Abstract
Use of the immunosuppressant mycophenolic acid (MPA) in cats is limited because MPA elimination depends on glucuronidation, which is deficient in cats. We evaluated formation of major (phenol glucuronide) and minor (acyl glucuronide, phenol glucoside, and acyl glucoside) MPA metabolites using liver microsomes from 16 cats, 26 dogs, and 48 humans. All MPA metabolites were formed by human liver microsomes, while dog and cat liver microsomes formed both MPA glucuronides, but only one MPA glucoside (phenol glucoside). Intrinsic clearance (CLint) of MPA for phenol glucuronidation by cat liver microsomes was only 15–17% that of dog and human liver microsomes. However CLint for acyl glucuronide and phenol glucoside formation in cat liver microsomes was similar to or greater than that for dog and human liver microsomes. While total MPA conjugation CLint was generally similar for cat liver microsomes compared with dog and human liver microsomes, relative contributions of each pathway varied between species with phenol glucuronidation predominating in dog and human liver microsomes and phenol glucosidation predominating in cat liver microsomes. MPA conjugation variation between cat liver microsomes was 3-fold for total conjugation and for phenol glucosidation, 6-fold for phenol glucuronidation, and 11-fold for acyl glucuronidation. Our results indicate that total MPA conjugation is quantitatively similar between liver microsomes from cats, dogs, and humans despite large differences in the conjugation pathways that are utilized by these species.
Keywords: Mycophenolate mofetil, mycophenolic acid, glucuronidation, glucosidation, cat, liver
INTRODUCTION
Mycophenolic acid (MPA) is an effective and widely used immunosuppressant in human medicine. It is a fermentation product of a Penicillium species that was discovered over a century ago, but wasn’t used for clinical purposes until the mid-1990s (Bulliham et al., 1998; Miles et al., 2005; Lange et al., 2008). Available pharmaceutical preparations include either mycophenolate mofetil (an ester prodrug) or mycophenolate sodium (salt). Clinical uses in dogs include prevention of organ transplant rejection and treatment of various autoimmune disorders (Whitley & Day, 2011). Mycophenolate mofetil has gained popularity in treating canine patients, as it is widely available in various oral and intravenous formulations, making it desirable for use in veterinary patients with immune mediated diseases (Wang et al., 2013; West & Hart, 2014). MPA has been studied extensively in humans, and to a lesser extent in dogs. However, apart from one case report of the clinical use of mycophenolate mofetil in two cats (Bacek & Macintire, 2011), and one in vitro study showing effects of MPA on feline lymphocytes (Kyles, et al., 2000) there are no studies establishing MPA safety or efficacy in cats.
MPA is a non-competitive reversible inhibitor of the enzyme, inosine monophosphate dehydrogenase (Whitley & Day, 2011; Abd Rahman et al., 2013). Inosine monophosphate dehydrogenase is a rate limiting enzyme in the de novo purine synthesis pathway, which is essential for T and B cell proliferation and differentiation of T-cytotoxic cells (Whitley & Day, 2011). The immunosuppressant effects of MPA are terminated by conjugative metabolism in liver and some other tissues, followed by excretion of these metabolites into urine and bile (Bullingham et al., 1998; Abd Rahman et al., 2013). In humans, MPA is primarily conjugated with uridine diphosphate (UDP)-glucuronic acid to form the stable pharmacologically inactive phenolic glucuronide and much smaller amounts of the acyl glucuronide (Miles et al., 2005; Picard et. al., 2005). In humans, minor MPA metabolic pathways include UDP-glucose conjugation to form phenolic and acyl glucosides (Shipkova et al., 2001; Picard et al., 2004). UDP-glucose acts as a co-factor by transferring D-glucose to the substrate, forming β-D-glucosides (Testa & Kramer, 2008). Glucosidation is an understudied metabolic pathway for drugs that occurs in species other than humans, and was notably described in the cat by Carro-Ciampi et al., 1985, when evaluating barbiturate metabolism.
Glucuronidation occurs primarily in the liver, and is catalyzed by a subset of the UDP-glycosyltransferase (UGT) enzymes that are all contained within the UGT1A, UGT2A, and UGT2B subfamilies (Court et al., 2012). Nineteen UGT1A, UGT2A, and UGT2B subfamily enzymes have been identified in humans, which differ in substrate selectivity and tissue expression levels (Court et al., 2012). In human liver, MPA phenol glucuronide is formed primarily by UGT1A9, while the acyl glucuronide is formed mainly by UGT2B7 (Shipkova et al., 2001; Girard et al., 2004). The enzymes mediating formation of the MPA glucosides remain to be determined.
Substantial differences in the presence and expression of UGT enzymes occur between species (Court et al., 2012; Court, 2013). Compared with humans and dogs, cats lack several UGTs normally expressed in liver including UGT1A6 and UGT1A9 which explains in large part their sensitivity to the adverse effects of aromatic medications such as acetaminophen since these enzymes are required for detoxification and elimination by glucuronidation (Court, 2013). Since UGT1A9 plays a major role in MPA glucuronidation in humans, it is possible that cats may lack the capacity for efficient MPA glucuronidation, thereby decreasing drug clearance and increasing the risk for adverse effects.
