Abstract
Renal sympathetic denervation (RDNx) has emerged as a novel therapy for hypertension; however, the therapeutic mechanisms remain unclear. Efferent renal sympathetic nerve activity (RSNA) has recently been implicated in trafficking renal inflammatory immune cells and inflammatory chemokine and cytokine release. Several of these inflammatory mediators are known to activate or sensitize afferent nerves. This study aimed to elucidate the roles of efferent and afferent renal nerves in renal inflammation and hypertension in the deoxycorticosterone acetate (DOCA)-salt rat model. Uninephrectomized male Sprague Dawley rats (275–300g) underwent selective afferent-selective RDNx (A-RDNx; n=10), total RDNx (T-RDNx; n=10), or Sham (n=10) and were instrumented for measurement of mean arterial pressure (MAP) and heart rate (HR) by radiotelemetry. Rats received 100mg DOCA (s.c.) and 0.9% saline for 21 days. Resting afferent renal nerve activity (ARNA) in DOCA and Vehicle animals was measured after the treatment protocol. Renal tissue inflammation was assessed by renal cytokine content and T-cell infiltration and activation. Resting ARNA, expressed as a percent of peak afferent nerve activity (%Amax), was substantially increased in DOCA vs. Vehicle (35.8±4.4 vs. 15.3±2.8%Amax). The DOCA-Sham hypertension (132±12 mmHg) was attenuated by ~50% in both T-RDNx (111±8) and A-RDNx (117±5mmHg) groups. Renal inflammation induced by DOCA-salt was attenuated by T-RDNx, and unaffected by A-RDNx. These data suggest ARNA may mediate the hypertensive response to DOCA-salt, but inflammation may be mediated primarily by efferent RSNA. Also, resting ARNA is elevated in DOCA-salt rats, which may highlight a crucial neural mechanism in the development and maintenance of hypertension.
Keywords: Hypertension, renal nerves, renal denervation, afferent, inflammation
INTRODUCTION
Arterial hypertension continues to be a primary predictor of future cardiovascular disease morbidity and mortality in the United States1, and worldwide2. Unfortunately, the development of effective treatment and preventative modalities have not met the increasing demand. While the pathogenesis of hypertension is complex, the contribution of renal dysfunction and increased activity of the sympathetic nervous system has been extensively documented and studied3, 4.
The concept that increased renal sympathetic nerve activity (SNA) contributes to hypertension development and maintenance is supported by studies showing renal denervation (RDNx) attenuates and/or reverses experimental hypertension4. These findings led to the first clinical trials5, 6 to treat patients with drug-resistant hypertension with RDNx, which reported an effective and sustained decrease in arterial pressure following RDNx. However, recent clinical trials7, 8 have failed to support this antihypertensive effect, which has led to an intense debate over the effectiveness of RDNx as well as the need for further preclinical study.
The antihypertensive effect of RDNx was initially hypothesized to result from ablation of renal efferent nerves, which contribute to tubular sodium reabsorption, renin release, and renal vascular resistance9. However, several studies conducted in the 1980’s suggested an important role for renal afferent nerves in the pathogenesis of hypertension, since interruption of visceral afferent input to the spinal cord by dorsal rhizotomy attenuated several preclinical models of hypertension10–13. These studies suggested increased afferent renal nerve activity (ARNA) contributes to an increase in SNA to renal and non-renal vascular beds, and resulting in an increase in arterial pressure. Interestingly, RDNx in patients with drug resistant hypertension reportedly reduces muscle SNA14, plasma glucose15, arrhythmias16, 17, and even episodes of sleep apnea18, 19. Altogether, these findings are consistent with the hypothesis that a portion of the beneficial responses to RDNx may be due, in part, to ablation of afferent rather than efferent renal nerves.
Our group has recently developed a novel method for selective renal afferent nerve ablation (A-RDNx)20. Also, we observed both total (T-RDNx) and A-RDNx attenuated DOCA-salt hypertension by approximately 50%, suggesting the antihypertensive response to renal denervation is primarily due to ablation of afferent nerves20. However, the question of how afferent-targeted RDNx attenuates hypertension remains unanswered.
Renal nerves, specifically efferent nerves21, reportedly mediate renal inflammation through the trafficking and activation of T cells and increased inflammatory cytokine content22. Further, several inflammatory proteins (e.g. IL-6, IL-1β, MCP-1, and GRO/KC23, 24) that are increased in models of hypertension may directly modify the sensitivity of peripheral sensory nerves25. Together, these data suggest the renal inflammation drives renal afferent activity, and in turn, contributes to the increase in arterial pressure.
The present study was designed to investigate the antihypertensive effect of RDNx on DOCA-salt hypertension, and the roles of efferent and afferent renal nerves in this response. We directly tested the hypothesis that afferent renal nerve discharge and sensitivity to stimulation would be increased after 21 days of DOCA-salt. Next, we tested the hypothesis that total RDNx (T-RDNx) and selective afferent RDNx (A-RDNx) would attenuate the development of DOCA-salt hypertension. Moreover, we hypothesized A-RDNx would lower arterial pressure independent of a decrease in renal inflammation.
METHODS
All procedures were approved by the University of Minnesota IACUC and were in compliance with the NIH Guide for Care and Use of Laboratory Animals. A detailed description of the methods and materials can be found in the Online Supplement.
