Abstract
Purpose
Histological markers that differentiate benign and malignant pediatric adrenocortical tumors (ACTs) are lacking. Previous studies have implicated an association of major histocompatibility complex (MHC) class II expression with ACT prognosis. Here we determined the expression of MHC class II as well as the cell of origin of these immunological markers in pediatric ACT. The impact of MHC class II gene expression on outcome was determined in a cohort of uniformly-treated children with adrenocortical carcinomas.
Experimental Design
We analyzed the expression of MHC class II and a selected cluster of differentiation genes in 63 pediatric ACTs by Affymetrix Human U133 Plus 2.0 or HT HG-U133+PM gene chip analyses. Cells expressing MHC class II were identified by morphologic and immunohistochemical assays.
Results
MHC class II expression was significantly greater in adrenocortical adenomas than in carcinomas (P = 4.8 × 10−6) and was associated with a higher progression-free survival (PFS) estimate (P = 0.003). Specifically, HLA-DPA1 expression was most significantly associated with PFS after adjustment for tumor weight and stage. HLA-DPA1 was predominantly expressed by hematopoietic infiltrating cells and undetectable in tumor cells in 23 of 26 cases (88%).
Conclusion
MHC class II expression, which is produced by tumor- infiltrating immune cells, is an indicator of disease aggressiveness in pediatric ACT. Our results suggest that immune responses modulate adrenocortical tumorigenesis, and may allow the refinement of risk stratification and treatment for this disease.
Keywords: MHC class II, pediatric adrenocortical tumors, HLA-DPA1, dendritic cells
Introduction
Adrenocortical tumors (ACTs) are rare in children accounting for approximately 0.2% of all pediatric malignancies (1). Currently, prognosis is based on tumor size, surgical resectability, and metastasis at diagnosis (2–5). Adrenocortical adenomas carry a good prognosis; however, the outcome is variable for the 80% of cases classified as carcinomas. Many small, completely excised adrenocortical carcinomas later relapse, while others are cured (2–5). To determine the clinical behavior of pediatric ACT according to histologic criteria is often challenging.
Previous studies have evaluated Major Histocompatibility Complex (MHC) class II expression as a prognostic marker for pediatric ACT (6–8). We previously demonstrated that the expression of specific MHC class II genes was higher in pediatric adrenocortical adenomas compared to carcinomas (6). Consistent with these findings, Leite et al reported that lower expression of HLA-DRA, HLA-DPA1, and HLA-DPB1 genes may contribute to more aggressive disease (7). However, Magro and colleagues concluded that MHC class II expression did not discriminate between benign and malignant pediatric ACTs (8). Therefore, the relevance of MHC class II as an indicator of outcome for pediatric ACT remains to be established.
MHC class II markers are expressed mainly by hematopoietic cells of the immune system, but have been observed in normal adult adrenal cortex cells with weak immunostaining in the inner fasciculate zone and strong staining in the reticularis zone (9). Conversely, fetal adrenocortical cells of both zones were invariably negative (9). Whether MHC class II gene expression in pediatric ACT arises from the adrenocortical tumor or infiltrating immune cells is uncertain.
Here we evaluated: 1) the expression of MHC class II genes; 2) the cell of origin expressing MHC class II; and 3) the impact of MHC class II expression on clinical outcome in a large group of uniformly-treated pediatric ACTs. High MHC class II expression, in particular HLA-DPA1, was found associated with tumor-infiltrating immune cells and a more favorable progression-free survival.
METHODS
Participants and Samples
The study included 52 participants in the St. Jude Children's Research Hospital (St. Jude) International Pediatric Adrenocortical Tumor Registry (IPACTR) (clinicaltrials.gov, ID number NCT00700414) (2). The tumors were of any histology (adenomas, carcinomas and undefined histology) and were not uniformly treated. The IPACTR patients typically underwent surgery, and chemotherapy for advanced stage disease (2–4). A second group comprised of 34 subjects with histologically-defined adrenocortical carcinomas were uniformly treated on the Children's Oncology Group (COG) ARAR0332 protocol (clinicaltrials.gov, ID number NCT00304070) (4), which is a non- randomized, single-arm, prospective study. Classification of adenoma, carcinoma or tumors of undetermined histology in the first cohort (IPACTR) was based on the modified criteria of Weiss and colleagues (10–12) by two independent pathologists. In the COG cohort, only cases with diagnosis of carcinoma, centrally reviewed by one of the authors (JH), were enrolled on this study. The criteria for carcinoma were based on a malignancy scoring system of nine gross and histopathological features, including tumor weight (>400 g), tumor size (>10.5 cm), extension into periadrenal soft tissue and/or adjacent organs, vena cava invasion, venous invasion, capsular invasion, mitotic index (>15 per 20 high power fields), and atypical mitotic figures, as previously defined by Wienecke (13). Each item present is given a score of 1. The identification of 4 or more of the above features is considered to be malignant. If there is metastatic disease to lymph nodes or distant sites, the tumor is considered malignant, as well. Written informed consent was obtained from parents or legal guardians in all cases. This study was approved by the St. Jude Institutional Review Board and by the Cancer Therapy Evaluation Program of the National Cancer Institute. Selected demographic and clinical features of the 86 patients are summarized in Table 1; detailed information is provided in Supplementary Table 1.