The aim of this study was to determine between and within species differences in MPA conjugation by glucuronidation and glucosidation using liver microsomes from humans, dogs, and cats. Our primary hypothesis was that cats would glucuronidate MPA more slowly than humans and dogs. We evaluated the formation of the known major (phenol glucuronide) and minor (acyl glucuronide, phenol glucoside) MPA conjugates.
MATERIALS & METHODS
Chemicals and Reagents
MPA, MPA phenol glucuronide, MPA phenol glucuronide-D3, MPA acyl glucuronide, and MPA phenol glucoside were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). The cofactors uridine 5’-diphosphoglucose disodium salt (UDP-glucose) and uridine 5’-diphosphoglucuronic acid disodium salt (UDP-glucuronic acid) and alamethicin a peptide that forms pores in lipid bilayer membranes to enhance co-factor availability, were purchased from Sigma-Aldrich (St. Louis, MO). Potassium phosphate monobasic and magnesium chloride were purchased from JT Baker (Austin, TX). Acetonitrile, acetic acid, formic acid and ammonia solution were purchased from Fisher Scientific (Pittsburgh, PA).
Human, dog, and cat pooled liver microsomes
Liver microsomes were prepared from frozen human, cat and dog livers by differential centrifugation as described by Court et al. 1997. The pooled liver microsomes were obtained from the Washington State University pharmacogenomics tissue bank with Institutional Animal Care and Use Committee and Institutional Review Board approval. The human samples (n = 48) were obtained from liver transplants that had failed or from surgical liver resections from the University of Chicago, the National Disease and Research Interchange, the International Institute for the Advancement of Medicine, and the University of Pittsburg (Court, 2010). There were 26 dog liver samples from Beagles, Greyhounds, and mixed breed dogs (19 males and 7 females). Sixteen cat liver samples were used from domestic short hair cats, (11 males and 5 females). The liver microsomes were obtained from these untreated purpose bred research animals or random sourced healthy animals that were euthanized for reasons unrelated to this study. All liver microsomes preparations were stored at −80°C until use.
Liver microsomes incubations
Human, dog, and cat liver microsomes 0.1mg/ml final volume with 50 mM pH 7.4 phosphate buffer) were incubated in 1.5 mL polypropylene tubes in the presence of MPA (10 to 1000 µM), alamethicin (50 µg / mL), magnesium chloride (5 mM) and either 5 mM UDP-glucuronic acid or 5 mM UDP-glucose (or both for individual cat liver microsomes) for 15 minutes at 37°C in a water bath. The reaction was stopped by removing the tubes from the water bath at 15 minutes and adding 200 µL of acetonitrile containing 5% acetic acid, vortexing and placing on ice. After adding 100 µL of a 20 µM solution of the internal standard (MPA phenol glucuronide-D3) dissolved in methanol, the mixture was vortexed and centrifuged for 10 minutes at 15,000 g. The supernatant was transferred to 2 mL polypropylene tubes and dried for approximately two hours in a centrifugal vacuum. Once dried, 200 µL of 2 mM ammonium formate (pH 4.5) in water with 25% acetonitrile was added. The samples were centrifuged for 10 minutes at 15,000 g and the supernatant was transferred to a 96 well polypropylene plate for analysis by HPLC-MS.
HPLC-MS quantitation of MPA glucuronide and glucoside
The HPLC apparatus consisted of an Agilent Model 1100 with Leap CTC-PAL autosampler and 2 × 150 mm Phenomenex Synergi 4 µ Fusion-RP 80A column. The MS detector was an AB-Sciex API4000 triple quadruple instrument and negative ion mass transitions monitored included 495.5→319 (MPA phenol glucuronide and MPA acyl glucuronide), 498.5→322 (MPA phenol glucuronide-D3), and 481.5→319 (MPA phenol glucoside). MPA was also detected with a negative ion mass of 319. The HPLC mobile phase consisted of a variable mixture of 2 mM ammonium formate buffer in water (mobile A) with acetonitrile (mobile B) run at a combined flow rate of 350 µL / minute. The initial mobile A:B mix (v/v) was 85:15 at the run start, which linearly changed with time to 10:90 at 5 minutes and returned to 85:15 at 6 minutes, and remained at that ratio until the end of the run at 10 minutes. Retention times after the start of the run were 4.7 minutes for MPA phenol glucuronide and MPA phenol glucuronide-D3, 5.1 minutes for MPA acyl glucuronide and MPA phenol glucoside, 5.5 minutes for MPA acyl glucoside, and 6.5 minutes for MPA. The lower limits of quantitation were 500 nM, 300 nM, and 300 nM concentration in the incubation for MPA phenol glucuronide, MPA acyl glucuronide, and MPA glucoside respectively. The limits of detection (defined as 10 times the baseline signal) were 50 nM, 75 nM, and 30 nM concentration in the incubation for MPA phenol glucuronide, MPA acyl glucuronide and MPA phenol glucoside.
Preliminary studies found that human liver microsomes produced small amounts of the MPA acyl glucoside, while cat and dog liver microsomes did not produce any detectable MPA acyl glucoside despite producing large amounts of the phenol glucoside. Consequently the MPA acyl glucoside was not evaluated further. The amount of MPA phenol glucuronide, MPA acyl glucuronide, and MPA phenol glucoside formed in the incubation were calculated from standard curves generated using samples containing known amounts of pure standards and internal standard.
