test profiles shown in Figure 2A) are shown. A scale bar is in the upper left corner of the panels.
Trichrome-stained sections of entire Yr 0 and Yr 5 biopsies captured at low magnification from Subject 4 (A) and 5 (C) show intact architecture. Higher magnification of representative areas of Yr 0 and Yr 5 biopsies are shown (B and D). Each inset shows higher magnification of representative portal tracts: all are devoid of inflammation. Finally, note that the thinner, 18 gauge Yr 0 biopsy is fragmented whereas the thicker, 16 gauge, Yr 5 biopsy is not (A).
Figure 5. Biopsies from three representative WISP-R subjects with abnormal liver test profiles as shown in Figures 2B and 2C.
Biopsies from three representative WISP-R participants are shown. The scale bar is shown in the upper left corner of the panels.
Two participants (Subjects 1 and 14; liver test profiles in Figure 2B) exhibited the most significant histopathological changes during the study had biliary obstruction secondary to anastomotic stricture that required intervention(s) during the five-year follow-up period.
A–B: Subject 1: Trichrome-stained sections of Yr 0 and Yr 5 biopsies: Severe NRH changes and biopsy fragmentation were already evident at baseline, prior to IS withdrawal. The Yr 5 biopsy shows increased subsinusoidal fibrosis and architectural distortion. No portal inflammation was evident in either the Yr 0 or Yr 5 biopsy (A: H&E-stained upper right insets).
C: Subject 14: Yr 0 and Yr 5 biopsies: This subject showed obvious obstructive cholangiopathic changes and increased portal/periportal fibrosis. The insets (40X magnification) show non-inflamed portal tracts.
The third participant (Subject 9; liver test profile in Figure 2C) exhibited fluctuations in GGT.
D: Subject 9: Yr 0 and Yr 5 biopsies showed neither inflammation nor noticeable change in fibrosis over five years.
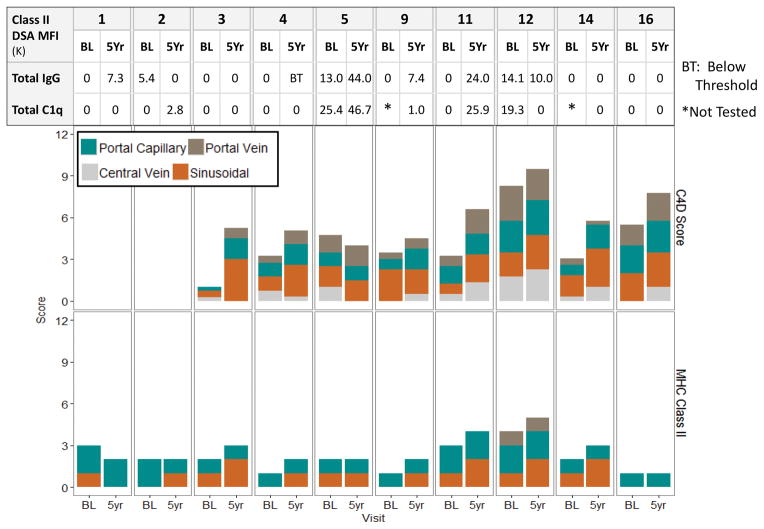
A separate needle biopsy fragment (0.5–1 cm) was snap frozen for optimal assessment of C4d deposition. Tissue handling and preservation artifacts resulted in the availability of only eight paired baseline and Yr 5 specimens for comparison (Figure 6). C4d scores summed over four compartments increased in seven of eight subjects by a median (range) score of 2.25 (1.0–4.25), primarily from increased sinusoidal staining. The expression of MHC Class II antigen, the putative target of Class II DSA, was quantified by compartment; portal-based dendritic cells served as internal positive controls. In contrast to kidney (18) and heart (19) allografts that display constitutive expression of MHC Class II on all interstitial capillaries, liver allografts displayed low to modest MHC class II expression, limited predominantly to occasional portal capillaries and focally on sinusoidal endothelium. MHC Class II staining scores also increased modestly, again predominantly in the sinusoidal compartment (Figure 6). Paired biopsies over five years showed increased sinusoidal staining for six of 10 subjects; portal capillary staining remained unchanged in nine and decreased in one subject.