Experiment 1: Direct Recording of Resting Afferent Renal Nerve Discharge in Vehicle and DOCA-Salt Rats
These experiments were conducted using the DOCA-salt rat, a well-established model that recapitulates moderate to severe human salt-sensitive hypertension and renal inflammation26. Twenty male Sprague Dawley rats (Weight: 275–300g; Age: 10–12 weeks) underwent the 21-day DOCA-salt treatment as previously described9, 20 (Figure 1). All rats were nephrectomized, and allowed three weeks to recover. Half of the rats were switched to 0.9% saline, and five days later, administered 100mg DOCA in a subcutaneous silicone implant (DOCA; n=10). Controls remained on distilled water and received a silicone vehicle implant (Vehicle; n=10). Following the 21-day DOCA or Vehicle treatment, multi-unit resting ARNA in each animal was measured in vivo under 2% isoflurane anesthesia. Additionally, afferent nerve sensitivity to mechano- and chemo-sensitive stimuli was assessed. Expanded methods for the ARNA recording protocol can be found in the Online Supplement.
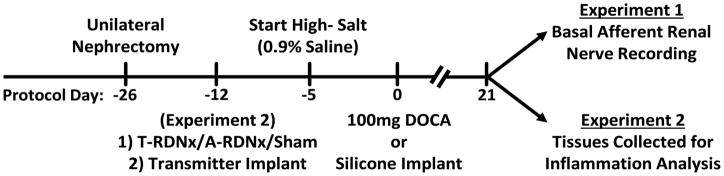
Figure 1.
The DOCA-salt protocol timeline for experiments 1 and 2.
The mean value of the 10-minute baseline integrated voltages (∫ARNA) was used to quantify resting ARNA. Further, to control for intra-experimental variability in the use of multi-unit nerve recording27, resting afferent nerve activity was also normalized to the peak ∫ARNA response to intrapelvic 50μM capsaicin (in 150mM NaCl), defined as the peak afferent activity (∫ARNApeak), and expressed as a percent of ∫ARNApeak using the following calculation:
Experiment 2: Effect of Total and Selective Afferent Renal Denervation on DOCA-Salt Hypertension and Renal Inflammation
A separate experiment was designed to determine the role of efferent and afferent renal nerves in the development of DOCA-salt hypertension and the associated renal inflammation. Thirty-eight male Sprague Dawley rats (Weight: 275–300g; Age: 10–12 weeks) underwent a unilateral nephrectomy 14 days prior to DOCA-salt treatment (see Figure 1). Rats were then randomly assigned to one of four experimental groups: (1) 100 mg DOCA-salt + total renal denervation (T-RDNx; n=12), (2) 100 mg DOCA-salt + selective afferent ablation (A-RDNx; n=11), (3) 100 mg DOCA-salt + sham denervation (DOCA-Sham; n=10), or (4) 0 mg DOCA + sham denervation (Vehicle-Sham; n=5). Total RDNx was achieved by surgical sectioning of renal nerves followed by a perivascular application of 10% phenol (in 100% ethanol). Afferent-specific RDNx was achieved by perivascular application 33mM capsaicin (in 5% Tween 80; 5% ethanol; 90% 150mM NaCl). Animals were instrumented with radiotelemeters for continuous measurement of arterial pressure and heart rate. After the 21-day treatment, tissues were collected for further analysis. For expanded methods, please see the Online Supplement.
Statistical Analysis
Temporal data were analyzed by a repeated measurement, two-way ANOVA with a Bonferroni post-hoc test (α=.05). All other data (2+ groups) were analyzed by one-way ANOVA with a Bonferroni post-hoc test (α=.05) or a Student’s t-test (2 groups). Statistical analyses were performed with GraphPad Prism 6.0 software. *p<.05 versus Vehicle-Sham; #p<.05 versus DOCA-Sham. Data is presented as mean ± SEM.
RESULTS
Experiment 1: Resting Afferent Renal Nerve Activity in the DOCA-Salt Model
Resting Afferent Nerve Activity and Sensitivity in DOCA-Salt Rats
Resting afferent renal nerve activity (ARNA) was measured under 2% isoflurane anesthesia. Mean arterial pressure (MAP) was elevated in anesthetized DOCA (151±5 mmHg) rats versus Vehicle (106±4 mmHg). Sample nerve tracings of resting RSNA, resting ARNA, and background noise signals from both vehicle and DOCA rats are depicted in Figure 2.
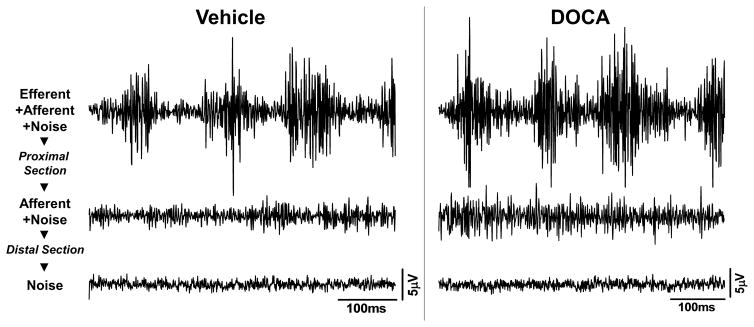
Figure 2.
Examples of original nerve tracings for total renal nerve activity (efferent+afferent+noise), afferent(+noise), and background noise from a vehicle and DOCA rat. Afferent signal was recorded after proximal sectioning of the isolated renal nerve. Background noise was recorded following a distal sectioning of the isolated nerve. Scale bars: 100ms (x-axis); 5μV (y-axis).
