Abstract
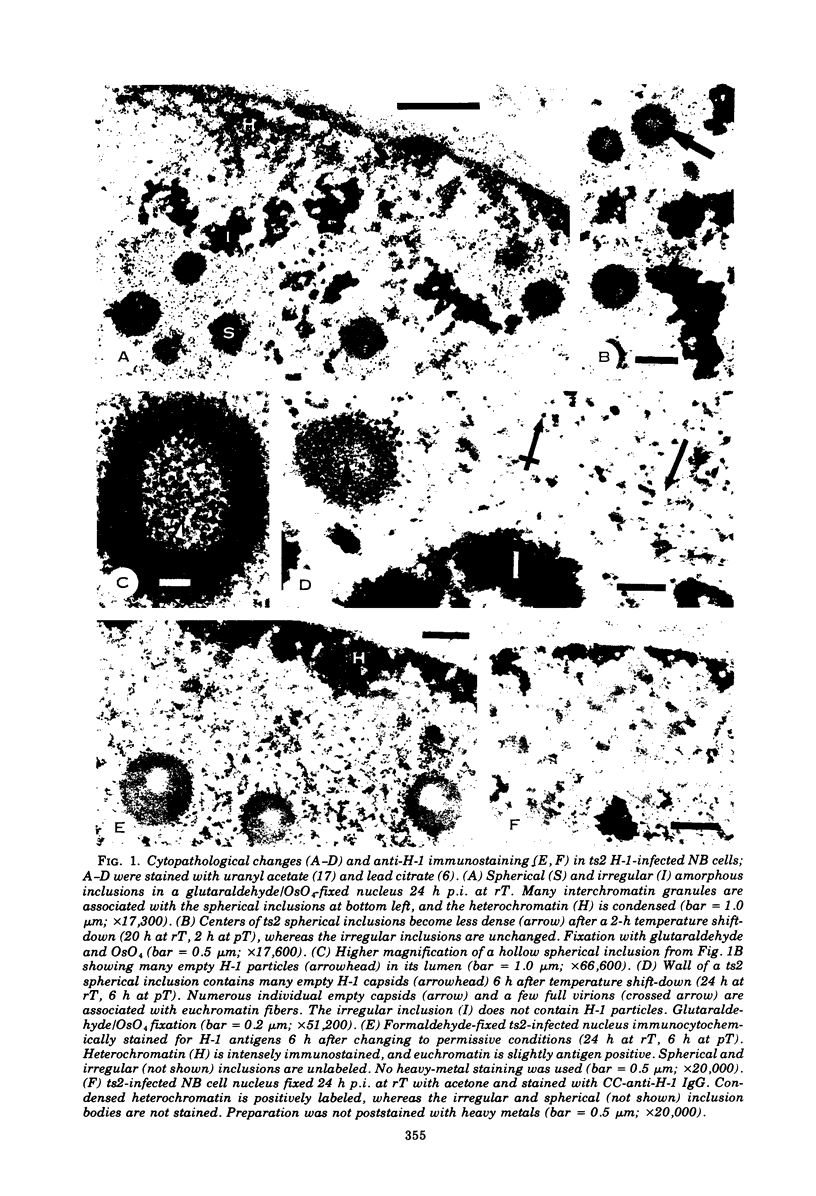
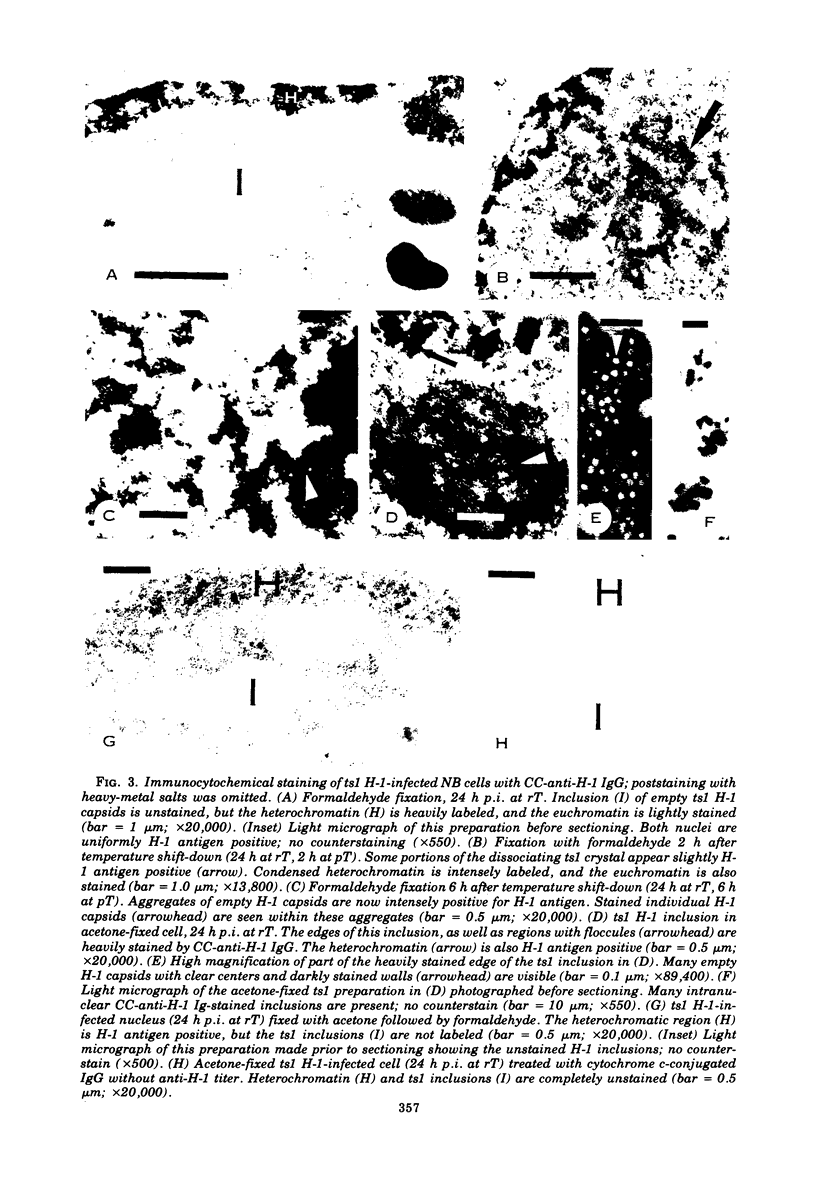
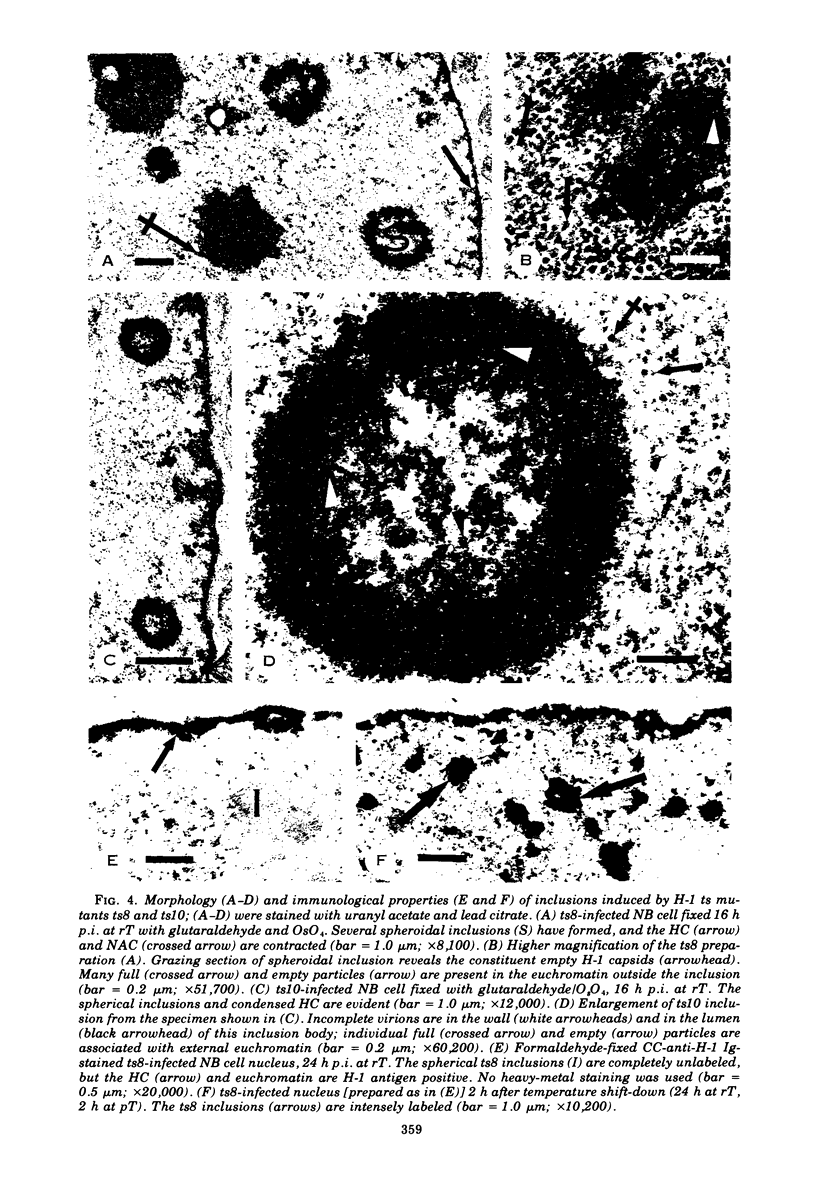
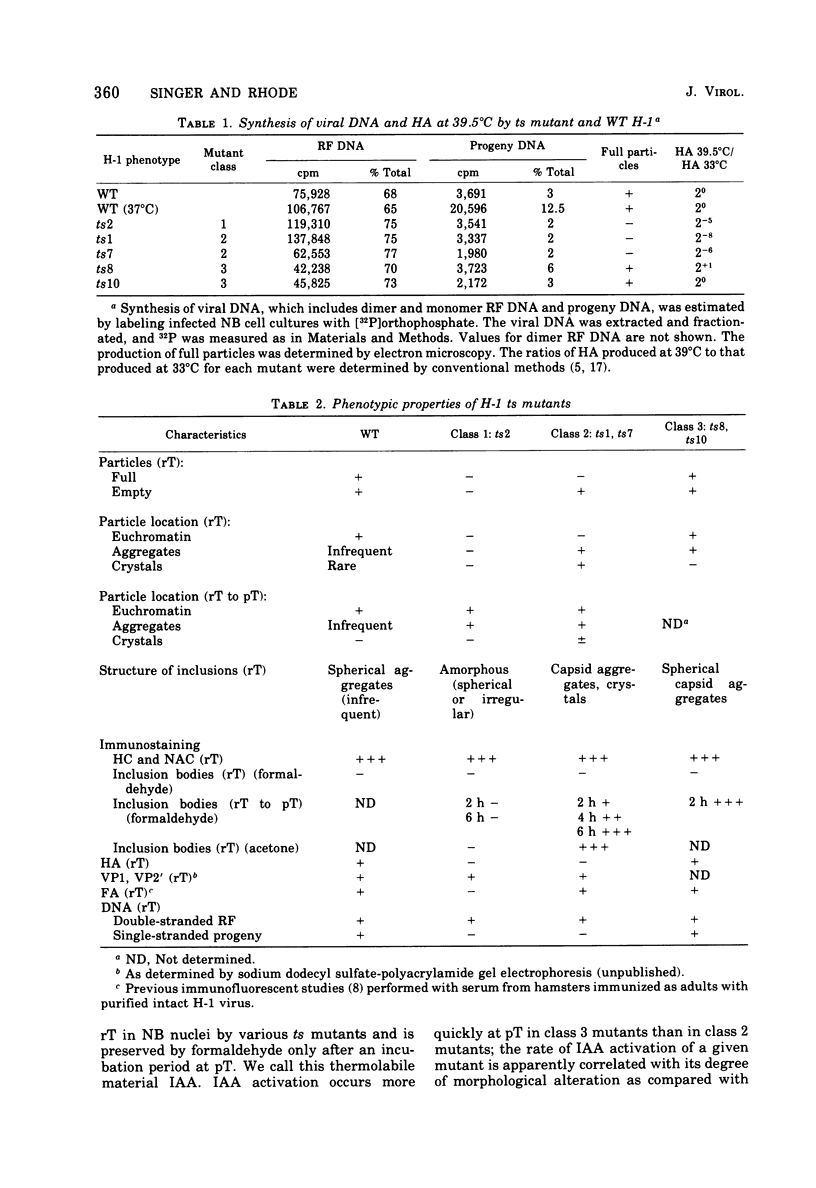
Electron microscopy and immunocytochrome c staining were used to define the phenotypes of several temperature-sensitive (ts) H-1 mutants. They were classified into three separate groups based on the properties of their capsids at the restrictive temperature (rT): (class 1) ts2 did not assemble capsids but produced spherical and irregular amorphous inclusions; (class 2) ts1 and ts7 exclusively synthesized empty particles which all aggregated and crystallized; and (class 3) ts8 and ts10 formed noncrystalline aggregates of empty virions, but many individual full, as well as empty, capsids were associated with euchromatin. Synthesis of progeny DNA and hemagglutinin at rT were normal for class 3 mutants, but defective for those in classes 1 and 2. The immunospecific staining patterns of these mutants indicated that the H-1 capsid proteins probably form two separate intranuclear antigens: (i) a thermostable chromatin-associated antigen present in proteins that have not formed capsids