Table 1.
Clinical Characteristics of 86 Pediatric Patients with Adrenocortical Tumors
| Characteristic | All cases | Non-uniformly treated cohort 1 (n = 52) | Uniformly treated cohort 2 (n = 34) |
|---|---|---|---|
|
| |||
| Gender | |||
| Female | 63 | 41 | 22 |
| Male | 23 | 11 | 12 |
|
| |||
| Age at presentation | |||
| Mean (mo) | 68.47 | 68.74 | 68.06 |
|
| |||
| Endocrine signs | |||
| Virilization | 47 | 27 | 20 |
| Mixed | 16 | 12 | 4 |
| Non-functioning | 14 | 7 | 7 |
| Other | 9 | 6 | 3 |
|
| |||
| Tumor histology | |||
| Adenoma | 13 | 13 | 0 |
| Carcinoma | 66 | 32 | 34 |
| Undefined | 7 | 7 | 0 |
|
| |||
| Disease stage | |||
| I | 28 | 18 | 10 |
| II | 18 | 11 | 7 |
| III | 28 | 18 | 10 |
| IV | 12 | 5 | 7 |
|
| |||
| Tumor weight (g) | |||
| < 200 | 49 | 28 | 21 |
| >200 | 32 | 22 | 10 |
|
| |||
| Relapse | |||
| (n) | 24 | 12 | 12 |
|
| |||
| Death | |||
| (n) | 21 | 13 | 8 |
|
| |||
| Survival time | |||
| Mean (mo) | 44.38 | 50.77 | 34.6 |
Abbreviations: IPACTR, International Pediatric Adrenocortical Tumor Registry (non-uniformly treated cohort); COG, Children's Oncology Group (uniformly-treated cohort).
Microarray Analysis
Total RNA from tumor samples were used for RNA probe preparation and hybridization to Affymetrix Human Genome U133 Plus 2.0 or HT HG-U133+PM gene chip arrays according to the manufacturer's guidelines (Affymetrix, Santa Clara, CA) (Data Supplement, section 1). Expression of MHC class II and selected cell surface marker genes (Supplementary Table 2) were evaluated in the two cohorts of pediatric adrenocortical tumors (Supplementary Table 1).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumor sections (4-μm) were stained for HLA-DPA1, HLA-DRA and cell surface markers (CD3, CD4, CD8, CD68 and CD163) using Benchmark XT (Ventana Medical, Tucson, AZ) or BondMax (Leica Microsystems, Bannockburn, IL) automated stainers and reagents as listed in Supplementary Table 3 according to the manufacturer's instructions.
Statistical Analysis
For each microarray, we used the Bioconductor affy software (www.bioconductor.org) to compute the mean values of robust multi-array average (RMA)-normalized log2-scale signals across all probe-sets annotated to MHC class II genes (14–15) for comparison of total MHC class II gene expression with histology and prognosis. The positive quantile transformation (PQT) (16) was used to further normalize microarray data for comparison of individual probe-set values with histology and prognosis. We used a discrimination gap statistic, as described in Data Supplement section 2, to select five individual probe-sets in the IPACTR cohort whose expression pattern most clearly distinguished adenomas vs. carcinomas, for further evaluation in the COG cohort. For each of the five selected probe-sets, a single-predictor Cox regression analysis was used to evaluate the univariate association of expression level with the estimate of progression-free survival (PFS) in the COG samples. These single-predictor Cox regression models were used to perform a one-sided test of the association of expression with PFS estimate at the P = 0.05/5 = 0.01 level (Bonferroni adjustment for multiple comparisons). Comparisons that showed a significant association (with the Bonferroni correction) were used in a two-predictor Cox regression model to compare expression with the PFS estimate after adjustment for tumor stage or tumor weight. PFS was defined as the time elapsed between protocol enrollment and the date of disease progression, death, or most recent follow-up. The Kaplan-Meier method was used to estimate PFS probability as a function of time. All statistical analyses were performed using R software (www.r-project.org) and all statistical tests are two-sided except where otherwise stated.