Enzyme activities, kinetics, and statistical analysis
Enzyme activities were calculated by dividing the amount of metabolite formed in each incubation tube by the amount of microsomal protein and the incubation time. The amount of metabolite formed was confirmed to be linear with respect to incubation time up to 30 minutes and protein concentration up to 0.4 mg / mL at 100 µM MPA concentration for pooled human, dog, and cat liver microsomes. Enzyme kinetic parameters including the Michaelis-Menten constant (Km) and the maximal enzyme velocity (Vmax) were derived from triplicate determinations by non-linear regression analysis (Sigmaplot version 12.0, Systat Software, San Jose, CA). Enzyme intrinsic clearance (CLint) was calculated by dividing the Vmax by the Km values for each activity and each species assayed. Differences in enzyme kinetic values between species for each activity were evaluated by analysis of variance (ANOVA) with multiple comparisons testing versus control (cat liver microsomes) using Dunnett’s method (Sigmaplot version 12.0). A p value less than 0.05 was considered statistically significant. Unless otherwise indicated the data are expressed as means ± one SD.
RESULTS
Comparative enzyme kinetics of MPA glucuronidation and glucosidation by liver microsomes
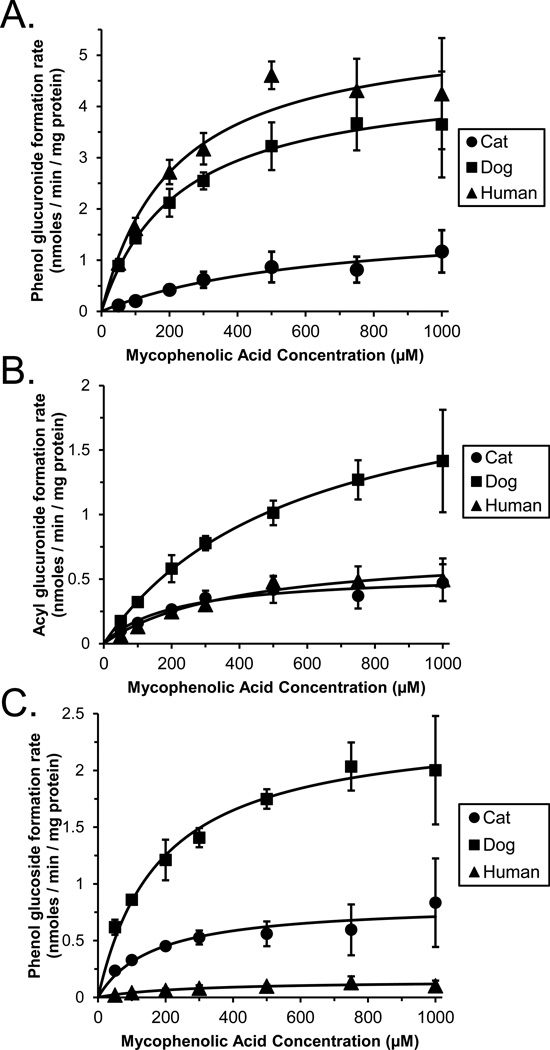
Figure 1 shows plots of MPA metabolite formation rates measured using substrate concentrations from 10 to 1000 µM in pooled cat (n = 16), dog (n = 26) and human (n = 48) liver microsomes as well as curves fitted to these data using the Michaelis-Menten model. All data were well described by the simple one enzyme Michaelis-Menten equation. Derived enzyme kinetic parameters (Vmax and Km) are given in Table 1 for pooled liver microsomes from each species studied. The calculated CLint values are expressed as both the absolute clearance (mL/kg/min) for each pathway, and also as the relative clearance (percentage of summed clearance by conjugation) to enable comparisons of each metabolic pathway within and between species.
Figure 1.
Enzyme kinetic plots of mycophenolic acid (MPA) substrate concentration versus formation rates of MPA phenol glucuronide (panel A), acyl glucuronide (panel B), and phenol glucoside (panel C) measured using pooled liver microsomes from 16 cats (filled circles), 26 dogs (filled squares) and 48 human donors (filled triangles). Data points are the mean (± standard error) values of 3 independent experiments. Also shown are the fitted curves derived by non-linear regression using the Michaelis-Menten model.
Table 1.
Enzyme kinetic parameter estimates (mean ± standard deviation for 3 independent experiments) determined for formation of mycophenolic acid phenol glucuronide, acyl glucuronide, and phenol glucoside by pooled liver microsomes from cats (n = 16), dogs (n = 26), and humans (n = 48). Also shown are the results of statistical comparisons between CLint values for cats compared with values for dogs and humans by ANOVA.
| Species | Metabolite formed | Vmax (nmoles/min/mg) |
Km (µM) |
CLint* (mL/kg/min) |
CLint** (% total) |
|---|---|---|---|---|---|
| Cats | Phenol glucuronide | 1.67 ± 1.31 | 510 ± 320 | 3.41 ± 1.171 | 23 ± 3 |
| Acyl glucuronide | 0.67 ± 0.17 | 327 ± 180 | 2.48 ± 1.242 | 17 ± 7 | |
| Phenol glucoside | 0.64 ± 0.29 | 84 ± 59 | 8.6 ± 2.43 | 60 ± 8 | |
| Total conjugation: | 15 ± 44 | ||||
| Dogs | Phenol glucuronide | 5.21 ± 2.33 | 280 ± 171 | 19.8 ± 4.0 | 56 ± 4 |
| Acyl glucuronide | 2.9 ± 1.0 | 854 ± 355 | 3.45 ± 0.65 | 10 ± 1 | |
| Phenol glucoside | 2.7 ± 0.55 | 228 ± 50 | 12 ± 0.2 | 34 ± 5 | |
| Total conjugation: | 35 ± 4 | ||||
| Humans | Phenol glucuronide | 6.86 ± 1.72 | 308 ± 130 | 23.4 ± 4.5 | 91 ± 2 |
| Acyl glucuronide | 0.99 ± 0.44 | 597 ± 294 | 1.68 ± 0.09 | 7 ± 1 | |
| Phenol glucoside | 0.18 ± 0.10 | 364 ± 72 | 0.49 ± 0.19 | 2 ± 1 | |
| Total conjugation: | 26 ± 4 | ||||
CLint = intrinsic clearance calculated by dividing the Vmax by the Km estimates.