Figure 6. Sum of Class II DSA MFIs and Biopsy C4d and MHC Class II Scores for Operationally Tolerant WISP-R Subjects.
For each operationally tolerant subject, data is shown at two time points: baseline (BL), prior to study entry and IS withdrawal and Yr 5, five years after study entry, corresponding to 4+ years after last dose of IS. The sum of Class II DSA single antigen and C1q binding MFIs and at the specified time point is shown, aligned with the C4d and MHC Class II scores by compartment.
Finally, multiplex labeling for α-SMA and CD34 was used to detect a shift toward a pathogenic phenotype in stellate (16) or sinusoidal endothelial cells (15). Despite the slight increase of sinusoidal C4d and MHC Class II scores, there were no changes in CD34 or α-SMA expression in peri-portal, sinusoidal, or peri-venular regions (data not shown).
Evolution of Auto-antibodies, DSAs, DSA IgG Subclass, and C1q Binding Activity over Time for Tolerant Pediatric Liver Transplant Recipients
Annual assessment of auto-antibodies and quantitative IgG over five years did not identify any trends among the operationally tolerant subjects over time of IS withdrawal (Figure 1; Supplementary Table 4). The presence and strength of Class I and II allo-antibodies in general, DSAs in particular, were also sequentially assessed. Eight of the 12 operationally tolerant subjects had no DSA at study entry. Three subjects (3, 7 and 14) never developed any detectable DSA, including Subject 14 who suffered cholangitis and recurrent episodes of biliary obstruction (Supplementary Table 2). At study entry, none of the 12 operationally tolerant subjects had detectable Class I DSA. Three subjects (5, 16 and 17) each developed a single Class I DSA of low MFI (2,000–5,000) during either IS withdrawal or follow-up (Table 2A). Five subjects (1, 4, 9, 11 and 16) developed de novo Class II DSA during IS withdrawal (Table 2B). Two developed a single anti-DR DSA and three developed a single anti-DQ DSA; de novo Class II DSA was often transient, occurring at a single time point for two of the five subjects (4 and 16). Notably, all seven Class II DSAs (four anti-DR and three anti-DQ) identified in the remaining four subjects prior to IS withdrawal, persisted during withdrawal and follow-up, without consistent or durable change in MFI over time (Table 2C).
Table 2a.
Evolution of Class I DSA during Long-term Follow-up of Operationally Tolerant Subjects*
| Pt ID | Baseline | Yr 1 | Yr 2 | Yr 3 | Yr 4 | Yr 5 |
|---|---|---|---|---|---|---|
| 5 | No Class I DSA | B*07:02 | No Class I DSA | No Class I DSA | No Class I DSA | No Class I DSA |
| 3,000 | ||||||
| 16 | No Class I DSA | A*02:01 | A*02:01 | No Class I DSA | No Class I DSA | No Class I DSA |
| 3,000 | 2,000 | |||||
| 17 | No Class I DSA | No Class I DSA | No Class I DSA | C*03:04 | C*03:04 | No Class I DSA |
| 2,200 | 2,000 |
Bold italicized entry denotes de novo Class I DSA.
The IgG subclass composition of Class II DSAs harbored by tolerant pediatric liver transplant recipients was determined to better delineate their functional nature. The four IgG subclasses, defined by their heavy chain gene usage, are well-known to differ in their ability to fix complement and affinity for Fc receptors. IgG3 is widely considered to be the most potent, followed closely by IgG1 while both IgG2 and IgG4 are considered weak (20). The prevalence of each subclass is also known to diminish sequentially with IgG1 being the most common and IgG4 the least common.
Baseline, Yr 3, and Yr 5 specimens with Class II DSA were subject to determination of IgG subclass. Seven of the 12 tolerant subjects exhibited at least one Class II DSA at one or more of these three time points. IgG subclass MFIs are shown, along with those from standard single antigen and C1q binding assays (Figure 3). The IgG1 subclass was indeed the most frequently identified, found in all subjects at for all Class II DSAs for all time points except one (Yr 5; Subject 12; DRB1*1501). IgG2 and IgG4 Class II DSAs were frequently found, in six and four of the seven participants, respectively. In three subjects (5, 9, and 11), IgG2 and IgG4 MFI far exceeded that of IgG1. In contrast, IgG3 DSA was rarely identified, found in a single participant at a single time-point for a single DSA (DQB1*0602; Subject 5; Yr 3).