RESULTS
Patients
The age at diagnosis of the 63 females and 23 males was 3.1 to 207.6 months (median, 38 months). Most patients presented with virilization (n=47), Cushing syndrome (n=7), aldosteronism (n=2), or mixed hormonal secretion (n=16). Fourteen patients had non- functional tumors. Disease was staged according to the COG ARAR0332 protocol (4): stage I (n=28), II (n=18), III (n=28) and IV (n=12). Primary tumor samples were obtained in all cases, with the exception of three biopsy samples (ACT080, ACT084 and ACT086) from COG patients who had stage IV disease, as the tumors were considered inoperable. The median follow-up time among progression-free patients was 52.3 months (range, 0.1 – 197.8 months). Clinical data for all patients are summarized in Table 1 and Supplementary Table 1.
Association of MHC Class II Gene Expression to Histology and Progression-free Survival
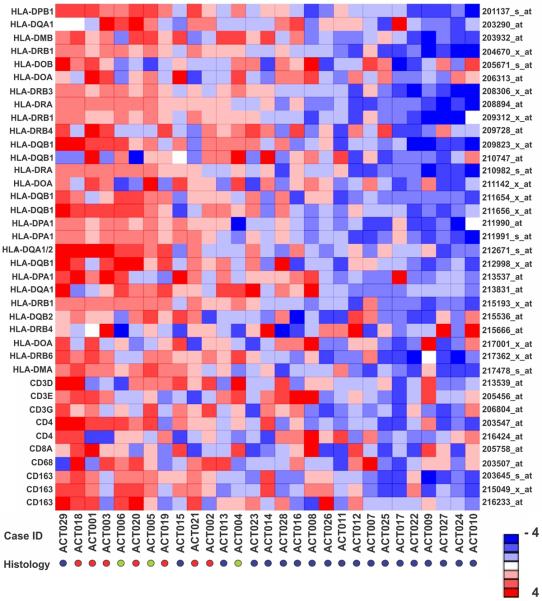
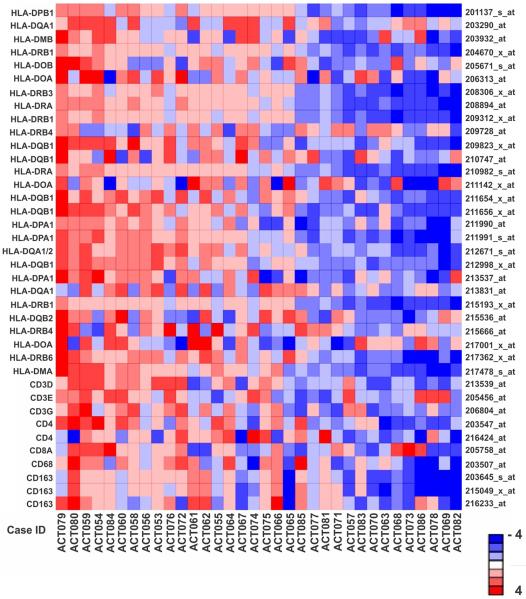
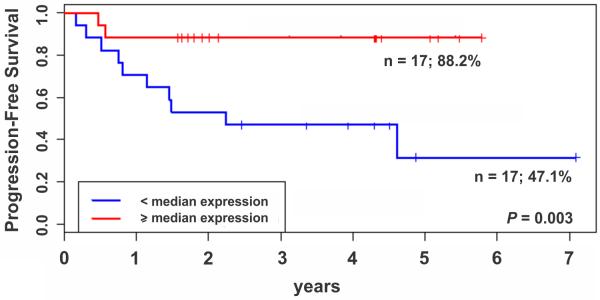
The median expression of 28 MHC class II probe sets was significantly higher in adenomas than in carcinomas (P = 4.8 × 10−6, Wilcoxon rank-sum test) in the IPACTR cohort (Fig 1). Additionally, greater MHC class II expression (Fig 2A) was associated with better progression-free survival (PFS) in the uniformly-treated pediatric adrenocortical carcinomas in the COG cohort (Fig 2B; P = 0.003, Cox regression). The three-year PFS of subjects with less than median MHC class II expression was 47.1% (95% CI: 28.4 – 77.9%) while that of subjects with greater than median MHC class II expression was 88.2% (95% CI: 74.2 – 100%) (Fig 2B).
Fig 1.
Gene expression profiling of MHC class II in the IPACTR cohort. Heat map of 38 probe-sets annotated to MHC class II and cluster of differentiation (CD) cell surface marker genes for 29 non-uniformly treated pediatric adrenocortical tumors. Red circles, adenomas; Green circles, undetermined histology; Blue circles, carcinomas. Probe-sets and corresponding genes are identified at right and left, respectively.
Fig 2.