CLint for each activity expressed as a percentage of the total summed CLint values for all activities within each species.
Phenol glucuronidation CLint of MPA in cats was only 17% that of dogs (p = 0.002, Dunnett’s test) and only 15% that of humans (p < 0.001, Dunnett’s test).
Acyl glucuronidation CLint of MPA in cats was not different from other species (p > 0.05, ANOVA).
Phenol glucosidation CLint of MPA in cats was over 20 times that of humans (p<0.001, Dunnett’s test) but similar to that of dogs (p > 0.05, Dunnett’s test).
Total conjugation for cats was 43% that of dogs (p = 0.002, Dunnett’s test) and 58% that of humans (p = 0.033, Dunnett’s test).
In agreement with our initial hypothesis (Table 1), phenol glucuronidation of MPA was substantially lower in cat livers (p = 0.001, ANOVA) with CLint values that averaged only 17% of dog livers (p = 0.002, Dunnett’s test) and 15% of human livers (p < 0.001, Dunnett’s test). However, acyl glucuronidation CLint of MPA in cat livers was not different from dog and human livers, (p > 0.05, ANOVA). Interestingly, phenol glucosidation CLint of MPA in cat livers was similar to that of dog livers (p > 0.05, Dunnett’s test) and more than 20 times that of human livers (p < 0.001, Dunnett’s test).
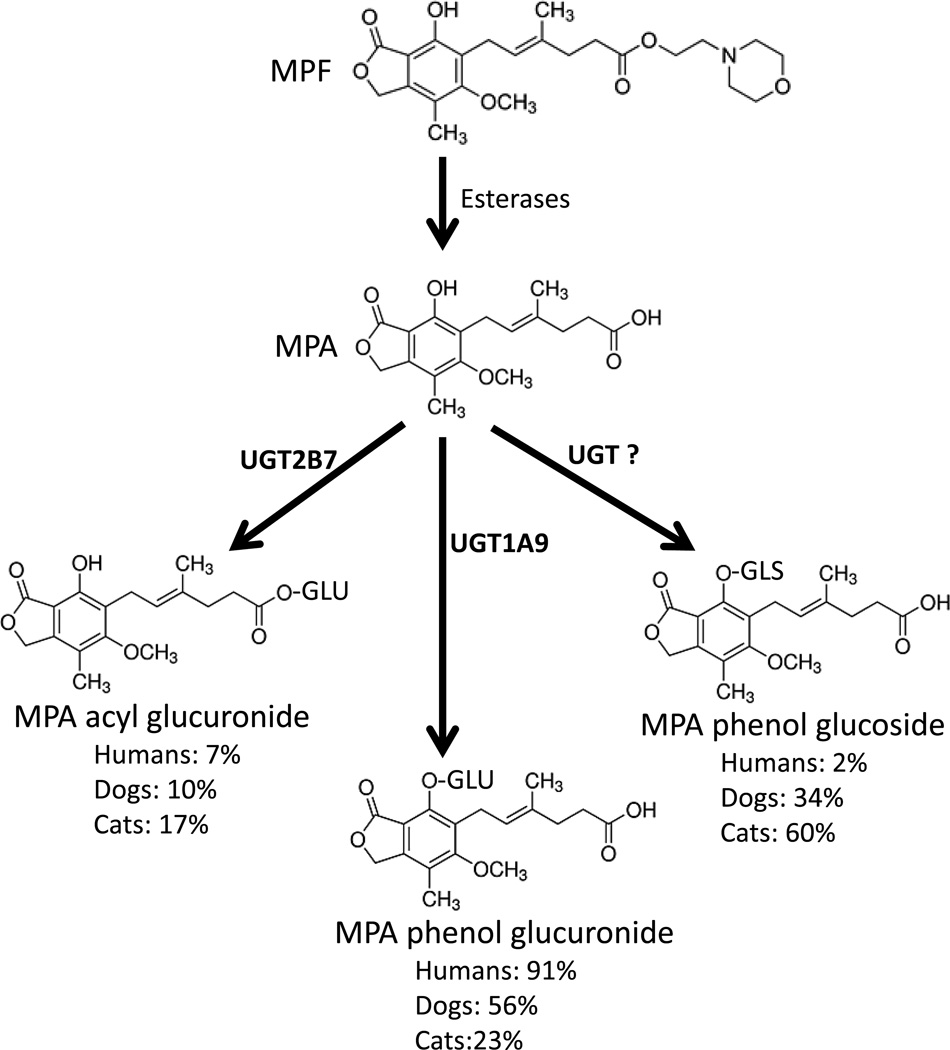
When MPA CLint values for all pathways evaluated were summed for each species (Table 1), the total predicted CLint for cat liver microsomes (15 mL/kg/min) was 43% that of dog (35 mL/kg/min) (p = 0.002, Dunnett’s test) and 58% of human (26 mL/kg/min) liver microsomes (p = 0.033, Dunnett’s test). The relative contribution of each pathway to total measured conjugation varied substantially between species in that phenol glucuronidation predominated in dog (56%) and human (91%) liver microsomes, while phenol glucosidation was the predominant pathway in cat (60%) liver microsomes (Figure 2).