Finally, all available baseline, Yr 3, and Yr 5 serum samples were tested, regardless of Class II DSA presence, for C1q binding activity. C1q binding is an early step in the classical complement cascade which culminates in the formation of the membrane attack complex and target cell destruction. A C1q binding MFI greater than 1,000 was considered positive. Of 10 baseline sera tested, three specimens (from subjects 5, 12 and 17), all with Class II DSA of MFI >10,000 fixed C1q; two baseline sera were unavailable (subjects 9 and 14) but had no detectable alloantibody (Figure 3). Over the course of five years, seven of the 12 tolerant subjects had serum that fixed C1q at one or more time point tested. C1q binding activity was never detected in the complete absence of Class II DSA. However, two Class II DSAs of MFI <2,000 did exhibit C1q binding activity (Subject 2, DQB1*05 01, MFI 1,300, Yr 5; subject 9, DQB1*03 01, MFI 1,900, Yr 3) (Figure 3). In contrast, several Class II DSAs of variable MFIs (4,500 – >20,000) did not exhibit C1q binding activity. Neither the presence nor strength of C1q binding activity appeared to be associated with progressive inflammation or fibrosis over time (Figure 3).
DISCUSSION
We have completed a prospective, multi-center, single arm cohort study of IS withdrawal for stable long-term pediatric recipients of parental living donor liver grafts. The strength of our trial lies in its prospective nature and the longitudinal collection of simultaneous protocol biopsies and peripheral blood, allowing us to assess whether immunological events in the periphery are reflected as allograft damage. Previously, we have reported on the trial’s primary endpoint – the proportion of participants who are operationally tolerant, defined as those who remain off IS for at least one year (14). We now report on the detailed histological and serological characterization of the operationally tolerant subjects over a five year period.
The only papers (two in total) that have assessed the long-term impact of IS withdrawal were in adults (2, 21). IS withdrawal, among recipients with hepatitis C virus, was associated with reduced prevalence of hyperglycemia, cardiovascular disease, and infection episodes (2). IS withdrawal, however, did not yield similar benefits in cohort without hepatitis C (21). Similarly, in our pediatric cohort, IS withdrawal did not mitigate components of metabolic syndrome (22). Since the benefit of remaining off IS has not been convincingly demonstrated for either adults or children, assessment of its safety over time is paramount.
The issue of greatest concern for operationally tolerant pediatric liver transplant recipients is that of allograft deterioration. Insufficient IS has been hypothesized as a possible etiology for the inflammation and/or fibrosis observed in long-surviving pediatric liver allograft recipients who have been maintained on standard of care IS (3–8) as well as those who have undergone IS withdrawal (9, 13). We have shown that, over five years – approximately one year of IS reduction plus four years of no IS, there has been no systematic or progressive increase in either inflammation or fibrosis by light microscopy. Overall, the absence of detectable allograft inflammation and progressive architectural distortion suggests operational tolerance. However, the frequent presence of DSA, commonly interpreted as an ongoing allo-immune response, contrasts with the apparent lack of damaging effector responses within the allograft. In order to further explore this conundrum, we interrogated both the antibody response and the allograft tissue in detail.
The majority of our tolerant subjects (eight of 12) initiated IS withdrawal without any DSA, consistent with reports that patients without DSA are more likely to be tolerant (23, 24). Notably, four tolerant subjects did have DSA at study entry indicating that the presence and persistence of DSA was not prohibitive of operational tolerance. Moreover, seven tolerant subjects developed de novo DSA (with either Class I or Class II specificity) that were often of high MFI and occasionally persistent. The DSA response of these operationally tolerant subjects, both pre-existing and de novo, exhibited a clear dominance of Class II over Class I specificity. Intriguingly, the emergence of de novo Class II DSA in was similarly observed in a recent pilot trial of tolerance induction in adult liver transplant recipients utilizing a regulatory T cell-enriched product (25). The preponderance of Class II DSA may be explained by the pattern of antigen expression and antigen clearance within the liver. In the quiescent, non-injured, non-inflamed liver, Class I antigens are constitutively expressed by all cells. The liver secretes Class I HLA antigens that can bind to and neutralize circulating Class I DSA to form immune complexes that are cleared by Kupffer cells [reviewed in (26)]. Preferential clearance of Class I compared to Class II DSA from the circulation has been reported after both liver transplantation alone and simultaneous liver and kidney transplantation (27–29). Class II expression within the quiescent, non-injured, and non-inflamed liver is largely restricted to hematolymphoid cells with only weak and focal microvascular endothelial expression (26, 30). The down-regulation of Class II expression has been attributed to lipopolysaccharide-induced production of IL-10 by Kupffer cells (31, 32). Notably, original descriptions of rodent liver allograft “tolerance” includes persistence of anti-class II DSA and paucity of donor class II antigen expression in the liver (33).