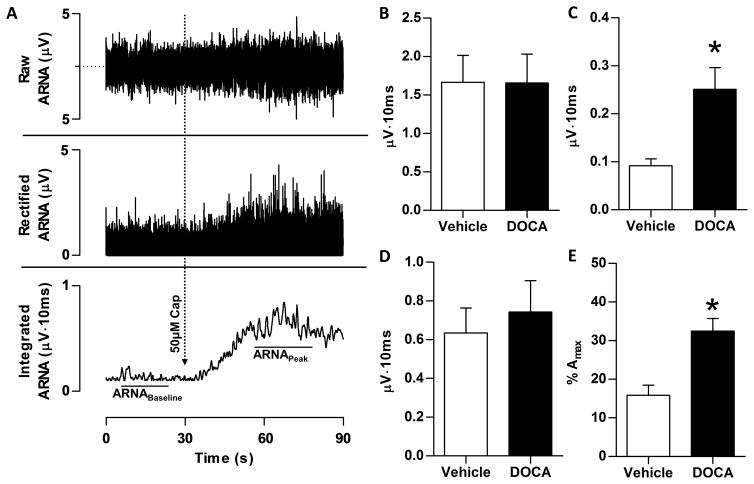
Prior to cutting the central end of the nerve, the integrated voltage of total multi-unit nerve activity (afferent + efferent) was no different between Vehicle and DOCA (Figure 3B). After sectioning the central end of the nerve, activity fell to less than 10% of this initial level, but note that resting ∫ARNA was higher in DOCA (Figure 3C). No difference was observed in peak response to intrapelvic capsaicin between groups (Figure 3D). Finally, when resting ∫ARNA is expressed as percentage of peak ∫ARNA to intrapelvic capsaicin (%Amax), resting ARNA was significantly higher in DOCA (Figure 3E). Notably, resting ARNA was approximately 2.5-fold higher in DOCA regardless of whether it was expressed as ∫ARNA or %Amax.
Figure 3.
Following DOCA-salt treatment, resting afferent renal nerve activity (ARNA) was measured in anesthetized rats. Panel A: A sample tracing of raw (top), rectified (middle), and integrated (bottom) nerve activity from a Vehicle-Sham animal is depicted, in which ARNA was recorded before and during the response to intrapelvic administration of 50μM capsaicin to establish the peak level of afferent renal nerve discharge. ARNA was quantified from the integrated (∫ARNA) signal. Panel B: Raw integrated signal of resting total renal nerve activity is no difference between Vehicle and DOCA rats. Panel C: Following proximal sectioning of the renal nerve, signal recorded represents only ∫ARNA. The resting ∫ARNA was elevated in DOCA versus Vehicle (0.24±0.04 versus 0.10±0.01 μV·sec). Panel D: Peak afferent nerve response to intrapelvic perfusion of 50μM capsaicin was no different between Vehicle and DOCA. Panel E: Basal ARNA, expressed as a percentage of peak ARNA response to intrapelvic capsaicin (%Amax). After normalization, resting activity remained increased in DOCA-salt rats compared Vehicle (32.0±5.7 versus 13.8±2.7%Amax). All data presented as mean±SEM (n=10/group). *p<.05 versus Vehicle-Sham.
In a subset of six rats in each group, the responses to isobaric intrapelvic perfusion of isotonic saline (150 mM NaCl), hypertonic saline (600 mM NaCl), and bradykinin (20 μg/mL) were assessed. Response to increased pelvic pressure from 0 to 20 mmHg was also measured. No difference between groups was observed in ARNA response to any stimuli (see Online Supplement).
Experiment 2: Response to Total and Selective Afferent Renal Denervation in DOCA-Salt Hypertension and Renal Inflammation
Cardiovascular Responses
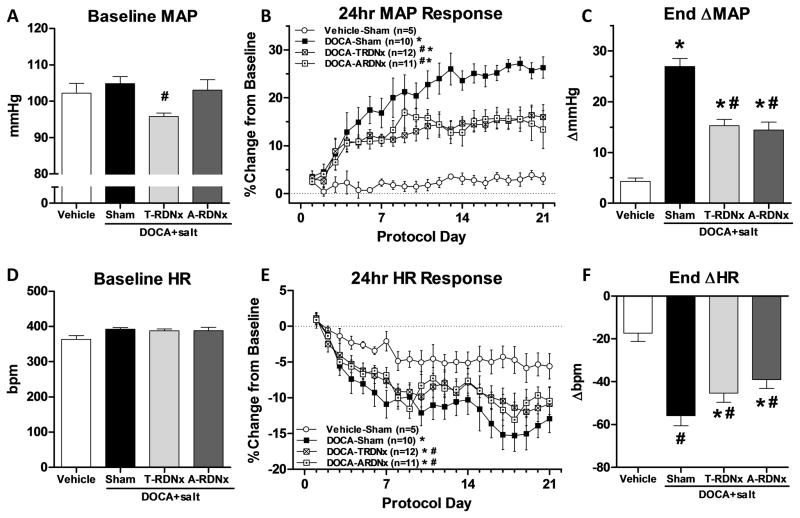
Prior to DOCA implantation, baseline mean arterial pressure (MAP) was lower in T-RDNx versus DOCA-Sham (96±1 versus 105±2mmHg) rats (Figure 4A). In contrast, basal MAP was unaffected by A-RDNx (103±4mmHg). The response to treatment was calculated as percent change from baseline (Figure 4B). The percent increase in MAP in DOCA-Sham was greater than Vehicle-Sham, which was attenuated equally by T-RDNx and A-RDNx (Figure 4B). The steady-state absolute change in MAP (Figure 4C), averaged over the final three days (days 19–21), was greater in DOCA-Sham versus Vehicle-Sham (Δ27±2 versus Δ4±1mmHg). Further, this response was halved by T-RDNx and A-RDNx (Δ15±1 and Δ14±2mmHg). Baseline HR prior to DOCA-salt treatment was similar across experimental groups (Figure 4D). The percent decrease in HR was greater in DOCA-Sham versus Vehicle-Sham (Figure 4E), and T-RDNx and A-RDNx also mitigated this response. Steady-state HR response (Figure 4F) to DOCA-sham (−56±5bpm) was also attenuated by T-RDNx and A-RDNx (−45±4 versus −39±4bpm). No effect of T-RDNx or A-RDNx versus DOCA-Sham was observed in daily or cumulative sodium intake (Figure S2).