and are concentrated on heterochromatin and nucleolar-associated chromatin and (ii) a thermolabile inclusion-associated antigen found in the proteins of assembled empty capsids that compose H-1 inclusions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Transcription in vivo of a defective parvovirus: sedimentation and electrophoretic analysis of RNA synthesized by adenovirus-associated virus and its helper adenovirus. Virology. 1974 Sep;61(1):182–199. doi: 10.1016/0042-6822(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Karasaki S. Size and ultrastructure of the H-viruses as determined with the use of specific antibodies. J Ultrastruct Res. 1966 Sep;16(1):109–122. doi: 10.1016/s0022-5320(66)80026-6. [DOI] [PubMed] [Google Scholar]
- Kongsvik J. R., Gierthy J. F., Rhode S. L., 3rd Replication process of the parvovirus H-1. IV. H-1-specific proteins synthesized in synchronized human NB kidney cells. J Virol. 1974 Dec;14(6):1600–1603. doi: 10.1128/jvi.14.6.1600-1603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsvik J. R., Toolan H. W. Effect of proteolytic enzymes on the hemagglutinating property of the parvoviruses, H-1, H-3, and RV. Proc Soc Exp Biol Med. 1972 May;140(1):140–144. doi: 10.3181/00379727-140-36411. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1 V. Isolation and characterization of temperature-sensitive H-1 mutants. J Virol. 1976 Feb;17(2):659–667. doi: 10.1128/jvi.17.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey R. E., Woodman D. R., Hetrick F. M. Studies on the natural infection of rats with the Kilham rat virus. Am J Epidemiol. 1968 Jul;88(1):139–143. doi: 10.1093/oxfordjournals.aje.a120863. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., Redler B. Synthesis of viral-specific RNA in cells infected with the parvovirus, Kilham rat virus. J Virol. 1974 Sep;14(3):434–440. doi: 10.1128/jvi.14.3.434-440.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. Multiplication of parvovirus LuIII in a synchronized culture system. III. Replication of viral DNA. J Virol. 1976 Mar;17(3):841–853. doi: 10.1128/jvi.17.3.841-853.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VII. Electron microscopy of replicative-form DNA synthesis. J Virol. 