Abstract
Bathochordaeus mcnutti sp. nov. is described from the mesopelagic northeast Pacific Ocean (Monterey Bay, California, USA). Larvaceans in the genus Bathochordaeus are large, often abundant zooplankters found throughout much of the world ocean, but until recently it was unclear whether more than a single species of Bathochordaeus existed. Using remotely operated vehicles, we have made hundreds of in situ observations, compiled two decades of time-series data, and carefully collected enough specimens to determine that three species of Bathochordaeus occur in Monterey Bay: B. charon (Chun), B. stygius (Garstang), and B. mcnutti sp. nov. Bathochordaeus mcnutti is readily distinguished from its two congeners by the distinct blue outline visible around the periphery of its tail, and by other aspects of its morphology, ecology, and genetics. The abundance of larvaceans means they are ecologically important as particle processors. Species within the genus, Bathochordaeus, comprise the largest of described larvaceans.
Electronic supplementary material
The online version of this article (doi:10.1007/s00227-016-3046-0) contains supplementary material, which is available to authorized users.
Introduction
The class Appendicularia, or Larvacea, is composed of three extant families: the Oikopleuridae, the Fritillaridae, and the Kowalevskiidae. Exclusively marine organisms, larvaceans are holoplanktonic pelagic tunicates. As a group, they are important members of the planktonic community, and are capable of having a grazing impact that exceeds that of copepods (Alldredge 1981; Deibel 1988; Fenaux et al. 1998; Gorsky and Fenaux 1998; Lombard et al. 2011). A few species have been well studied, particularly those that are easily kept alive in the laboratory, like Oikopleura dioica.
The majority of larvacean species have not been subject to investigation. Larvaceans occur throughout the world ocean, yet their published diversity remains remarkably, and likely inaccurately, low (Hopcroft 2005). Only about 70 species are currently recognized, with 43 of those found in the Pacific Ocean (Fenaux et al. 1998; Castellanos et al. 2009; Deibel and Lowen 2012). In plankton tows, the small species can be very abundant, often rivaling copepods in number, but their fragility makes larvaceans notoriously difficult to collect and study (Bückmann and Kapp 1975; Fenaux 1993). Nowhere is this more clearly the case than in the open ocean and deep sea where the expense and difficulty of sampling are greatly increased (Hopcroft and Robison 1999; Hopcroft 2005).
Consequently, it is likely that numerous groups—larvaceans among them—in these extensive habitats have many species yet to be discovered and formally described (Appeltans et al. 2012). The relative dearth of deep-sea specimens may mean that some described species are in need of more careful examination, particularly those that are cryptic (Pimm 2012). In Monterey Bay, larvaceans of various species are found throughout the water column and the numbers of individuals, if not species (predominantly undescribed) increases as we go through the benthic boundary layer as deep as 3500 m (Robison et al. 2010).
As particle processors, larvaceans affect both the pelagic environment in which they live as well as the seafloor beneath them. The active houses of giant larvaceans function as oases for zooplankton in the mesopelagic habitat (Steinberg et al. 1994) while their abandoned houses, or “sinkers,” are estimated to contribute up to one-third of the vertical carbon flux to the deep seafloor in Monterey Bay (Robison et al. 2005). Because they can transform small, suspended particles into pellets or aggregates that sink quickly, organic carbon processed by larvaceans can shortcut the microbial loop (Deibel 1988; Fenaux et al. 1998). Lombard et al. (2011) showed that the size spectrum of prey for the oikopleurid Oikopleura dioica ranges from 0.4 to 20% of their body size—a broader range than most planktivorous organisms—as O. dioica feeds on microphagous as well as macrophagous organisms. Resolving the identities of these animals is thus of ecological as well as taxonomic significance.
Giant larvaceans are oikopleurids in the subfamily Bathochordaeinae. Chun (1900) described the first giant larvacean from animals he collected during the Valdivia expedition that was made over 100 years ago. He named them Bathochordaeus charon after the mythical ferryman who carries the souls of the dead across the River Styx. During the Valdivia expedition, two specimens were collected from the South Atlantic and two more from the Indian Ocean. This is noteworthy because of the relative rarity with which these animals are collected using modern trawl equipment (Hopcroft 2005). It was not until three decades after their original description that Garstang (1936) collected two more giant larvaceans in good condition. His specimens differed from those of Chun, and since he had no access to the original specimens, Garstang (1937) rather reluctantly described a new species: Bathochordaeus stygius.