Figure 4.
Panel A: Baseline mean arterial pressure (MAP) prior to DOCA treatment, was lower in T-RDNx group versus DOCA-Sham (*96±1 versus 105±2mmHg) rats and A-RDNx had no effect (103±4mmHg). Panel B: The percent increase from baseline MAP in DOCA-Sham rats was attenuated equally by T-RDNx and A-RDNx. Panel C: The end ΔMAP was averaged from the final three days of treatment (days 19–21) in the four treatment groups. The ΔMAP DOCA-Sham (27±2mmHg) was halved by T-RDNx and A-RDNx (15±1 and 14±2mmHg). Panel D: No differences in baseline HR was observed. Panel E: The percent change in heart rate from baseline was reduced in DOCA-Sham compared to Vehicle-Sham rats, and both T-RDNx and A-RDNx mitigated this effect. Panel F: End ΔHR averaged of the final three days of treatment was decreased in DOCA-sham versus Vehicle-Sham (−56±5 versus −17±4bpm), and T-RDNx and A-RDNx attenuated this response (−45±4 versus −9±4bpm). All data presented as mean±SEM. *p<.05 versus Vehicle-Sham; #p<.05 versus DOCA-Sham.
Renal Inflammatory Chemokine and Cytokine Content
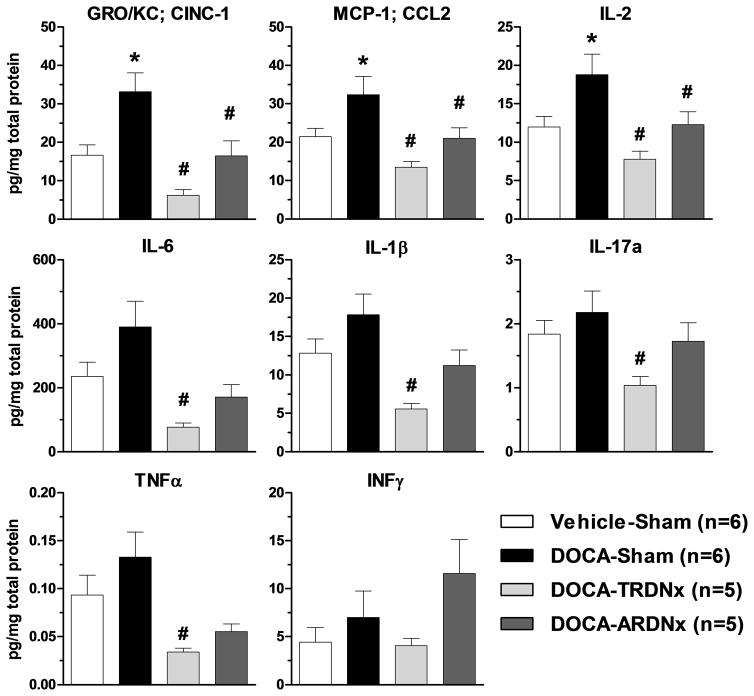
As shown in Figure 5, several pro-inflammatory markers (GRO/KC, MCP-1, IL-2, and IL-6) were increased in DOCA-Sham versus Vehicle-Sham. Moreover, compared to DOCA-Sham, T-RDNx decreased nearly all inflammatory markers (GRO/KC, MCP-1, IL-1β, IL-2, IL-6, IL-17a, and TNFα). Similarly, A-RDNx attenuated several pro-inflammatory cytokines (GRO/KC, MCP-1, IL-2), but fewer markers and to a lesser extent than T-RDNx. Within DOCA treated rats (DOCA-Sham, T-RDNx, and A-RDNx), no significant correlation between arterial pressure and cytokine content was observed (see Online Supplement)
Figure 5.
Renal cortical and medullary (mixed) tissue inflammatory marker content was measured by Luminex multiplex immunoassay. Several pro-inflammatory markers (GRO/KC, MCP-1, IL-2, and IL-6) were increased (*p<.05) in DOCA-Sham rats versus Vehicle-Sham. T-RDNx greatly decreased (#p<.05) the levels of GRO/KC, MCP-1, IL-1β, IL-2, IL-6, IL-17a, and TNFα versus DOCA-Sham. A-RDNx attenuated fewer pro-inflammatory cytokines (GRO/KC, MCP-1, IL-2) versus DOCA-Sham. All data presented as mean±SEM. *p<.05 versus Vehicle-Sham; #p<.05 versus DOCA-Sham.