Gene expression profiling of MHC class II and outcome in the COG cohort. (A) Heat map of 38 probe-sets annotated to MHC class II and cluster differentiation (CD) genes for 34 uniformly-treated pediatric adrenocortical tumors (COG cohort). Probe-sets and corresponding genes are identified at right and left, respectively. (B) Kaplan-Meier estimates of progression-free survival of subjects with tumor expression of MHC II genes less than vs. greater than the median in the uniformly-treated pediatric adrenocortical carcinomas in the COG cohort.
Expression of Specific MHC Class II Genes is Associated with Histology and Progression-free Survival
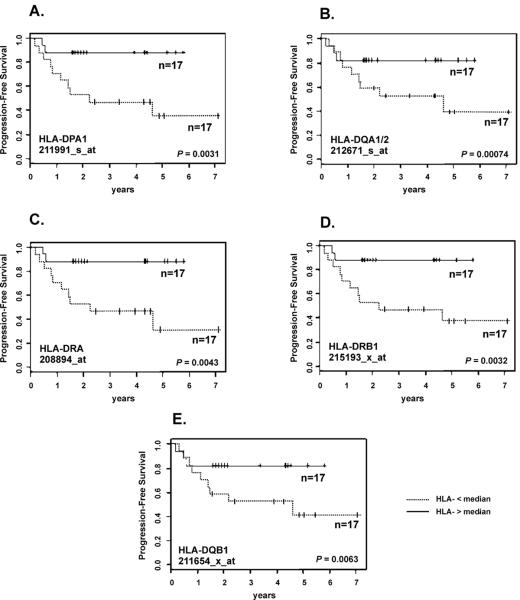
To identify the individual MHC class II probe sets associated with histology and PFS, we computed a discriminating gap size (DGS) criterion (16) for differential gene expression in adenomas and carcinomas in the IPACTR cohort. The DGS criterion can also identify a gap in the expression distribution that may distinguish between two distinct biological processes (see Data Supplement section 2 for details). The probe sets were ranked according to the value of this DGS criterion, and the top 5 were selected (HLA-DPA1, HLA-DQA1/2, HLA-DRA, HLA-DRB1 and HLA-DQB1) (Supplementary Fig 1).
Expression (< vs. ≥ median) of each of the five probe-sets with greatest DGS was then compared to the PFS in the COG cohort (Fig 3). We performed one-sided statistical tests to determine whether greater expression of each probe set was associated with improved PFS, with the Bonferroni adjustment for multiple tests. Greater expression of four of the five probe-sets was significantly associated with greater PFS (one-sided raw p-values < .01, or 0.05/5) (Supplementary Table 4).
Fig 3.
Identification of MHC class II genes associated with progression-free survival (PFS) of pediatric ACT. The five MHC class II probe-sets most differentially expressed in adenomas vs. carcinomas (IPACTR cohort) were used to assess PFS in the uniformly-treated COG group of pediatric adrenocortical carcinomas (n = 34).
In particular, expression of HLA-DPA1 (probe-set 211991_s_at) was significantly associated with tumor weight (P < 0.0001) and disease stage (P = 0.032) but not with age (P = 0.397), gender (P = 0.179), endocrine syndrome (P = 0.071), surgery (P = 0.864) or chemotherapy (P = 0.106) (Supplementary Table 5).
Association of HLA-DPA1 Expression with Progression-free Survival after Adjustment for Disease Stage
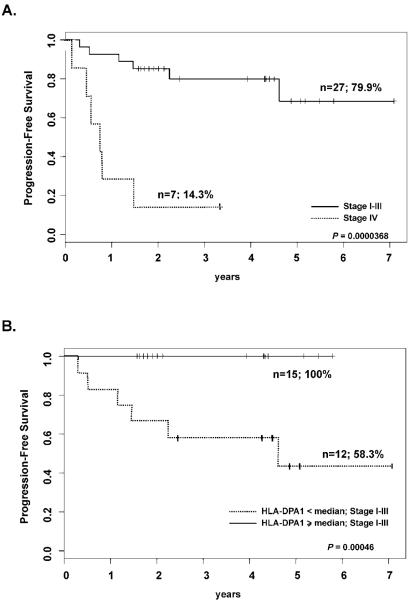
The prognostic value of HLA-DPA1 (probe-set 211991_s_at) expression after adjustment for disease stage in the uniformly-treated COG cohort was evaluated. As expected, advanced stage was associated with poor progression-free survival (PFS). Five of seven stage IV patients had progressive disease within one year and a sixth patient had disease progression within two years (Fig 4A). To date, none of the 15 stage I-III patients with greater than median expression of HLA-DPA1 have died or experienced progressive disease while the 12 stage I-III patients with less than median expression of HLA-DPA1 had a three-year PFS of 58.3% (95% CI: 36.2–94.1%; Fig 4B).