Over the subsequent years, giant larvaceans were infrequently collected. When specimens were found, most seemed to fit Garstang’s (1937) description of B. stygius. The two species were alternately distinguished then synonymized, with the result that confusion remains in the modern literature over the actual number of extant giant larvacean species (Fenaux 1966, 1993; Flood 2005; Hopcroft 2005; Sherlock et al. 2016).
The advent of tools like remotely operated vehicles (ROVs) and specialized sampling equipment allows us to both observe and carefully collect pelagic specimens, providing the opportunity to better study the diversity of fragile organisms like larvaceans. We have made thousands of in situ observations and numerous collections of these delicate animals. Nearly two decades of data from MBARI’s Mesopelagic Time Series have provided insight into their ecology and distribution. From these data, we have learned that Bathochordaeus exhibits a seasonal peak in abundance in Monterey Bay and has a reproductive strategy that differs markedly from that of other oikopleurids.
Within our video recordings, the presence of a vivid blue line surrounding the tails of some Bathochordaeus suggested that there were at least two different forms of giant larvaceans in Monterey Bay. However, carefully collected specimens have allowed us to make detailed laboratory observations and to use modern molecular techniques in order to determine that, in fact, three giant larvacean species occur in Monterey Bay. The most common species is Bathochordaeus stygius Garstang (1937). A second much less common species fits well with Chun’s (1900) original description of B. charon (Sherlock et al. 2016). The third is a new species of giant larvacean in the genus Bathochordaeus that we describe here based on morphological, ecological, and molecular evidence: Bathochordaeus mcnutti sp. nov.
Materials and methods
The Monterey Bay Aquarium Research Institute (MBARI) has relied upon three ROVs, Ventana, Tiburon, and Doc Ricketts, to make sample collections and video observations of fragile, pelagic organisms since 1988. All three ROVs have been equipped with either standard or high-definition video cameras (Robison 1993; Robison et al. 2010). Between them, these vehicles have amassed close to 22,000 h of video footage, annotated by biologists and organized in a database called VARS, the Video Annotation and Reference System (Connor 2005; Schlining and Stout 2006).
A mesopelagic time series for Monterey Bay that spans approximately two decades was used to compare ecological data for both types of Bathochordaeus. During ROV dives, data for depth (m), temperature (°C), salinity (psu), and oxygen concentration (ml/l) were recorded continuously with sensors installed on the ROVs. These data are merged with each annotated record of Bathochordaeus spp. in VARS and provide in situ environmental data for all observations.
Giant larvaceans are often visible from several meters away because of the outer filter or “house” that surrounds them, which is often greater than a meter in longest dimension (Barham 1979; Hamner and Robison 1992). The blue outline on the tail of Bathochordaeus mcnutti is difficult to see if the animal is more than a meter away from the ROV’s camera, and specimens close to the camera may be difficult to see clearly if the ROV is moving fast. Therefore, only video footage that was clear and close-up was utilized for enumeration and to determine the depth distribution of B. mcnutti and B. stygius.
Physical descriptions are based on in situ observations made from 1991 to 2014 and on laboratory examinations of dozens of specimens collected over that time period. Preservation for morphological examination was typically done with 5% formalin; however, cacodylate-buffered 2% glutaraldehyde was occasionally used as well. All measurements reported here were made on formalin-preserved animals. The type locality for holotype and paratype specimens of B. mcnutti sp. nov., and two congeners are given (Table 1).
Table 1.