The liver’s unique ability to clear anti-Class I antibodies, as discussed above, may explain the absence of circulating Class I DSA and the presence of Class II antibodies in liver transplant recipients. However, essentially all organ transplant recipients, not just liver recipients, exhibit a dominance of Class II DSA. Moreover, the presence of α-DQ DSA has been consistently associated with poor allograft outcomes after renal, cardiac and liver transplantation (11, 34–38). The biological rationale for why Class II antigens in general and DQ antigens in particular elicit the strongest antibody responses is unknown. Poor donor-recipient DQ matching may also be an explanation for kidney transplant recipients, since the DR but not DQ loci are considered in kidney allocation. However, this is an unlikely explanation for liver transplant recipients as HLA matching does not enter into either organ allocation or acceptance. Among our operationally tolerant pediatric liver transplant recipients, Class II DSA, including α-DQ DSA was common.
To further explore the DSA response of our operationally tolerant subjects, we characterized DSA by IgG subclass and C1q binding activity. In our cohort, IgG1 was pervasive, IgG2 was common, IgG4 was frequent, but IgG3 was rare, identified only in a single subject at a single time point. The relative stability of allograft histopathology may be partially attributable to the rarity of IgG3 DSA, which has been associated with inferior outcomes for both kidney and liver transplant recipients (39, 40). Among adult, non-HLA identical, primary kidney transplant recipients, IgG3 DSA was associated with increased rates of rejection and graft loss as well as lower glomerular filtration rates at last follow-up (39). Among adult, primary liver transplant recipients, pre-formed IgG3 DSA independently predicted death [hazard ratio (HR) 2.4, p<0.001]; among those without pre-formed DSA who survived for at least one year, de novo IgG3 DSA also independently predicted death (HR 2.1, p=0.004) (40).
In our operationally tolerant subjects, the identified Class II DSA frequently exhibited C1q binding activity. The clinical importance of complement binding has been reported for various allografts (11, 35, 40–42). In the kidney, C1q+ DSA has been associated with unfavorable histopathologic features including microvascular inflammation, C4d deposition, transplant glomerulopathy, interstitial and tubular inflammation, and interstitial fibrosis and tubular atrophy. Moreover, those with C1q+ DSA suffered poor five year graft survival (54%), compared to those without DSA (94%) and those with non–complement binding DSA (93%) (P <0.001 for both comparisons) (41). In contrast, the impact of C1q+ DSA on liver transplant outcomes is less clear with conflicting reports in adult recipients (40, 43). A single report regarding pediatric liver transplant recipients found that C1q binding activity often coincided with high MFI and correlated with a non-tolerant phenotype (11).
Despite the presence of high MFI DSA with C1q positivity, the “signature” lesion of antibody-mediated rejection in all allografts, microvascular inflammation (44), was not observed in our withdrawal trial or in the tolerance induction trial (25). We did however note an increased number of Kupffer cells after IS withdrawal which, along with LSECs, exhibit known scavenger functions. Efficient clearance of immune complexes, activated complement components, and platelet aggregates by these scavenger cells may protect the liver allograft from antibody-mediated injury [reviewed in (26)]. Furthermore we did not observe phenotypic changes in LSECs characteristic of their response to injury or fibrogenesis (45–48). This finding is wholly consistent with the lack of progressive fibrosis observed by light microscopy. We did observe a modest increase in sinusoidal C4d deposits. Whether undetectable arterial intimal LSEC injury or microvascular endothelial cell alterations (e.g. upregulation of complement regulatory proteins or cytoprotective molecules) are occurring is currently being investigated.