Fig 4.
Association of stage disease and expression of HLA-DPA1 with progression-free survival (PFS). (A) Kaplan-Meier estimates of PFS according to stage disease-only (stage I-III versus IV). (B) Kaplan-Meier estimates of PFS for cases with stage I-III disease according to expression of HLA-DPA1.
Cells of Origin of MHC Class II Expression
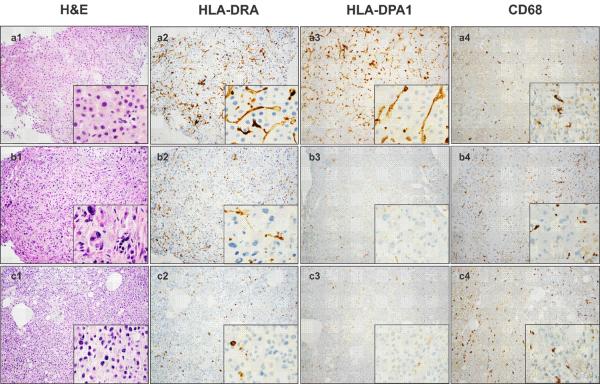
Archival tumor sections of 26 IPACTR cases (15 carcinomas, 6 adenomas, 5 of undetermined histology) were stained for hematoxylin and eosin (H&E) to confirm the pathological diagnosis. Eight ACTs (ACT005, ACT008, ACT012, ACT030, ACT031, ACT032, ACT033 and ACT034) were stained with antibodies specific to HLA-DPA1 and HLA-DRA and markers of mononuclear hematopoietic cells (CD3, CD4, CD8, CD68 and CD163) (Supplementary Table 3). H&E and immunostaining results for a representative adenoma (ACT030, row a), early-stage carcinoma (ACT012, row b) and late-stage carcinoma (ACT008, row c) are shown in Figure 5A. HLA staining was predominantly cytoplasmic, with HLA-DRA brighter than HLA-DPA1 (Fig 5A, columns 2 and 3). MHC class II staining was observed in two morphologic cell types, one that stained intensely for HLA-DRA and HLA-DPA1 and had relatively short cytoplasmic processes and one that stained less intensely and had longer cytoplasmic processes (Fig 5A inset, columns 2 and 3). The cells with short cytoplasmic processes were also strongly positive for CD68 (Fig 5A, column 4) and CD163 (not shown) but less positive for CD4 (Supplementary Fig 2), suggesting tumor-infiltrating macrophages. Other cells showing long cytoplasmic processes were negative for CD68 (Fig 5A, column 4) and CD163 but dimly positive for CD4, consistent with dendritic cells (Supplementary Fig 2). Staining of sequential sections of the same tissue block for CD3, CD4 and CD8 revealed both types of T lymphocytes. Approximately 5–10% of the infiltrating cells were T- lymphocytes (CD3-positive). Moreover, many of the tumor-infiltrating cells dimly positive for CD4 in the adenoma had morphologic characteristic of histiocytes and dendritic cells (Supplementary Fig 2).
Fig 5.
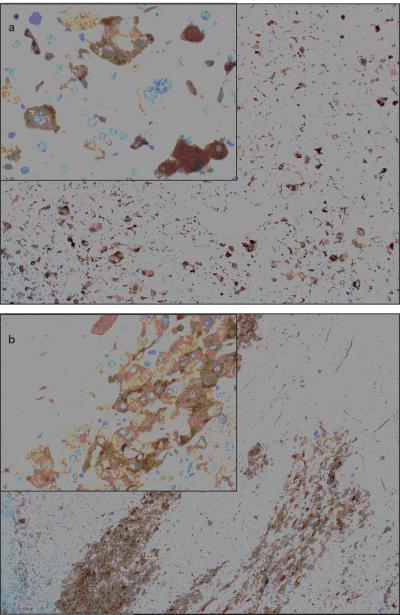
(A) Immunohistochemical analysis of MHC class II protein expression in adrenocortical tumors. Row a: adenoma; row b: stage I carcinoma; row c: stage III carcinoma. Note high expression of HLA-DRA and HLA-DPA1 in the adenoma and low expression in the two carcinomas. CD68 is a cytoplasmic marker for cells of the macrophage lineage. (B) Immunohistochemical analysis reveals HLA-DPA1 expression by adrenocortical tumor cells in two pediatric ACTs: (a) ACT047 and (b) ACT048 (Magnification, 100×; inset, 400×).
Because of the strong association between HLA-DPA1 gene expression and outcome, we analyzed the remaining 18 IPACTR ACTs (10 ACC, 5 ACA and 3 of undetermined histology) with anti–HLA-DPA1 antibody. HLA-DPA1 protein was absent or undetectable in the tumor cells in 15 of these cases (83%). However, three cases (ACT040, ACT047 and ACT048) (17%) showed focal HLA-DPA1 immunostaining within the adrenocortical tumor cells (Fig 5B and Supplementary Table 6).