Collection information for type specimens of Bathochordaeus mcnutti sp. nov., B. stygius (Garstang 1937) and B. charon (Chun, 1903)
| Specimen | Date | Dive# | ROV | Depth (m) | Lat. | Long. | USNM# |
|---|---|---|---|---|---|---|---|
| B. mcnutti | 8/20/2013 | 3730 | Ventana | 135 | 36.7587 | −122.1042 | 1422057 |
| B. mcnutti | 8/20/2013 | 3730 | Ventana | 99 | 36.7595 | −122.1033 | 1422056 |
| B. mcnutti | 8/20/2013 | 3730 | Ventana | 93 | 36.7603 | −122.1028 | 1422058 |
| B. mcnutti | 8/27/2009 | 3410 | Ventana | 91 | 36.7495 | −122.1050 | 1422059 |
| B. charon | 11/11/2013 | 548 | Doc Ricketts | 598 | 36.5409 | −122.5205 | 1251906 |
| B. charon | 3/28/2013 | 457 | Doc Ricketts | 281 | 36.6887 | −122.0438 | 1251907 |
| B. stygius | 8/20/2013 | 3730 | Ventana | 285 | 36.7507 | −122.1033 | 1251908 |
| B. stygius | 8/20/2013 | 3730 | Ventana | 270 | 36.7544 | −122.1055 | 1251909 |
| B. stygius | 11/18/2013 | 545 | Doc Ricketts | 215 | 36.7480 | −122.1022 | 1251910 |
The catalog numbers (USNM #) for the Smithsonian Institution, National Museum of Natural History are given
For molecular work, animals were placed in 95% ethanol or liquid nitrogen for initial preservation and stored at −80 °C until extraction. Samples of DNA were extracted from tissue using the DNeasy® Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Traditional DNA barcoding primers such as those for cytochrome c oxidase 1 (Folmer et al. 1994), 12S, cytochrome b, and H3 did not amplify genes for Bathochordaeus spp., and therefore we selected two tunicate primers used by Hirose et al. (2009), that were constructed specifically for tunicates, salps, doliolids, and larvaceans:
5′-CATTTWTTTTGATTWTTTRGWCATCCNGA-3′ (UroCox1-244F).
5′-GCWCYTATWSWWAAWACATAATGAA ARTG-3′ (UroCox1-387R).
These primers amplified a 400-base-pair section of the mitochondrial cytochrome c oxidase subunit I (COI) gene with the following PCR parameters: 35 cycles of 94 °C for 1 min, 40 °C for 1 min, 72 °C for 1 min. Amplification of an 1800-base-pair fragment of small subunit ribosomal DNA (18 S) was done using the modified universal primers mitchA and mitchB from Medlin et al. (1988) with the following PCR parameters: 4 cycles of 94 °C for 1 min, 54 °C for 1 min, a step of 0.1 °C/second from 54–72, 72 °C for 2 min, followed by 29 cycles of 94 °C for 1 min, 58 °C for 1 min, 72 °C for 1:30 min. All products were bidirectionally sequenced using BigDye® Terminator v3.1 Cycle Sequencing Kit on an ABI 3100 or ABI 3500xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequences were edited and aligned using Geneious version 6.0.5 created by Biomatters (Auckland, New Zealand, http://www.geneious.com/), and submitted to GenBank (Accession Numbers: KX599256-KX599281). Haplotype networks were created in TCS v1.21. Distance matrix was generated in Mega v6.
Results
Systematics
Type material
The holotype, collected from a depth of 99 m (where the seafloor is 1058 m) in Monterey Bay, California (36.759570°N, −122.103366°W) is deposited at the Smithsonian Institution, National Museum of Natural History, USA (catalog number USNM 1422056).
Diagnosis
Adult giant larvaceans in the genus Bathochordaeus are larger than most other adult larvaceans by close to an order of magnitude. In our samples, adult specimens (based on the presence of eggs and testes) range in size from approximately 3–10 cm in total length. From a distance in situ, size can be a distinguishing characteristic for the genus. Observed under a dissecting microscope, even a small Bathochordaeus specimen is readily separated from all other oikopleuridae by its asymmetrical mid-dorsal field cells, i.e., the large cells in the oikoblastic layer on either side of the animal’s midline are not mirror images of each other (Flood and Deibel 1998).