In summary, five year follow-up of our tolerant pediatric cohort is notable for a consistent disparity between ongoing peripheral allo-antibody responses and evidence of allograft damage. The immunological mechanisms that underlie these provocative observations remain to be elucidated, but “tolerogenic” properties unique to the liver are likely contributors. Traditional explanations for the muting of T cell responses within the liver include a bias of liver antigen presenting cells towards eliciting regulatory rather than effector responses, the predisposition of activated T cells towards exhaustion and/or apoptosis due to the expression of negative co-stimulatory molecules and anti-inflammatory cytokines (IL-10 and TGF-β) by multiple intrahepatic cell populations, the production of soluble MHC Class I, and the reduced expression of MHC Class II (49, 50). Other possible explanations include the scarcity of IgG3 DSA subclass, activation of liver protective mechanisms (e.g. increasing the number of Kupffer cells to phagocytize the products of DSA reactions), or up-regulation of complement regulatory or cytoprotective molecules on the endothelium. Our observations suggest that some or all of these mechanisms explain the failure of the immune response to cause clinical or histopathologic damage to in operationally tolerant allografts. We, however, cannot exclude the possibility that damage to the allograft might be too subtle to detect during the five year interval examined.
Although we have comprehensively characterized a unique cohort of operationally tolerant pediatric liver transplant recipients, we recognize that our study does have important weaknesses. First and perhaps foremost, the cohort is modest in size as it derives from a pilot study of IS withdrawal. Unfortunately, it is impossible to enlarge the cohort as it is the operationally tolerant subset of subjects enrolled in WISP-R. However, the modest number of subjects allowed us to study and present each in great detail. Second, our subjects are highly selected and relatively homogeneous, all pediatric recipients of parental living donor allografts. Our findings may, therefore, have limited generalizability. Relevance to pediatric recipients of deceased donor allografts who are identified as operationally tolerant may emerge once data is available from a currently ongoing trial of IS withdrawal for 88 pediatric deceased and living donor liver transplant recipients at 12 transplant centers in North America (iWITH; NCT01638559). Third, our study which was focused on identifying and studying operationally tolerant subjects did not have a control cohort. As such, it is impossible to determine whether the evolution of DSA, IgG subclass and C1q binding profiles specifically reflect the withdrawal and/or the absence of IS. Finally, as already mentioned above, although our detailed and comprehensive five-year follow-up data of an operationally tolerant cohort is unique, even longer follow-up to delineate the clinical and histological impact of IS withdrawal is critically important to maximize the longevity of pediatric liver transplant recipients.
In conclusion we have demonstrated that IS discontinuation has been safe in a closely monitored cohort of operationally tolerant pediatric recipients has not been associated with histological deterioration of allografts over five years of follow-up. Despite the persistence or development of DSA and modest increases in sinusoidal C4d staining, the allografts did not evidence damage from either humoral or cellular effector mechanisms. Microvascular inflammation, progressive fibrosis, or changes in LSEC or stellate cell phenotype did not develop over more than five years of follow-up. Although it is possible that progressive pathology may emerge with longer follow-up, progressive histological deterioration of allografts has been well-described in patients maintained on IS. Regardless, limitations imposed by the size and homogeneity of this cohort - pediatric recipients of parental living donor liver grafts - are being addressed in a similar but substantially larger study [NCT01638559; Immunosuppression Withdrawal for Stable Pediatric Liver Transplant Recipients (iWITH)] inclusive of deceased donor transplant recipients with the expectation that a better understanding of the mechanistic underpinnings of “liver allograft acceptance” will be achieved.
Supplementary Material
Table 2b.
Evolution of Class II DSA during Long-term Follow-up of Operationally Tolerant Subjects without Class II DSA at Baseline*
Bold italicized entry denotes de novo Class II DSA.
Baseline, Yr 3, and Yr 5 (highlighted) specimens were tested for IgG subclass as shown in Figure 3.
Table 2c.
Evolution of Class II DSA during Long-term Follow-up of Operationally Tolerant Subjects with Class II DSA at Baseline
Baseline, Yr 3, and Yr 5 (highlighted) specimens were tested for IgG subclass as shown in Figure 3.