DISCUSSION
Greater expression of MHC class II genes, HLA-DPA1 in particular, is associated with better prognosis for pediatric adrenocortical tumors independent of histopathological diagnosis. In most cases, MHC class II genes were expressed by tumor-infiltrating hematopoietic cells rather than adrenocortical tumor cells. These results suggest that immune surveillance, evident by elevated expression of MHC class II and the presence of tumor-associated macrophages and dendritic cells, may limit adrenocortical progression leading to less aggressive disease.
The histological features of ACTs often do not allow unequivocal classification as adenomas or carcinomas, and such cases are currently classified as tumors of indeterminate histology, undetermined malignant potential, or simply “adrenocortical neoplasms” (13,17–18). In our IPACTR series, seven of 52 tumors (13%) had undetermined histological findings. Three of these cases expressed high levels of MHC class II based on microarray (ACT005 and ACT006) and IHC (ACT047), which is characteristic of pediatric adrenocortical adenomas. All three patients experienced tumor spillage during surgery, but did not relapse and remained disease-free at the time of the last follow up (range 20–56 months). Three of the remaining four ACTs of undetermined histology (ACT034, ACT039 and ACT046) expressed high levels of HLA-DPA1 and are free of disease for more than 2 years. These clinical findings are consistent with the observed high MHC class II expression and less aggressive disease.
Prognostic information is also lacking for pediatric adrenocortical tumors classified as carcinomas, as their outcome varies substantially after complete resection (3–5; 10–13). Tumor size (weight or volume) is associated with prognosis, but small adrenocortical carcinomas too often behave aggressively (early local and distant relapse), while large carcinomas may have an indolent course. We suggest that the expression of MHC class II genes can be used to refine the risk classification of ACTs. Overexpression of MHC class II genes could more decisively identify patients with undetermined or carcinoma histology who are unlikely to require adjuvant chemotherapy after complete surgical resection. Like childhood ACTs with a Weiss score of 0 or 1 (10–13, 17) those with relatively large, completely excised carcinomas (stage II–III) with high expression of MHC class II genes could be spared intensive chemotherapy. Completely resected carcinomas with low MHC class II gene expression could be treated with adjuvant chemotherapy irrespective of their size.
Overexpression of IGF2 and BUB1B is associated with poor prognosis in adult ACT (19–20). In pediatric ACT, IGF2 is overexpressed in both adenomas and carcinomas (21) and therefore does not correlate with HLA-DPA1 levels or outcome (P > 0.10; Supplementary Table 5). BUBR1, the protein encoded by BUB1B, has a critical role in regulating the spindle-assembly checkpoint and implicated in chromosome instability and cancer progression (22). Similar to adult ACT and other adult cancers (23–24) overexpression of BUB1B inversely correlated with progression-free survival in pediatric ACT from both cohorts (P = 0.003) (Supplementary Table 5).
MHC class II molecules are surface glycoproteins on antigen-presenting cells (e.g., dendritic cells, monocyte/macrophages, and B-lymphocytes) that presumably present tumor peptides to CD4+ T cells (25). Previous studies of pediatric ACT have not definitively addressed the cell of origin expressing MHC class II, although it was suggested to originate from adrenocortical tumor cells and downregulated concurrently with tumor progression (6–8). However, MHC class II is not expressed in the fetal adrenal cortex (26–27), the putative origin of pediatric ACT (28), but rather, predominantly by tissue macrophages and a small population of CD45R+, IgM+ lymphoid cells (28). Our findings demonstrate that the cells expressing MHC class II genes in pediatric ACTs, primarily adenomas, are macrophages and dendritic cells. Dendritic cells are capable of initiating innate and adaptive tumor responses (29), suggesting a mechanism for suppressing pediatric ACT progression. In contrast, three ACTs (ACT40, ACT047 and ACT048) displayed focal tumor-cell expression of HLA-DPA1 in addition to the infiltrating hematopoietic cells. Two of these patients (ACT047, ACT048) were more than 7 years of age at diagnosis and presented with virilization and large tumors. Interestingly, these cases experienced tumor spillage during surgery, but did not relapse during more than 20 months of follow up. Whether tumor cells expressing MHC class II are capable of participating in regulatory immune mechanisms and have prognostic implications remains to be determined.