Figure 2.
The structures and known metabolic pathways responsible for activation of mycophenolate mofetil (MPF) to mycophenolic acid (MPA) by esterases, and conjugative metabolism of MPA to the phenol glucuronide by UGT1A9, acyl glucuronide by UGT2B7, and phenol glucoside by an undetermined UGT. Also shown are the relative amounts of each conjugated metabolite formed by pooled human, dog, and cat liver microsomes in this study.
Variation in MPA conjugation between individual cat liver microsomes
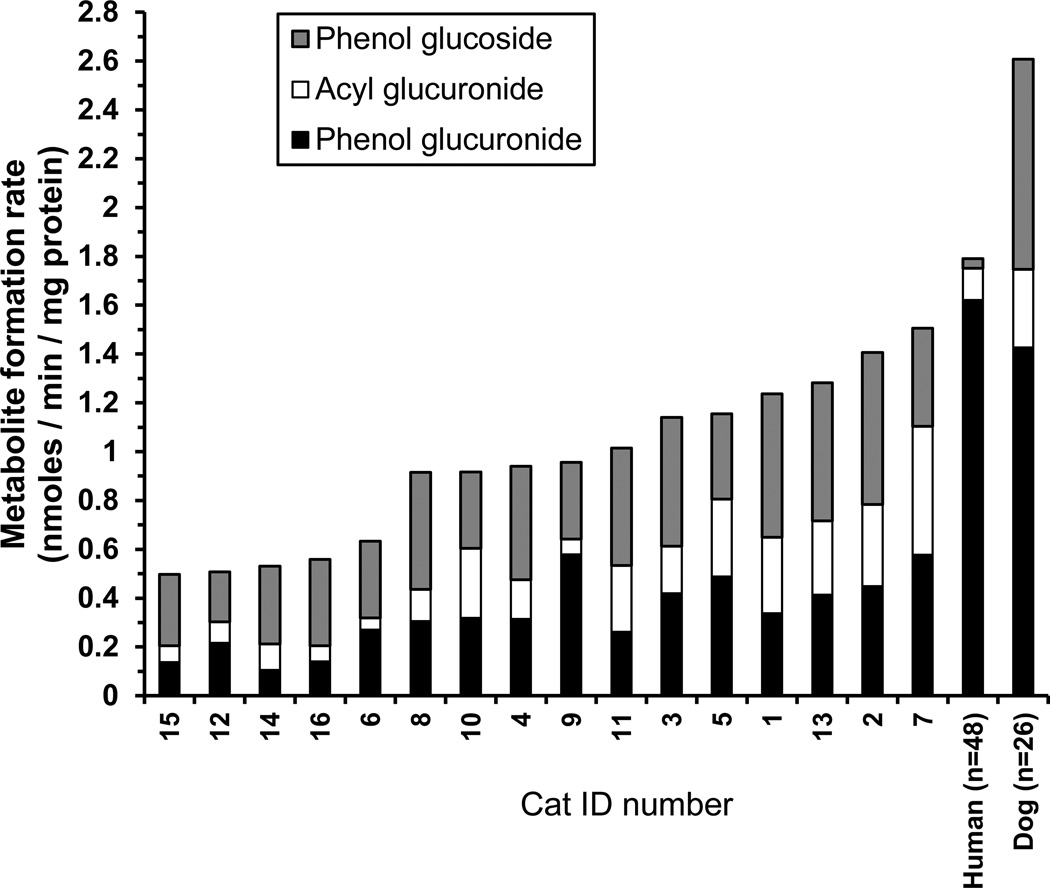
Metabolite formation rates were then determined for the 16 individual cat liver microsome fractions that constituted the pooled microsomes at a substrate concentration of 100 µM to evaluate individual variability (Figure 3). Activities determined for pooled dog and human liver microsomes using the same MPA concentration are included for comparison. Mean ± SD (coefficient of variation as a percent; CV%) activities for formation of MPA phenol glucuronide, MPA acyl glucuronide, and MPA phenol glucoside were 0.33 ± 0.15 (44%), 0.20 ± 0.14 (67%), and 0.41 ± 0.12 (30%) nmoles/ min / mg protein, respectively. Maximum over minimum fold variation in activities were highest for acyl glucuronidation (11-fold), intermediate for phenol glucuronidation (5.5-fold) and lowest for phenol glucosidation (3-fold). However, when the three conjugation pathways were summed the fold variation was modest (3-fold), ranging from 0.5 to 1.5 nmoles / min / mg protein with a mean ± SD (CV%) of 0.95 ± 0.33 (35%) nmoles / min / mg protein.
Figure 3.