Renal T-Cell Infiltration and Activation
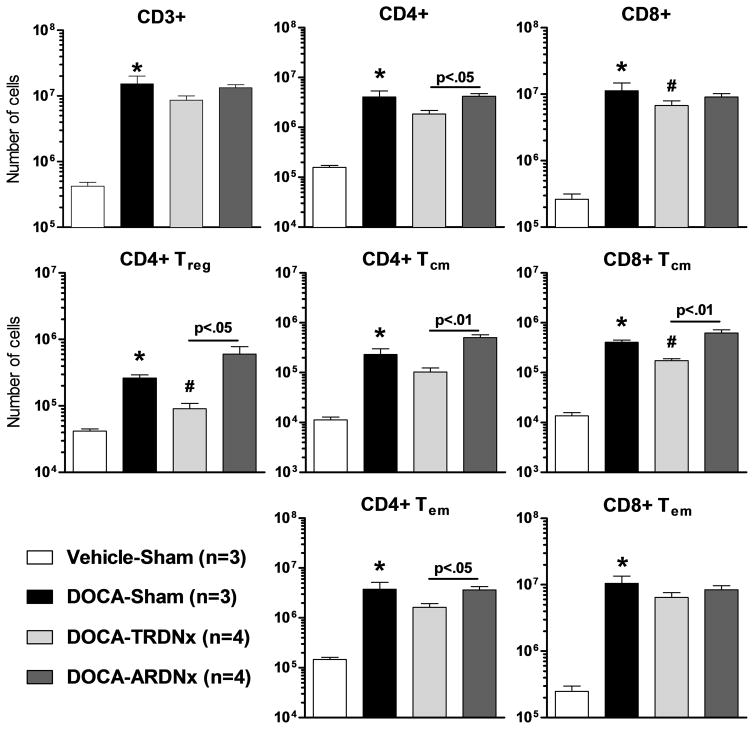
To elucidate the role of renal efferent and afferent nerves in renal inflammation, renal single-cell lymphocyte suspensions were analyzed by flow cytometry. Renal T-cells and sub-populations, including CD4+ and CD8+ subsets (Figure 6), are nearly all increased in DOCA-Sham rats. T-RDNx tended to prevent this effect, where CD4+ regulatory T cells (CD25high), CD8+ T-cells, and CD8+ central memory T-cells (CD44high/CD62Lhigh) were lowered (p<.05) by T-RDNx versus DOCA-Sham. No differences were observed between A-RDNx and DOCA-Sham.
Figure 6.
Renal T-cell infiltration and activation were assessed by flow cytometry. Nearly all T-cells subtypes were elevated with DOCA-salt treatment. Total renal nerve ablation (T-RDNx) tended to lower renal T-cell infiltration and activation versus DOCA-Sham. Compared to afferent-targeted RDNx (A-RDNx), CD4+ central memory (Tcm), effector memory (Tem), and regulatory (Treg) were lower in T-RDNx animals. All data presented as mean±SEM. *p<.05 versus Vehicle-Sham; #p<.05 versus DOCA-Sham.
Renal Denervation Efficacy
T-RDNx reduced renal NE content to nearly zero (<5% DOCA-Sham). In contrast, NE content in A-RDNx remained similar to DOCA-Sham. T-RDNx and A-RDNx markedly and similarly reduced CGRP content, indicating a similar efficacy was achieved. Together, these data indicate T-RDNx and A-RDNx techniques ablated the majority of the targeted nerves. Data is available in the Online Supplement.
DISCUSSION
The objective of the present study was to investigate the role of afferent and efferent renal nerves in the pathogenesis of a model of salt-sensitive hypertension that is associated with renal inflammation – the DOCA-salt rat. There were four important findings: First, using direct multiunit in vivo recording, we observed resting afferent nerve activity (ARNA) was elevated in the DOCA-salt rat. Secondly, total (T-RDNx) and selective afferent renal denervation (A-RDNx) mitigated both the rate and magnitude of DOCA-salt hypertension development equally. Third, nearly all activated renal T-cells were lowered by T-RDNx compared to DOCA-Sham rats, but not A-RDNx. Lastly, T-RDNx also lowered the renal pro-inflammatory marker content versus DOCA-Sham, whereas A-RDNx attenuated or had no effect.
Collectively, these findings support the hypothesis that afferent and efferent renal nerves both play a central role in the pathogenesis of DOCA-salt hypertension and renal inflammation. Our interpretation of these findings in the context of previous studies is discussed in greater detail below.
Renal Nerves and Hypertension: Disparate Roles for Afferent and Efferent Sympathetic Nerves
Increased RSNA and renal inflammation are both recently hypothesized to contribute to the development and maintenance of many forms of hypertension. In this study, as well as previous reports from our laboratory9, 20, total renal denervation (T-RDNx) abated the rate of development of DOCA-salt hypertension by approximately 50%. In addition, as we have previously observed28, T-RDNx decreased the basal level of arterial pressure (prior to DOCA-salt) by approximately 10mmHg, suggesting renal efferent nerves contribute to blood pressure homeostasis under normotensive conditions in the rat. In contrast, whereas A-RDNx had no effect on the basal level of arterial pressure, it attenuated the rate of development and magnitude of hypertension in a temporal pattern identical to T-RDNx. This finding, combined with the observation that the resting level of afferent renal nerve activity (ARNA) was substantially increased in DOCA-salt, leads us to conclude the renal nerve-dependent component of DOCA-salt hypertension is primarily mediated by increased afferent, rather than efferent, renal nerve activity.
To our knowledge, there is only one other report where resting ARNA was measured in a model of hypertension. In agreement with the present study, Moss and Stoltock observed an increased resting firing rate of single-unit afferent renal nerves in the spontaneously hypertensive rat (SHR) compared to a normotensive control29. While single-unit recordings are favorable due to simple quantification and low-experimental variability, this technique is dependent on large sample sizes to appropriately represent whole-organ activity. Alternatively, multi-unit recording offers general sampling of multiple units within a bundle that may be missed using single-unit recording. Also, we feel the use of normalization to a maximal response avoids the traditional complication of intra-experimental variability and group comparison. Importantly, the observation from this study using multi-unit recordings and the previous use of single unit recordings both confirm an elevation in ARNA in hypertensive models.
Despite the paucity of direct evidence linking a chronic increase in ARNA to the hypertension etiology, this hypothesis is supported by studies in which renal deafferentation was achieved by dorsal rhizotomy (DRZ). Campese and colleagues used this approach to elucidate the role of renal afferent nerves in the hypertensive response following renal injury10. DRZ also attenuates the hypertensive response in the SHR11, renovascular hypertension12, and chronic renal failure13. It is important to note DRZ ablates not only renal, but all visceral and somatic sensory input to the spinal cord. Moreover, DRZ reportedly causes salt-sensitive hypertension by disrupting the reno-renal reflex30. Our recent studies show the targeted ablation of renal afferent nerves by periaxonal capsaicin does not affect sodium handling or salt-sensitivity20. Whether these disparate reports are due to the ablation technique or blood pressure measurement methodology remains to be studied.