1977 Feb;21(2):713–723. doi: 10.1128/jvi.21.2.713-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Ultrastructural studies of H-1 parvovirus replication. IV. Crystal development and structure with the temperature-sensitive mutant ts1. J Virol. 1977 Oct;24(1):343–352. doi: 10.1128/jvi.24.1.343-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Toolan H. W. Ultrastructural studies of H-1 parvovirus replication. I. Cytopathology produced in human NB epithelial cells and hamster embryo fibroblasts. Virology. 1975 May;65(1):40–54. doi: 10.1016/0042-6822(75)90005-7. [DOI] [PubMed] [Google Scholar]
- Singer I. I. Ultrastructural studies of H-1 parvovirus replication. II. Induced changes in the deoxyribonucleoprotein and ribonucleoprotein components of human NB cell nuclei. Exp Cell Res. 1975 Oct 1;95(1):205–217. doi: 10.1016/0014-4827(75)90625-4. [DOI] [PubMed] [Google Scholar]
- Singer I. I. Ultrastructural studies of H-1 parvovirus replication. III. Intracellular localization of viral antigens with immunocytochrome c. Exp Cell Res. 1976 May;99(2):346–356. doi: 10.1016/0014-4827(76)90592-9. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Toolan H. W. The picodna viruses. H, RV, and AAV. Int Rev Exp Pathol. 1968;6:135–180. [PubMed] [Google Scholar]