Stacked bar chart showing the formation rates of mycophenolic acid (MPA) phenol glucuronide (black bars), acyl glucuronide (white bars), and phenol glucoside (gray bars) measured at 100 µM MPA concentration using microsomes prepared from 16 different domestic short hair cat livers in triplicate in 1 experiment. Individual sample results (identification (ID) numbers 1 to 16) were sorted from lowest to highest (left to right) total conjugation activity. Also shown for comparison are the same activities for pooled dog and human liver microsomes measured at the same MPA concentration. The mean values for the dog and human were derived from 3 experiments.
DISCUSSION
MPA is a potentially useful immunosuppressant drug in cats (Kyles, et al., 2000; Bacek & Macintire, 2011). However, given the major role for glucuronidation in MPA metabolism in humans, there are concerns that the known deficiencies of UGT1A9 (and other UGTs) in cats could also cause deficient MPA glucuronidation, potentially delaying MPA clearance and leading to MPA accumulation and toxicity. Without a good understanding of MPA clearance in cats, it is not possible to determine the dose required to achieve an effective and safe blood concentration in the feline species. In support of our primary hypothesis, we did find significantly (over 5 times) slower formation of the MPA phenol glucuronide in cats versus dogs and humans. However, we also found a much smaller difference (about 2 times) in overall metabolism of MPA by conjugation (glucuronidation plus glucosidation) when comparing cat liver microsomes with dog and human liver microsomes despite significantly reduced MPA phenol glucuronide formation in cats. This occurred in large part because of the substantially higher relative contribution of glucosidation to total MPA conjugation in cat liver microsomes (60%) when compared with dog (34%) and especially human (2%) liver microsomes.
Although glucosidation is the major pathway for conjugative metabolism of xenobiotics in non-vertebrate species (including insects and plants), glucuronidation is the dominant conjugative clearance mechanism in all vertebrates studied to date (Shipkova et al., 2001; Huang, et al., 2008; Wang et al, 2014; Bock, 2015). This is despite the ready availability of UDP-glucose as a cofactor in hepatocytes. Although there are relatively few examples of drugs that are glucosidated to any significant extent in mammals, some that are include: morphine, phenobarbital, amobarbital, ibuprofen and varenicline (Kalow et al., 1979; Arima & Kato, 1990; Bernus et al., 1994; Nandi & Soine, 1997; Chau et al., 2013). In most instances, the same drugs are also metabolized by glucuronidation with higher efficiency, although a few exceptions have been identified, including acyl glucosidation of panoprofen in mice (Arima & Kato, 1990), and N-glucosidation of amobarbital and phenobarbital in humans (Kalow et al., 1979; Bernus et al., 1994; Nandi & Soine, 1997). In future studies, one should consider the potential for glucosidation (versus glucuronidation) as a major alternate clearance pathway for drugs used in cats.
The specific enzyme mediating glucosidation of MPA (either phenol or acyl) has not yet been determined. Results so far implicate UGT2B7 as the main enzyme that glucosidates morphine (Chau et al., 2014), ibuprofen (Buchheit et al., 2011), an experimental endothelin antagonist (Tang, et al., 2003), and hyodeoxycholic acid (Mackenzie et al., 2003), while UGT2B15 was found to glucosidate an experimental aldose reductase inhibitor (Toide et al., 2004). Our results indicate that cats may express a UGT that has a higher catalytic efficiency for glucosidation of MPA than those expressed by humans and dogs. Apart from the UGT1 and UGT2 family of enzymes that most efficiently use UDP-glucuronic acid as the co-substrate, several UGTs have been identified in humans that primarily use other UDP-sugars as the co-substrate including UGT3A1, UGT3A2 and UGT8. Of these, UGT3A2 appears to have the highest efficiency in using UDP-glucose for conjugating xenobiotics (Mackenzie et al., 2011). However, although human UGT3A2 is expressed in the kidney, it is poorly expressed in the liver and intestines.
We also quantified differences in MPA metabolism between different cat livers. Although the variability in total conjugation metabolism was quite modest (only about 3-fold), the variation in MPA acyl glucuronide was quite substantial (11-fold). Acyl glucuronide metabolites tend to be unstable and capable of covalently binding to proteins (Picard et al., 2004). MPA acyl glucuronide, unlike MPA phenol glucuronide, acts as an uncompetitive IMPDH inhibitor but has lower enzyme inhibitory potential than MPA (Gensburger et al., 2009). Acyl glucuronide metabolites can be secreted into the bile and then re-deposited into the intestines versus eliminated, thereby increasing exposure of this active metabolite to the intestine, possibly increasing the chance of gastro-intestinal side effects (Picard et al., 2004). Furthermore differences in MPA acyl glucuronide have been associated with the occurrence of MPA associated diarrhea in people (Picard et al., 2004). Consequently, if diarrhea is also found to occur as a side effect in some cats, this could be explained by variability in acyl glucuronide formation.
There are several limitations to our studies that should be noted. Our study involved liver microsomes since the liver is a primary organ involved in drug glucuronidation and MPA metabolism. However, significant MPA conjugation has also been reported for human kidney and intestinal mucosa microsomes with specific activities that are similar to liver activities (Bowalgaha & Miners, 2001; Shipkova et al., 2001). Consequently, it has been suggested that conjugation in intestines and kidney may be important for pre-systemic extraction or urinary clearance of MPA, respectively. However, extrapolation of data obtained from experiments using kidney and intestinal microsomes to the whole organ indicates that MPA clearance is 20 to 40 times greater in the liver compared with kidney and intestines suggesting that the effect on extraction or clearance may be limited, at least in people (Bowalgaha & Miners, 2001). Finally, although our data suggests that MPA should be cleared by conjugation in the liver in cats at a rate that is only about half of the rate in dogs and humans, the predictive value of this model is limited. Thus, pharmacokinetic studies will be needed to ascertain whether there are species differences in MPA disposition and clearance.