We have demonstrated that MHC class II gene expression in pediatric ACTs originates from tumor-infiltrating immune cells and is associated with more favorable prognosis. Our data supports the inclusion of MHC class II expression in addition to standard criteria (e.g., tumor size, mitotic activity, atypical mitoses and other histological features) as a tool for determining pediatric ACT diagnosis. Future studies will define the interaction of infiltrating hematopoietic cells with the pediatric adrenocortical tumor microenvironment, which may refine risk classification and facilitate the development of immunotherapies for these tumors.
Supplementary Material
Translational Relevance.
Current prognostic stratification of pediatric adrenocortical tumors (ACTs) is imprecise. Large tumors, incomplete surgical resection and regional and distant metastasis are associated with poor prognosis. However, a substantial number of cases without these features relapse and eventually die from progressive disease. Developing new molecular prognostic criteria would assist pathologists and oncologists to refine the histopathological classification and clinical management of pediatric ACT. Here we report that the expression of MHC class II genes is significantly associated with prognosis, hence a potential biomarker to assist in differentiating benign from malignant ACTs. We further identified tumor infiltrating dendritic cells and macrophages, but not the tumor cells, as the source of MHC class II antigen expression. Collectively, these findings provide an opportunity to improve the risk stratification and outcome for pediatric ACT.
Acknowledgments
We thank the study participants and the clinicians and research staff at participating institutions. We thank Jay Morris and Melanie Loyd for assistance with the expression arrays; Charlene Henry, Raven Childers and the St. Jude anatomic pathology staff for assistance with immunostaining studies. We thank Sharon Naron for editing the manuscript.
Grant Support This work was supported by a grant from Centocor, Inc., National Institutes of Health Cancer Center Support Grant CA21765, and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed
Authors' Contributions Conception and design: Emilia Modolo Pinto, Raul C. Ribeiro and Gerard P. Zambetti.
Provision of study materials or patients: Carlos Rodriguez-Galindo, Bonald Figueiredo, Raul C. Ribeiro.
Collection and assembly of data: Emilia Modolo Pinto, Stanley Pounds, John Kim Choi, Zhifa Liu, Geoff Neale, David Finkelstein, Raul C. Ribeiro, Gerard P. Zambetti
Data analysis and interpretation: Emilia Modolo Pinto, Stanley Pounds, John Kim Choi, John Hicks, Alberto Pappo, Zhifa Liu, Raul C. Ribeiro, Gerard P. Zambetti
Writing, critical review, and/or revision of the manuscript: all authors.
REFERENCES
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD: 2010. http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 2.Michalkiewicz E, Sandrini R, Figueiredo B, Miranda EC, Caran E, Oliveira-Filho AG, et al. Clinical and outcome characteristics of children with adrenocortical tumors: A report from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004;22:838–845. doi: 10.1200/JCO.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro RC, Michalikiewicz E, Figueiredo BC, DeLacerda L, Sandrini F, Pianovsky MD, et al. Adrenocortical tumors in children. Brazil J Med Biol Res. 2000;33:1225–1234. doi: 10.1590/s0100-879x2000001000013. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro RC, Pinto EM, Zambetti GP, Rodriguez-Galindo C. The International Pediatric Adrenocortical Tumor Registry initiative: Contributions to clinical, biological, and treatment advances in pediatric adrenocortical tumors. Mol Cell Endocrinol. 2012;351:37–43. doi: 10.1016/j.mce.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 5.McAteer JP, Huaco JA, Gow KW. Predictors of survival in pediatric adrenocortical carcinoma: A Surveillance, Epidemiology, and End Results (SEER) program study. J Pediatr Surg. 2013;48:1025–1031. doi: 10.1016/j.jpedsurg.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 6.West AN, Neale GA, Pounds S, Figueiredo B, Rodriguez-Galindo C, Pianoviski MA, et al. Gene expression profiling of childhood adrenocortical tumors. Cancer Res. 2007;67:600–608. doi: 10.1158/0008-5472.CAN-06-3767. [DOI] [PubMed] [Google Scholar]
- 7.Leite FA, Lira RCP, Fedatto PF, Antonini SR, Martinelli CE, Jr, de Castro M, et al. Low expression of HLA-DRA, HLA-DPA1, and HLA-DPB1 is associated with poor prognosis in pediatric adrenocortical tumors (ACT) Pediatr Blood Cancer. 2014;6:1940–1948. doi: 10.1002/pbc.25118. [DOI] [PubMed] [Google Scholar]