Acknowledgments
Financial Support: This research was performed as a project of the Immune Tolerance Network, an international clinical research consortium headquartered at the Benaroya Research Institute and supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1AI109565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We gratefully acknowledge the contributions of R. A. Bray, Ph.D. and H. M. Gebel, Ph.D. for HLA typing, allo-antibody detection, and DSA determination; A. L. Girnita, M.D. for IgG subclass determination; and D. B. Tyan, Ph.D. for performing C1q binding activity assays.
ABBREVIATIONS
- IS
Immunosuppression
- IgG
Immunoglobulin G
- DSA
Donor specific antibody
- SMA
Smooth muscle actin
- LSEC
Liver sinusoidal endothelial cell
- MFI
Mean fluorescence intensity
- MHC
Major histocompatibility complex
- HLA
Human leukocyte antigen
- AE
Adverse event
- SAE
Serious adverse event
- NRH
Nodular regenerative hyperplasia
- HR
Hazard ratio
References
- 1.Londono MC, Rimola A, O’Grady J, Sanchez-Fueyo A. Immunosuppression minimization vs. complete drug withdrawal in liver transplantation. J Hepatol. 2013;59:872–879. doi: 10.1016/j.jhep.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Orlando G, Manzia T, Baiocchi L, Sanchez-Fueyo A, Angelico M, Tisone G. The Tor Vergata weaning off immunosuppression protocol in stable HCV liver transplant patients: the updated follow up at 78 months. Transpl Immunol. 2008;20:43–47. doi: 10.1016/j.trim.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Ekong UD, Melin-Aldana H, Seshadri R, Lokar J, Harris D, Whitington PF, Alonso EM. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver Transpl. 2008;14:1582–1587. doi: 10.1002/lt.21549. [DOI] [PubMed] [Google Scholar]
- 4.Evans HM, Kelly DA, McKiernan PJ, Hubscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43:1109–1117. doi: 10.1002/hep.21152. [DOI] [PubMed] [Google Scholar]
- 5.Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, Egawa H, Yurugi K, Masuda S, Minamiguchi S, et al. Progressive graft fibrosis and donor-specific human leukocyte antigen antibodies in pediatric late liver allografts. Liver Transpl. 2012;18:1333–1342. doi: 10.1002/lt.23534. [DOI] [PubMed] [Google Scholar]
- 6.Sanada Y, Matsumoto K, Urahashi T, Ihara Y, Wakiya T, Okada N, Yamada N, et al. Protocol liver biopsy is the only examination that can detect mid-term graft fibrosis after pediatric liver transplantation. World J Gastroenterol. 2014;20:6638–6650. doi: 10.3748/wjg.v20.i21.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheenstra R, Peeters PM, Verkade HJ, Gouw AS. Graft fibrosis after pediatric liver transplantation: ten years of follow-up. Hepatology. 2009;49:880–886. doi: 10.1002/hep.22686. [DOI] [PubMed] [Google Scholar]
- 8.Venturi C, Sempoux C, Quinones JA, Bourdeaux C, Hoyos SP, Sokal E, Reding R. Dynamics of allograft fibrosis in pediatric liver transplantation. Am J Transplant. 2014;14:1648–1656. doi: 10.1111/ajt.12740. [DOI] [PubMed] [Google Scholar]
- 9.Yoshitomi M, Koshiba T, Haga H, Li Y, Zhao X, Cheng D, Miyagawa A, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87:606–614. doi: 10.1097/TP.0b013e318195a7cb. [DOI] [PubMed] [Google Scholar]
- 10.Grabhorn E, Binder TM, Obrecht D, Brinkert F, Lehnhardt A, Herden U, Peine S, et al. Long-term Clinical Relevance of De Novo Donor-Specific Antibodies After Pediatric Liver Transplantation. Transplantation. 2015;99:1876–1881. doi: 10.1097/TP.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 11.Wozniak LJ, Hickey MJ, Venick RS, Vargas JH, Farmer DG, Busuttil RW, McDiarmid SV, et al. Donor-specific HLA Antibodies Are Associated With Late Allograft Dysfunction After Pediatric Liver Transplantation. Transplantation. 2015;99:1416–1422. doi: 10.1097/TP.0000000000000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada H, Kondou H, Kimura T, Ikeda K, Tachibana M, Hasegawa Y, Kiyohara Y, et al. Humoral immunity is involved in the development of pericentral fibrosis after pediatric live donor liver transplantation. Pediatr Transplant. 2012;16:858–865. doi: 10.1111/j.1399-3046.2012.01781.x. [DOI] [PubMed] [Google Scholar]