Acknowledgments
Dr Court was supported by the US National Institutes of Health grant (GM102130) and the William R. Jones endowment to Washington State University College of Veterinary Medicine.
REFERENCES
- Abd Rahman AN, Tett SE, Staatz CE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in patients with autoimmune disease. Clinical Pharmacokinetics. 2013;52(5):303–331. doi: 10.1007/s40262-013-0039-8. [DOI] [PubMed] [Google Scholar]
- Arima N, Kato Y. Dose-dependent shift in acyl glucuronidation and glucosidation of pranoprofen, a 2-arylpropionic acid derivative, in mice in vivo. Journal of Pharmacobiodynamics. 1990;13(12):719–723. doi: 10.1248/bpb1978.13.719. [DOI] [PubMed] [Google Scholar]
- Bacek LM, Macintire DK. Treatment of primary immune-mediated hemolytic anemia with mycophenolate mofetil in two cats. Journal of Veterinary Emergency and Critical Care (San Antonio) 2011;21(1):45–49. doi: 10.1111/j.1476-4431.2010.00606.x. [DOI] [PubMed] [Google Scholar]
- Bernus I, Dickinson RG, Hooper WD, Eadie MJ. Inhibition of phenobarbitone N-glucosidation by valproate. British Journal of Clinical Pharmacology. 1994;38(5):411–416. doi: 10.1111/j.1365-2125.1994.tb04375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW. The UDP-glycosyltransferase (UGT) superfamily expressed in humans, insects and plants: Animal-plant arms-race and co-evolution. Biochemical Pharmacology. 2016;99:11–17. doi: 10.1016/j.bcp.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Bowalgaha K, Miners JO. The glucuronidation of mycophenolic acid by human liver, kidney and jejunum microsomes. British Journal of Clinical Pharmacology. 2001;52(5):605–609. doi: 10.1046/j.0306-5251.2001.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit D, Dragan CA, Schmitt EI, Bureik M. Production of ibuprofen acyl glucosides by human UGT2B7. Drug Metabolism and Disposition. 2011;39(12):2174–2181. doi: 10.1124/dmd.111.041640. [DOI] [PubMed] [Google Scholar]
- Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clinical Pharmacokinetics. 1998;34(6):429–455. doi: 10.2165/00003088-199834060-00002. [DOI] [PubMed] [Google Scholar]
- Carro-Ciampi G, Jurima M, Kadar D, Tang BK, Kalow W. N-Glucosidation of amobarbital in the cat. Canadian Journal of Physiology and Pharmacology. 1985;63(10):1263–1266. doi: 10.1139/y85-209. [DOI] [PubMed] [Google Scholar]
- Chau N, Elliot DJ, Lewis BC, Burns K, Johnston MR, Mackenzie PI, Miners JO. Morphine glucuronidation and glucosidation represent complementary metabolic pathways that are both catalyzed by UDP-glucuronosyltransferase 2B7: kinetic, inhibition, and molecular modeling studies. Journal of Pharmacology and Experimental Therapeutics. 2014;349(1):126–137. doi: 10.1124/jpet.113.212258. [DOI] [PubMed] [Google Scholar]
- Court MH. Interindividual variability in hepatic drug glucuronidation: studies into the role of age, sex, enzyme inducers, and genetic polymorphism using the human liver bank as a model system. Drug Metabolism Review. 2010;42(1):209–224. doi: 10.3109/03602530903209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court MH, Zhang X, Ding X, Yee KK, Hesse LM, Finel M. Quantitative distribution of mRNAs encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica. 2012;42(3):266–277. doi: 10.3109/00498254.2011.618954. [DOI] [PubMed] [Google Scholar]
- Court MH. Feline drug metabolism and disposition: pharmacokinetic evidence for species differences and molecular mechanisms. Veterinary Clinics of North American Small Animal Practice. 2013;43(5):1039–1054. doi: 10.1016/j.cvsm.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensburger O, Picard N, Marquet P. Effect of mycophenolate acyl-glucuronide on human recombinant type 2 inosine monophosphate dehydrogenase. Clinical Chemistry. 2009;55(5):986–993. doi: 10.1373/clinchem.2008.113936. [DOI] [PubMed] [Google Scholar]
- Girard H, Court MH, Bernard O, Fortier LC, Villeneuve L, Hao Q, Greenblatt DJ, von Moltke LL, Perussed L, Guillemette C. Identification of common polymorphisms in the promoter of the UGT1A9 gene: evidence that UGT1A9 protein and activity levels are strongly genetically controlled in the liver. Pharmacogenetics. 2004;14(8):501–515. doi: 10.1097/01.fpc.0000114754.08559.27. [DOI] [PubMed] [Google Scholar]
- Huang FF, Chai CL, Zhang Z, Liu ZH, Dai FY, Lu C, Xiang ZH. The UDP-glucosyltransferase multigene family in Bombyx mori. BMC Genomics. 2008;9:563. doi: 10.1186/1471-2164-9-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalow W, Tang BK, Kadar D, Endrenyi L, Chan FY. A method for studying drug metabolism in populations: racial differences in amobarbital metabolism. Clinical Pharmacology and Therapeutics. 1979;26(6):766–776. doi: 10.1002/cpt1979266766. [DOI] [PubMed] [Google Scholar]