The shape and size of the houses of B. mcnutti and B. stygius observed in situ are very similar to each other but are quite different from B. charon (Sherlock et al. 2016). Unless gravid, the trunk (body) and tail of Bathochordaeus species in Monterey Bay are largely transparent. The most conspicuous pigmentation in B. stygius, if present, occurs in the gut and varies with the food consumed. In contrast, specimens of B. mcnutti bear a striking, iridescent blue outline around the periphery of their tails (Fig. 1). This is visible in the lights of a ROV but also under a microscope and in daylight. The outline remains visible even after preservation in formaldehyde, although the blue color turns an opaque white (Fig. 2). When examined closely, key differences in the morphology of their pharynx, spiracles, and esophagus combine to further distinguish B. mcnutti from its congeners.
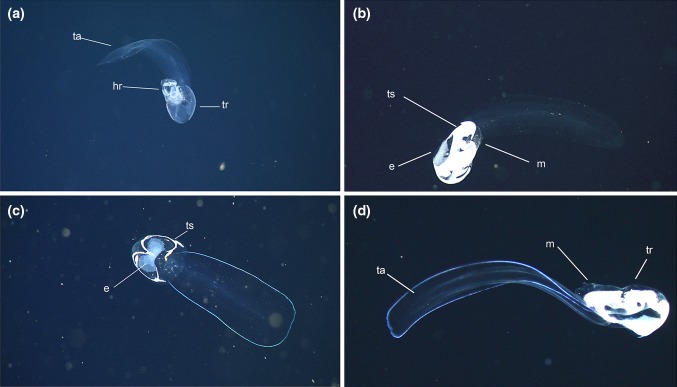
Fig. 1.
In situ comparison of B. stygius (a, b) to B. mcnutti sp. nov. (c, d). The tail (ta) and trunk (tr) of all specimens are clearly visible. One animal is beginning to inflate its “house” (house-rudiment), (h) but the rest are fecund adults with well-developed testes (ts), and eggs (e), that no longer occupy a house
Fig. 2.
Comparison of B. stygius (top row, a) and B. mcnutti sp. nov. (bottom row, b) preserved in 5% formalin. All 10 specimens were collected by ROV (remotely operated vehicle) on August 30, 2013. The outline around the tail of B. mcnutti has lost its blue coloration but remains readily apparent over the large size-range of specimens (scale 2 cm)
Etymology
The specific name mcnutti serves as an honorific to Marcia McNutt, Ph.D. MBARI’s President/CEO at the time this species was discovered.
Orientation to the animal: The mouth of Bathochordaeus opens dorsally, near the anterior end of the animal and the anus voids ventrally just right of the midline and behind the spiracles. The trunk of the animal embodies the mouth, spiracles, both lobes of the stomach, the digestive tract, gonads, brain, and heart. In comparison, the tail appears almost without structure and attaches to the trunk ventrally.
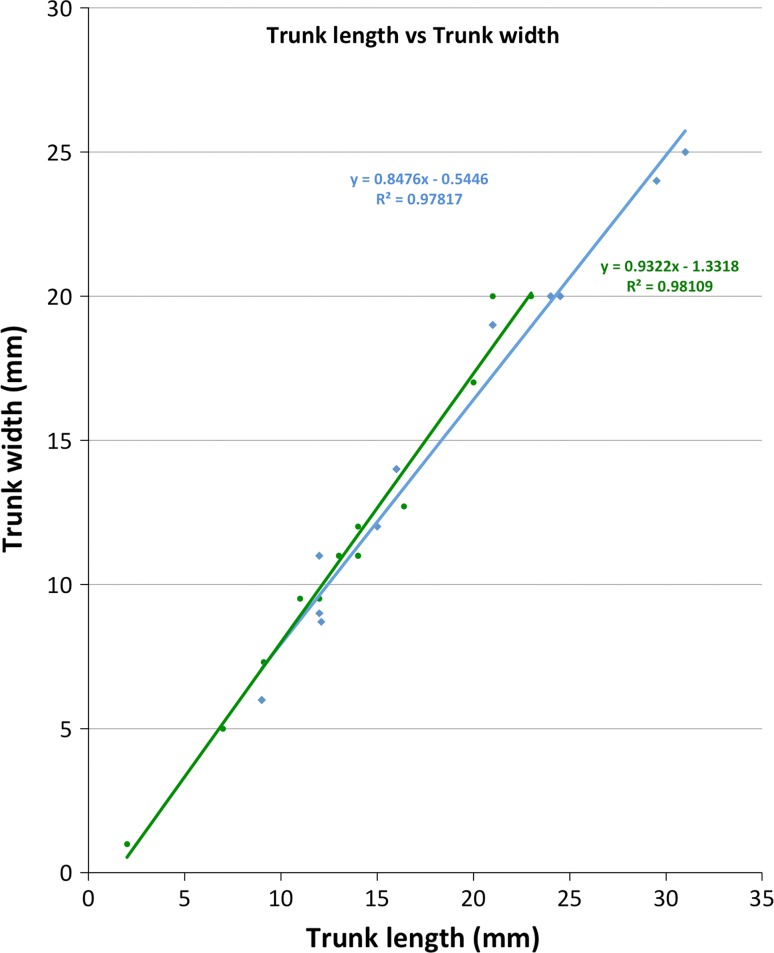
Trunk: The trunk of Bathochordaeus mcnutti is slightly longer than wide (1.2:1.0) as is that of B. stygius (Fig. 3), and the trunks of both species appear oval when viewed from above or below (Fig. 2). The height of the trunk tapers and at its thickest, most posterior point, it is slightly less thick than wide. The anterior end of the trunk is narrower by approximately half. These features combine to give the trunk a blunt, slightly wedge-shaped profile that is similar for both species (Fig. 1).