- 13.Egawa H, Miyagawa-Hayashino A, Haga H, Teramukai S, Yoshizawa A, Ogawa K, Ogura Y, et al. Non-inflammatory centrilobular sinusoidal fibrosis in pediatric liver transplant recipients under tacrolimus withdrawal. Hepatol Res. 2012;42:895–903. doi: 10.1111/j.1872-034X.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 14.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 15.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;61:1740–1746. doi: 10.1002/hep.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Tyan DB. C1q assay for the detection of complement fixing antibody to HLA antigens. Methods Mol Biol. 2013;1034:305–311. doi: 10.1007/978-1-62703-493-7_16. [DOI] [PubMed] [Google Scholar]
- 18.Muczynski KA, Ekle DM, Coder DM, Anderson SK. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. J Am Soc Nephrol. 2003;14:1336–1348. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- 19.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–683. [PMC free article] [PubMed] [Google Scholar]
- 20.Valenzuela NM, Mulder A, Reed EF. HLA class I antibodies trigger increased adherence of monocytes to endothelial cells by eliciting an increase in endothelial P-selectin and, depending on subclass, by engaging FcgammaRs. J Immunol. 2013;190:6635–6650. doi: 10.4049/jimmunol.1201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tryphonopoulos P, Ruiz P, Weppler D, Nishida S, Levi DM, Moon J, Tekin A, et al. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation. 2010;90:1556–1561. doi: 10.1097/TP.0b013e3182003db7. [DOI] [PubMed] [Google Scholar]
- 22.Perito ER, Mohammad S, Rosenthal P, Alonso EM, Ekong UD, Lobritto SJ, Feng S. Posttransplant metabolic syndrome in the withdrawal of immunosuppression in Pediatric Liver Transplant Recipients (WISP-R) pilot trial. Am J Transplant. 2015;15:779–785. doi: 10.1111/ajt.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girnita A, Mazariegos GV, Castellaneta A, Reyes J, Bentlejewski C, Thomson AW, Zeevi A. Liver transplant recipients weaned off immunosuppression lack circulating donor-specific antibodies. Hum Immunol. 2010;71:274–276. doi: 10.1016/j.humimm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Ohe H, Waki K, Yoshitomi M, Morimoto T, Nafady-Hego H, Satoda N, Li Y, et al. Factors affecting operational tolerance after pediatric living-donor liver transplantation: impact of early post-transplant events and HLA match. Transpl Int. 2012;25:97–106. doi: 10.1111/j.1432-2277.2011.01389.x. [DOI] [PubMed] [Google Scholar]
- 25.Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, Watanabe M, et al. A Pilot Study of Operational Tolerance with a Regulatory T Cell-Based Cell Therapy in Living Donor Liver Transplantation. Hepatology. 2016 doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 26.Demetris AJ, Zeevi A, O’Leary JG. ABO-compatible liver allograft antibody-mediated rejection: an update. Curr Opin Organ Transplant. 2015;20:314–324. doi: 10.1097/MOT.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dar W, Agarwal A, Watkins C, Gebel HM, Bray RA, Kokko KE, Pearson TC, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–847. doi: 10.1111/j.1600-6143.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 28.Olausson M, Mjornstedt L, Norden G, Rydberg L, Molne J, Backman L, Friman S. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant. 2007;7:130–136. doi: 10.1111/j.1600-6143.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 29.Taner T, Gandhi MJ, Sanderson SO, Poterucha CR, De Goey SR, Stegall MD, Heimbach JK. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–1510. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 30.Rouger P, Gugenheim J, Gane P, Capron-Landereau M, Michel F, Reynes M, Bismuth J. Distribution of the MHC antigens after liver transplantation: relationship with biochemical and histological parameters. Clin Exp Immunol. 1990;80:404–408. doi: 10.1111/j.1365-2249.1990.tb03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 32.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 33.Kamada N, Shinomiya T. Serology of liver transplantation in the rat. I. Alloantibody responses and evidence for tolerance in a nonrejector combination. Transplantation. 1986;42:7–13. doi: 10.1097/00007890-198607000-00002. [DOI] [PubMed] [Google Scholar]