A more direct approach to elucidate this mechanism is the monitoring of arterial pressure response to ARNA stimulation. Notably, Kottke and colleagues reported in 1945 that long-term direct stimulation of renal nerves produced chronic hypertension in dogs31. Although efferent renal nerve stimulation was the presumed cause, the role afferent renal nerve stimulation cannot be ruled out. More recently, studies from Patel and Knuepfer32 and Simon et al.33 found that acute stimulation of renal afferent nerves in conscious rats elicited an increase in vasopressin and arterial pressure. However, no follow-up studies examined whether this response is maintained longer than one hour, so the arterial pressure response to chronic ARNA stimulation remains speculative.
Afferent renal nerves modulate autonomic neural pathways in the brain integral in neuroendocrine control of cardiovascular function. For example, afferent renal nerve stimulation was recently reported to directly modulate the activity of pre-motor autonomic neurons in the rostral ventrolateral (RVLM) and paraventricular nucleus (PVN) in rats34. Moreover, hypothalamic lesions attenuate DOCA-salt hypertension35, 36 as well as the two-kidney-one-clip model of hypertension37, 38, a model which is also proposed to be dependent on afferent renal nerves12. While peripheral sympathetic activity or neural signaling in the hypothalamus was not measured in the current study, we hypothesize the attenuation of DOCA-salt hypertension by A-RDNx is likely due to a disruption of afferent signal to the PVN and RVLM resulting in an attenuation of sympathetic activity and/or vasopressin release – both of which are reported to be elevated in DOCA-salt hypertension39, 40. The neural and hormonal mechanisms by which A-RDNx attenuates DOCA-salt hypertension remained to be established.
Notably, this study contrast with our recent report using a similar approach in the Dahl salt-sensitive (Dahl S) rat on high salt diet28. Although T-RDNx decreased arterial pressure similarly in both the early and late phases of Dahl salt-sensitive hypertension, A-RDNx had no effect on arterial pressure. Based on these findings, we concluded afferent renal nerves do not contribute to the hypertension in the Dahl S rat. Importantly, it is clear that A-RDNx does not uniformly prevent all experimental models of hypertension. The explanation for these differences is unfortunately unknown. One possibility is the renal inflammation, which hypothetically drives ARNA, is less pronounced in the Dahl S rat compared to the DOCA-salt model. In other words, despite reports of renal inflammation in the Dahl S rats41, it may not be of the type or magnitude that assumingly alters ARNA. Additional examination of the mechanistic relationship between renal inflammatory mediators and renal afferent nerves is currently underway.
Renal Nerves and Renal Inflammation: A Bidirectional Communication?
Renal nerve ablation is reported to have an anti-inflammatory effect21, 22, a concept that is also supported by the present study. We found the combination of efferent and afferent denervation (T-RDNx) abated the renal inflammation induced by DOCA-salt. This is consistent with the previous report that T-RDNx attenuated the development of hypertension and renal inflammation in the Ang-II mouse model21. Moreover, these studies suggest the renal efferent nerves regulate the trafficking of inflammatory mediators. Interestingly, in contrast to the previous study where Xiao and colleagues observed A-RDNx had no effect on the renal inflammation or hypertension in the Ang-II mouse model21, we observed an equal attenuation of the hypertension with A-RDNx and T-RDNx. It is important to note this crucial difference can not be directly attributed to the renal inflammation, as A-RDNx had a lesser effect on renal inflammatory cytokine content or T-cell activation versus T-RDNx. Moreover, based on the current study’s findings, we conclude the afferent renal nerves directly contribute to the DOCA-salt hypertension, and the pro-hypertensive effects of renal inflammation may be relayed by the afferent renal nerves. While the role of afferent nerves in the blood pressure effect is clear, the mechanism linking afferent nerves and renal inflammation remains speculative.
Peripheral and central sensitization to afferent sensory input has been reported in models of systemic inflammation, as well as inflammation isolated in the dorsal root ganglion23, 24, 42. This raises the question of whether nerves can become activated or sensitized through the interaction with pro-inflammatory mediators. Though we did not observe an increased sensitivity, we can not rule out tonic activation since we observed ARNA was elevated under resting conditions in the DOCA-salt rat. Moreover, in a study by Veelken and colleagues using a rat model of glomerulonephritis, the investigators suggested inflammatory immune cells (e.g. macrophage and dendritic cells) may be indirectly communicating with the central nervous system through the direct interaction with renal afferent nerves22. When considering our inflammatory protein content data, which suggests a myriad of pro-inflammatory cell types and signals are contributing to this cytokine storm, several candidates for the chronic sensory nerve stimuli remain targets for future study.
Regarding the anti-inflammatory effect of T-RDNx and partial effect A-RDNx, we believe this is due in part to a reduced renal perfusion pressure43. Klanke and colleagues44 elegantly demonstrated a pressure-mediated renal inflammatory response in the DOCA-salt rat. These investigators reported that both mineralocorticoid and pressure effects individually contribute to the renal inflammation induced by DOCA-salt in rats. While we observed a greater reduction in renal inflammation was observed following T-RDNx compared to A-RDNx, it is important to note the blood pressure reduction remained similar. Interestingly, the pro-hypertensive effect of renal inflammation may be mediated through renal afferent nerves, as indicated by the anti-hypertensive effect of A-RDNx and the increased resting ARNA in DOCA-salt rats. Further studies are required to elucidate the direct activation of renal afferent nerves by renal inflammation, and its role in chronic blood pressure control.