Fig. 3.
Trunk length versus width. Measurements were made on preserved (5% formalin) specimens of various sizes (n = 10 B. mcnutti, 10 B. stygius). For both species, the trunk is slightly longer than wide
Tail: Blue outline encircles the periphery of the entire tail of B. mcnutti (Figs. 1, 2). The broad tail widens as it progresses from the trunk to its distal end, at which point it is blunt with rounded corners.
Mouth and lips: The dorsal lip of B. mcnutti is semi-circular, smooth or very slightly scalloped (Fig. 4). The dorsal lip of B. stygius is usually distinguished from B. mcnutti because the former typically has a prominent band of densely packed cells, just above the ciliary funnel (cf) sometimes giving the dorsal lip a slightly scalloped look that Garstang referred to as “corrugated” (Fig. 4). More rarely, we have observed similar cells along the dorsal lip of B. mcnutti, but when present, they are not so densely packed as in B. stygius. In Oikopleura dioca, similar cells aid in particle rejection by causing ciliary reversal in the spiracles (Galt and Mackie 1971; Fenaux 1986; Bassham and Postlethwait 2005), but in B. stygius their function is not known. The conspicuous ciliated funnel itself is a prominent pharyngeal feature just below the dorsal lip, and it lies in approximately the same position for B. mcnutti as B. stygius, just right of the midline and brain. However, the ciliated funnel of blue-tailed larvaceans usually appears larger relative to their mouth than does that of B. stygius because the ciliated funnel of B. mcnutti is approximately two-thirds the diameter of the mouth while that of B. stygius is generally only half the diameter of the mouth or less (Fig. 4). Both of these species are markedly different in the orientation and aspect of the ciliated funnel than their congener B. charon (Sherlock et al. 2016). The ventral lip is pronounced in the genus, Bathochordaeus, but the inner sensory cells just below it are diminished in B. mcnutti compared to B. stygius and may even appear to be absent in smaller B. mcnutti (Fig. 4). The function of the inner sensory cells is unknown, but they seem likely to also play a role in particle rejection.
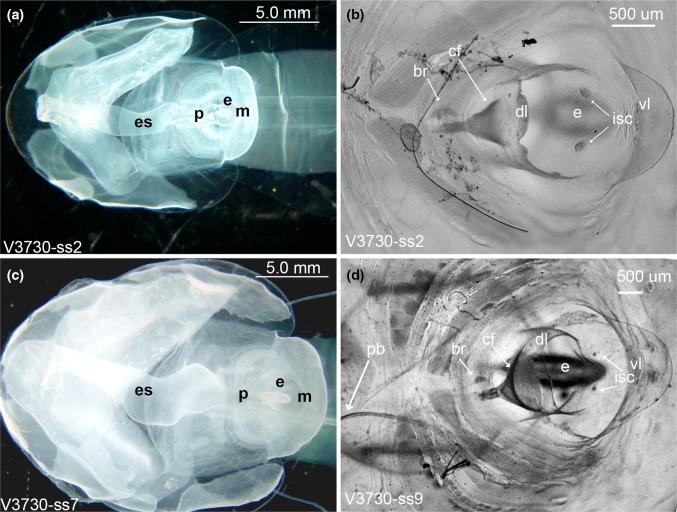
Fig. 4.