- 34.DeVos JM, Gaber AO, Knight RJ, Land GA, Suki WN, Gaber LW, Patel SJ. Donor-specific HLA-DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int. 2012;82:598–604. doi: 10.1038/ki.2012.190. [DOI] [PubMed] [Google Scholar]
- 35.Freitas MC, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, Briley KP, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95:1113–1119. doi: 10.1097/TP.0b013e3182888db6. [DOI] [PubMed] [Google Scholar]
- 36.Tagliamacco A, Cioni M, Comoli P, Ramondetta M, Brambilla C, Trivelli A, Magnasco A, et al. DQ molecules are the principal stimulators of de novo donor-specific antibodies in nonsensitized pediatric recipients receiving a first kidney transplant. Transpl Int. 2014;27:667–673. doi: 10.1111/tri.12316. [DOI] [PubMed] [Google Scholar]
- 37.Willicombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Galliford J, McLean A, et al. De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation. 2012;94:172–177. doi: 10.1097/TP.0b013e3182543950. [DOI] [PubMed] [Google Scholar]
- 38.Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, Terasaki PI. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013;13:1541–1548. doi: 10.1002/ajt.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everly MJ, Rebellato LM, Haisch CE, Briley KP, Bolin P, Kendrick WT, Kendrick SA, et al. Impact of IgM and IgG3 anti-HLA alloantibodies in primary renal allograft recipients. Transplantation. 2014;97:494–501. doi: 10.1097/01.TP.0000441362.11232.48. [DOI] [PubMed] [Google Scholar]
- 40.O’Leary JG, Kaneku H, Banuelos N, Jennings LW, Klintmalm GB, Terasaki PI. Impact of IgG3 subclass and C1q-fixing donor-specific HLA alloantibodies on rejection and survival in liver transplantation. Am J Transplant. 2015;15:1003–1013. doi: 10.1111/ajt.13153. [DOI] [PubMed] [Google Scholar]
- 41.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215–1226. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 42.Chin C, Chen G, Sequeria F, Berry G, Siehr S, Bernstein D, Rosenthal D, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant. 2011;30:158–163. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Kubal CA, Mangus RS, Saxena R, Lobashevsky A, Higgins N, Agarwal A, Fridell JA, et al. Crossmatch-positive liver transplantation in patients receiving thymoglobulin-rituximab induction. Transplantation. 2014;97:56–63. doi: 10.1097/TP.0b013e3182a688c0. [DOI] [PubMed] [Google Scholar]
- 44.Drachenberg CB, Papadimitriou JC. Endothelial injury in renal antibody-mediated allograft rejection: a schematic view based on pathogenesis. Transplantation. 2013;95:1073–1083. doi: 10.1097/TP.0b013e31827e6b45. [DOI] [PubMed] [Google Scholar]
- 45.Brunt EM, Gouw AS, Hubscher SG, Tiniakos DG, Bedossa P, Burt AD, Callea F, et al. Pathology of the liver sinusoids. Histopathology. 2014;64:907–920. doi: 10.1111/his.12364. [DOI] [PubMed] [Google Scholar]
- 46.Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalor PF, Lai WK, Curbishley SM, Shetty S, Adams DH. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J Gastroenterol. 2006;12:5429–5439. doi: 10.3748/wjg.v12.i34.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu B, Broome U, Uzunel M, Nava S, Ge X, Kumagai-Braesch M, Hultenby K, et al. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 2003;163:1275–1289. doi: 10.1016/S0002-9440(10)63487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benseler V, Tay SS, Bowen DG, Bertolino P. Role of the hepatic parenchyma in liver transplant tolerance: a paradigm revisited. Dig Dis. 2011;29:391–401. doi: 10.1159/000329802. [DOI] [PubMed] [Google Scholar]
- 50.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60:2109–2117. doi: 10.1002/hep.27254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.