- 8.Magro G, Esposito G, Cecchetto G, Dall'lgna P, Marcato R, Gambini C, et al. Pediatric adrenocortical tumors: Morphological diagnostic criteria and immunohistochemical expression of matrix metalloproteinase type 2 and human leucocyte-associated antigen (HLA) class II antigens. Results from the Italian Pediatric Rare Tumor (TREP) Study project. Hum Pathol. 2012;43:31–39. doi: 10.1016/j.humpath.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Khoury EL, Greenspan JS, Greenspan FS. Adrenocortical cells of the zona reticularis normally express HLA-DR antigenic determinants. Am J Pathol. 1987;127:580–591. [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss LM, Medeiros LJ, Vickery AL., Jr Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. 1989;13:202–206. doi: 10.1097/00000478-198903000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8:163–169. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Bugg MF, Ribeiro RC, Roberson PK, Lloyd RV, Sandrini R, Silva JB, et al. Correlation of pathologic features with clinical outcome in pediatric adrenocortical neoplasia. A study of a Brazilian population. Brazilian Group for Treatment of Childhood Adrenocortical Tumors. Am J Clin Pathol. 1994;101:625–629. doi: 10.1093/ajcp/101.5.625. [DOI] [PubMed] [Google Scholar]
- 13.Wieneke J, Thompson L, Heffess C. Adrenal cortical neoplasms in the pediatric population: a clinicopathologic and immunophenotypic analysis of 83 patients. Am J Surg Pathol. 2003;27:867–881. doi: 10.1097/00000478-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 15.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Pawlikowska I, Wu G, Edmonson M, Liu Z, Gruber T, Zhang J, et al. The most informative spacing test effectively discovers biologically relevant outliers or multiple modes in expression. Bioinformatics. 2014;30:1400–1408. doi: 10.1093/bioinformatics/btu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee G, DasGupta S, Mukherjee G, Sengupta M, Roy P, Arun I, et al. Usefulness of Wieneke criteria in assessing morphologic characteristics of adrenocortical tumors in children. Pediatr Surg Int. 2015;31:563–571. doi: 10.1007/s00383-015-3708-x. [DOI] [PubMed] [Google Scholar]
- 18.Sakoda A, Mushtaq I, Levitt G, Sebire NJ. Clinical and histopathological features of adrenocortical neoplasms in children: retrospective review from a single specialist center. J Pediatr Surg. 2014;49:410–415. doi: 10.1016/j.jpedsurg.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Drelon C, Berthon A, Val P. Adrenocortical cancer and IGF2: is the game over or our experimental models limited? J Clin Endocrinol Metab. 2013;98:505–507. doi: 10.1210/jc.2012-3310. [DOI] [PubMed] [Google Scholar]
- 20.de Reynies A, Assie G, Rickman DS, Tissier F, Groussin L, Rene-Corail F, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27:1108–1115. doi: 10.1200/JCO.2008.18.5678. [DOI] [PubMed] [Google Scholar]
- 21.Pinto EM, Chen X, Easton J, Finkelstein D, Liu Z, Pounds S, et al. Genomic landscape of paediatric adrenocortical tumours. Nat Commun. 2015;6:6302. doi: 10.1038/ncomms7302. doi: 10.1038/ncomms7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker DJ, Jin F, Jeganathan KB, van Deursen JM. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell. 2009;16:475–486. doi: 10.1016/j.ccr.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Ando K, Oki E, Ikawa-Yoshida A, Ida S, Kimura Y, et al. Aberrations of BUBR1 and TP53 gene mutually associated with chromosomal instability in human colorectal cancer. Anticancer Res. 2014;34:5421–5428. [PubMed] [Google Scholar]
- 24.Chen H, Lee J, Kljavin NM, Haley B, Daemen A, Johnson L, et al. Requirement for BUB1B/BUBR1 in tumor progression of lung adenocarcinoma. Genes & Cancer. 2015;6:106–118. doi: 10.18632/genesandcancer.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holling TM, Schooten E, van Den Elsen PJ. Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Hum Immunol. 2004;65:282–290. doi: 10.1016/j.humimm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Marx C, Wolkersdörfer GW, Brown JW, Scherbaum WA, Bornstein SR. MHC class II expression-a new tool to assess dignity in adrenocortical tumours. J Clin Endocrinol Metab. 1996;81:4488–4491. doi: 10.1210/jcem.81.12.8954065. [DOI] [PubMed] [Google Scholar]
- 27.Oliver AM, Thomson AW, Sewell HF, Abramovich DR. Major histocompatibility complex (MHC) class II antigen (HLA-DR, DQ, and DP) expression in human fetal endocrine organs and gut. Scand J Immunol. 1988;27:731–737. doi: 10.1111/j.1365-3083.1988.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 28.Coulter CL. Fetal adrenal development: Insight gained from adrenal tumors. Trends Endocrinol Metab. 2005;16:235–242. doi: 10.1016/j.tem.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Tel J, Anguille S, Waterborg CE, Smits EL, Figdor CG, de Vries IJ. Tumoricidal activity of human dendritic cells. Trends in Imm. 2014;35:38–46. doi: 10.1016/j.it.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.