Summary and Clinical Perspectives
With the recent conflicting clinical reports of the efficacy of RDNx5–8, it is crucial to elucidate the mechanisms of the effect of RDNx and if it may be improved or tailored for the treatment of hypertension complicated by renal disease. Collectively, the data from the present study suggests the DOCA-salt hypertension is mediated by both efferent and afferent renal nerves. Firstly, efferent renal nerves appear to contribute to renal inflammation development, and the pro-hypertensive effect of renal inflammation is likely mediated through the presence and activation of afferent renal nerves. In concert with our other recent findings28 where no preventative or reversal effect of afferent-specific denervation was observed in Dahl S rats, perhaps these data collectively suggest RDNx is not a universal treatment for all hypertension types. Further, perhaps RDNx would be most effective in patients with confirmed renal inflammation and elevated peripheral or renal SNA. Indeed, development of diagnostic tests for renal inflammation and elevated RSNA could isolate this patient population. Furthermore, our current study highlights an important role for renal afferent nerves to consider in the future study of renal denervation.
Limitations
Anesthesia
Measurement and analysis of RSNA is potentially complicated by anesthesia. It is not clear whether renal afferent nerve activity is affected since recordings were made directly from axonal projections of sensory nerves, where anesthetic modulation of synaptic transmission is not an issue. Although we did employ a normalization procedure to minimize this confounder, we cannot rule out the possibility that isoflurane modulates afferent nerve discharge. We are currently developing a method to record afferent renal nerve activity in conscious rats to explore this possibility.
Denervation Efficacy
Based on our previous study28, we have validated the efficacy of periaxonal capsaicin for targeted ablation of renal sensory neurons. We observed pelvic CGRP content is undetectable 10 days post-capsaicin, but becomes detectable after four weeks. This time-dependent recovery of neurotransmitter content suggests reinnervation may be occuring; however, it is not clear whether the detectable levels of neurotransmitter content in this study is due to partial denervation attainment or reinnervation. Moreover, although the loss of pelvic CGRP content is consistent with ablation of sensory nerve terminals, it is not known whether a single capsaicin treatment results in the loss of renal dorsal root ganglion cell bodies and axons. Further studies are needed to more clearly establish the extent and duration of targeted renal afferent denervation using this method.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
We report resting afferent renal nerve activity is elevated in the DOCA-salt model of hypertension in the rat.
Afferent renal denervation attenuated the development of salt-sensitive hypertension independent of a decrease in renal inflammation.
What is Relevant?
The present study suggests that afferent renal nerves may contribute directly to the development and maintenance of salt-sensitive hypertension complicated by renal inflammation.
Summary
Resting afferent renal nerve activity (ARNA) was elevated in the hypertensive rat. Total and afferent-specific renal denervation similarly attenuated the development of the hypertension by approximately 50%. While total nerve ablation largely blunted renal inflammation and afferent-specific denervation had a lesser effect, the blood pressure effects were equal. Together, these data indicate afferent renal nerves mediate the antihypertensive response to total renal denervation in this model. Also, these data suggest the pro-inflammatory response is chiefly mediated primarily by efferent renal nerves, whereas the hypertensive response is mediated largely by afferent renal nerves.
Acknowledgments
We are grateful for the assistance from Dr. Angela Panoskaltsis-Mortari and the Cytokine Reference Laboratory in the measurement of the tissue inflammatory protein markers.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health R01 grant HL116476 (PI: J.W. Osborn). C.T. Banek was supported by NIHLBI T32 Training Grant 2T32HL7741-21 (PI: David Ingbar).
Footnotes
DISCLOSURES
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Davis MI, Filion KB, Zhang D, Eisenberg MJ, Afilalo J, Schiffrin EL, Joyal D. Effectiveness of renal denervation therapy for resistant hypertension: A systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:231–241. doi: 10.1016/j.jacc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 4.DiBona GF, Esler M. Translational medicine: The antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 5.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 6.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 8.Desch S, Okon T, Heinemann D, Kulle K, Rohnert K, Sonnabend M, Petzold M, Muller U, Schuler G, Eitel I, Thiele H, Lurz P. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65:1202–1208. doi: 10.1161/HYPERTENSIONAHA.115.05283. [DOI] [PubMed] [Google Scholar]
- 9.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in doca-salt model in rats: A telemetric approach. Am J Physiol Heart Circ Physiol. 2005;289:H1519–1529. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 10.Ye S, Gamburd M, Mozayeni P, Koss M, Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens. 1998;11:723–728. doi: 10.1016/s0895-7061(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 11.Janssen BJ, Debets JJ, Struyker-Boudier HA, Smits JF. Role of sensory renal nerves in the development of spontaneous hypertension in rats. Clinical and Experimental Hypertension. 1987;9(Suppl 1):227–239. doi: 10.3109/10641968709160176. [DOI] [PubMed] [Google Scholar]
- 12.Wyss JM, Aboukarsh N, Oparil S. Sensory denervation of the kidney attenuates renovascular hypertension in the rat. Am J Physiol. 1986;250:H82–86. doi: 10.1152/ajpheart.1986.250.1.H82. [DOI] [PubMed] [Google Scholar]