- Kyles AE, Gregory CR, Craigmill AL. Comparison of the in vitro antiproliferative effects of five immunosuppressive drugs on lymphocytes in whole blood from cats. American Journal of Veterinary Research. 2000;61(8):906–909. doi: 10.2460/ajvr.2000.61.906. [DOI] [PubMed] [Google Scholar]
- Lange S, Mueller SC, Altmann S, Dahlhaus M, Drewelow B, Freund M, Junghanss C. Pharmacokinetics of oral mycophenolate mofetil in combination with CsA in dogs after nonmyeloablative allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(7):667–674. doi: 10.1038/sj.bmt.1705958. [DOI] [PubMed] [Google Scholar]
- Mackenzie P, Little JM, Radominska-Pandya A. Glucosidation of hyodeoxycholic acid by UDP-glucuronosyltransferase 2B7. Biochemical Pharmacology. 2003;65(3):417–421. doi: 10.1016/s0006-2952(02)01522-8. [DOI] [PubMed] [Google Scholar]
- MacKenzie PI, Rogers A, Elliot DJ, Chau N, Hulin JA, Miners JO, Meech R. The novel UDP glycosyltransferase 3A2: cloning, catalytic properties, and tissue distribution. Molecular Pharmacology. 2011;79(3):472–478. doi: 10.1124/mol.110.069336. [DOI] [PubMed] [Google Scholar]
- Miles KK, Stern ST, Smith PC, Kessler FK, Ali S, Ritter JK. An investigation of human and rat liver microsomal mycophenolic acid glucuronidation: evidence for a principal role of UGT1A enzymes and species differences in UGT1A specificity. Drug Metabolism and Disposition. 2005;33(10):1513–1520. doi: 10.1124/dmd.105.004663. [DOI] [PubMed] [Google Scholar]
- Nandi V, Soine WH. HPLC analysis for amobarbital N-glycosides in urine. Journal of Pharmaceutical Biomedical Analysus. 1997;15(8):1187–1195. doi: 10.1016/s0731-7085(96)01936-x. [DOI] [PubMed] [Google Scholar]
- Picard N, Ratanasavanh D, Premaud A, Le Meur Y, Marquet P. Identification of the UDP-glucuronosyltransferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metabolism Disposition. 2005;33(1):139–146. doi: 10.1124/dmd.104.001651. [DOI] [PubMed] [Google Scholar]
- Shipkova M, Strassburg CP, Braun F, Streit F, Grone HJ, Armstrong VW, Tukey RH, Oellerich M, Wieland E. Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes. British Journal of Pharmacology. 2001;132(5):1027–1034. doi: 10.1038/sj.bjp.0703898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Hochman JH, Ma B, Subramanian R, Vyas KP. Acyl glucuronidation and glucosidation of a new and selective endothelin ET(A) receptor antagonist in human liver microsomes. Drug Metabolism and Disposition. 2003;31(1):37–45. doi: 10.1124/dmd.31.1.37. [DOI] [PubMed] [Google Scholar]
- Testa B, Kramer SD. The biochemistry of drug metabolism--an introduction: part 4. reactions of conjugation and their enzymes. Chemistry and Biodiversity. 2008;5(11):2171–2336. doi: 10.1002/cbdv.200890199. [DOI] [PubMed] [Google Scholar]
- Toide K, Terauchi Y, Fujii T, Yamazaki H, Kamataki T. Uridine diphosphate sugar-selective conjugation of an aldose reductase inhibitor (AS-3201) by UDP-glucuronosyltransferase 2B subfamily in human liver microsomes. Biochemical Pharmacology. 2004;67(7):1269–1278. doi: 10.1016/j.bcp.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Wagner M, Earley AK, Webster AC, Schmid CH, Balk EM, Uhlig K. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Systematic Review. 2015;12:Cd007746. doi: 10.1002/14651858.CD007746.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Smith JR, Creevy KE. Treatment of canine idiopathic immune-mediated haemolytic anaemia with mycophenolate mofetil and glucocorticoids: 30 cases (2007 to 2011) Journal of Small Animal Practice. 2013;54(8):399–404. doi: 10.1111/jsap.12107. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang H, Wu Q. Characterization of the zebrafish Ugt repertoire reveals a new class of drug-metabolizing UDP glucuronosyltransferases. Molecular Pharmacology. 2014;86(1):62–75. doi: 10.1124/mol.113.091462. [DOI] [PubMed] [Google Scholar]
- West LD, Hart JR. Treatment of idiopathic immune-mediated hemolytic anemia with mycophenolate mofetil in five dogs. Journal of Veterinray Emergency and Critical Care (San Antonio) 2014;24(2):226–231. doi: 10.1111/vec.12121. [DOI] [PubMed] [Google Scholar]
- Whitley NT, Day MJ. Immunomodulatory drugs and their application to the management of canine immune-mediated disease. Journal of Small Animal Practice. 2011;52(2):70–85. doi: 10.1111/j.1748-5827.2011.01024.x. [DOI] [PubMed] [Google Scholar]