- 13.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25:878–882. doi: 10.1161/01.hyp.25.4.878. [DOI] [PubMed] [Google Scholar]
- 14.Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension. 2014;64:118–124. doi: 10.1161/HYPERTENSIONAHA.113.03098. [DOI] [PubMed] [Google Scholar]
- 15.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Bohm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: A pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 16.Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Bayramova S, Losik D, Baranova V, Karaskov A, Steinberg JS. Renal denervation for improving outcomes of catheter ablation in patients with atrial fibrillation and hypertension: Early experience. Heart Rhythm. 2014;11:1131–1138. doi: 10.1016/j.hrthm.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 18.Kario K, Ikemoto T, Kuwabara M, Ishiyama H, Saito K, Hoshide S. Catheter-based renal denervation reduces hypoxia-triggered nocturnal blood pressure peak in obstructive sleep apnea syndrome. J Clin Hypertens (Greenwich) 2016;18:707–709. doi: 10.1111/jch.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witkowski A, Kadziela J. Obstructive sleep apnoea, resistant hypertension and renal denervation. EuroIntervention. 2013;9(Suppl R):R105–109. doi: 10.4244/EIJV9SRA18. [DOI] [PubMed] [Google Scholar]
- 20.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–122. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J, 2nd, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin ii-induced hypertension. Circ Res. 2015;117:547–557. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veelken R, Vogel EM, Hilgers K, Amann K, Hartner A, Sass G, Neuhuber W, Tiegs G. Autonomic renal denervation ameliorates experimental glomerulonephritis. J Am Soc Nephrol. 2008;19:1371–1378. doi: 10.1681/ASN.2007050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handbook of Experimental Pharmacology. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JM, An J. Cytokines, inflammation, and pain. International Anesthesiology Clinics. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 26.Iyer A, Chan V, Brown L. The doca-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Current Cardiology Reviews. 2010;6:291–297. doi: 10.2174/157340310793566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke SL, Lim K, Moretti JL, Head GA. Comparison of sympathetic nerve activity normalization procedures in conscious rabbits. Am J Physiol Heart Circ Physiol. 2016;310:H1222–1232. doi: 10.1152/ajpheart.00866.2015. [DOI] [PubMed] [Google Scholar]
- 28.Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase dahl s hypertension. Am J Physiol Regul Integr Comp Physiol. 2016;310:R262–267. doi: 10.1152/ajpregu.00408.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss NG, Scoltock AB. Age-dependent changes in afferent renal nerve activity in genetically hypertensive rats. Am J Physiol. 1992;262:R834–841. doi: 10.1152/ajpregu.1992.262.5.R834. [DOI] [PubMed] [Google Scholar]
- 30.Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension. 2003;42:968–973. doi: 10.1161/01.HYP.0000097549.70134.D8. [DOI] [PubMed] [Google Scholar]
- 31.Kottke FJ, Kubicek WG, Visscher MB. The production of arterial hypertension by chronic renal artery-nerve stimulation. Am J Physiol. 1945;145:38–47. doi: 10.1152/ajplegacy.1945.145.1.38. [DOI] [PubMed] [Google Scholar]
- 32.Patel KP, Knuepfer MM. Effect of afferent renal nerve stimulation on blood pressure, heart rate and noradrenergic activity in conscious rats. Journal of the Autonomic Nervous System. 1986;17:121–130. doi: 10.1016/0165-1838(86)90087-1. [DOI] [PubMed] [Google Scholar]
- 33.Simon JK, Kasting NW, Ciriello J. Afferent renal nerve effects on plasma vasopressin and oxytocin in conscious rats. Am J Physiol. 1989;256:R1240–1244. doi: 10.1152/ajpregu.1989.256.6.R1240. [DOI] [PubMed] [Google Scholar]
- 34.Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates rvlm-projecting pvn neurons. Am J Physiol Heart Circ Physiol. 2015;308:H1103–1111. doi: 10.1152/ajpheart.00862.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciriello J. Contribution of forebrain mechanisms in the maintenance of deoxycorticosterone acetate-salt hypertension. Clinical and Experimental Hypertension. 1988;10(Suppl 1):169–178. doi: 10.3109/10641968809075970. [DOI] [PubMed] [Google Scholar]
- 36.Bealer SL. Hypothalamic knife cuts attenuate maintenance of deoxycorticosterone acetate-salt induced hypertension. Brain Research. 1984;309:192–195. doi: 10.1016/0006-8993(84)91029-1. [DOI] [PubMed] [Google Scholar]
- 37.Castro AL, Almeida EF, Vadenal R, Lopes OU. Effects of anterior hypothalamic disconnection on the evolution of goldblatt renal hypertension. A dual response. Hypertension. 1983;5:V85–89. doi: 10.1161/01.hyp.5.6_pt_3.v85. [DOI] [PubMed] [Google Scholar]
- 38.Marson O, Saragoca MA, Ribeiro AB, Bossolan D, Tufik S, Ramos OL. Anteroventral third ventricle and renin-angiotensin system interaction in the two-kidney, one clip hypertensive rat. Hypertension. 1983;5:V90–93. doi: 10.1161/01.hyp.5.6_pt_3.v90. [DOI] [PubMed] [Google Scholar]
- 39.Crofton JT, Share L, Shade RE, Lee-Kwon WJ, Manning M, Sawyer WH. The importance of vasopressin in the development and maintenance of doc-salt hypertension in the rat. Hypertension. 1979;1:31–38. doi: 10.1161/01.hyp.1.1.31. [DOI] [PubMed] [Google Scholar]
- 40.Katholi RE, Naftilan AJ, Oparil S. Importance of renal sympathetic tone in the development of doca-salt hypertension in the rat. Hypertension. 1980;2:266–273. doi: 10.1161/01.hyp.2.3.266. [DOI] [PubMed] [Google Scholar]
- 41.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 42.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: The brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 43.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 44.Klanke B, Cordasic N, Hartner A, Schmieder RE, Veelken R, Hilgers KF. Blood pressure versus direct mineralocorticoid effects on kidney inflammation and fibrosis in doca-salt hypertension. Nephrology, Dialysis, Transplantation. 2008;23:3456–3463. doi: 10.1093/ndt/gfn301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.