Dorsal views of B. stygius (top row, a, b) and B. mcnutti sp. nov. (bottom row, c, d). In B. mcnutti, the pharynx (p) is broad and the esophagus (es) appears distinctly kinked (c). Both structures are more slender in B. stygius, and the esophagus is almost tubular (a). To accommodate the bulkier pharynx, the endostyle (e) of B. mcnutti is pushed down, closer to the floor of the trunk. Its more horizontal aspect makes the endostyle of B. mcnutti appear elongate in comparison with B. stygius when viewed from above or below. In both species, the ventral lip (vl) is pronounced (b, d). The dorsal lip (dl) of B. mcnutti is generally smooth and uniform (d), while that of B. stygius usually looks more scalloped, due in part to the presence of densely packed cells at its apex (b). These same cells are less conspicuous in B. mcnutti and may be absent, entirely (d). The ciliated funnel (cf) of B. mcnutti (d) is typically more pronounced than that of B. stygius (b) because it is usually larger relative to the mouth (m). The inner sensory cells (isc) lie just below the ventral lip and in B. mcnutti (d) are small relative to B. stygius (b) of similar size and may be absent. Specimen identification numbers begin with “V” for the ROV Ventana and are followed by the dive number. In plate (b) a fiber is attached to the trunk and is not part of the specimen
Pharyngeal region (including endostyle and esophagus): The pharynx of B. mcnutti is larger and more robust than that of B. stygius (Fig. 4) and is encircled by peripharyngeal bands that when viewed dorsally, describe a distinct hourglass shape before taking a slightly convoluted path to the esophagus. On the other hand, the pharynx of B. stygius is narrow in comparison and gracefully follows a straighter path to its more tubular esophagus. Where the pharynx meets the esophagus of B. mcnutti, it appears kinked, from above angling briefly right at its junction with the pharynx, then veering to the left as the esophagus continues toward the stomach (Figs. 4, 5). In both species, there is a junction where the peripharyngeal bands meet dorsally, prior to the twisting of the pharynx, but in B. mcnutti, their sinuous outline contributes to the Fig. 8 like shape of the pharynx (online supporting video). Perhaps in order to accommodate its bulkier pharynx, the endostyle of B. mcnutti appears to have rotated from the more vertical (dorsal–ventral) orientation of B. stygius to a more horizontal (posterior–anterior) orientation, such that when viewed directly from above or below, the endostyle appears quite long relative to that of B. stygius (Figs. 4, 5).
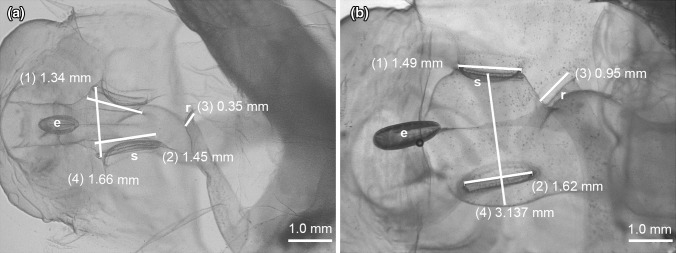
Fig. 5.
Ventral view of the trunk. The inner, pharyngeal openings to the spiracles (s) of B. stygius (a) and of B. mcnutti sp. nov. (b) are approximately equal in diameter to their outer openings. The spiracles of B. mcnutti (b: 1, 2) are about half the diameter of the pharynx at their midpoint (4). The slightly cupped spiracles of B. stygius (a: 1, 2) are more dorsoventrally flattened and in widest diameter approximate the diameter of the pharynx (4). Observed ventrally, the endostyle (e) of B. mcnutti appears more elongate than that of B. stygius. The rectum (r) voids slightly left of the mid-line in each species
Fig. 8.
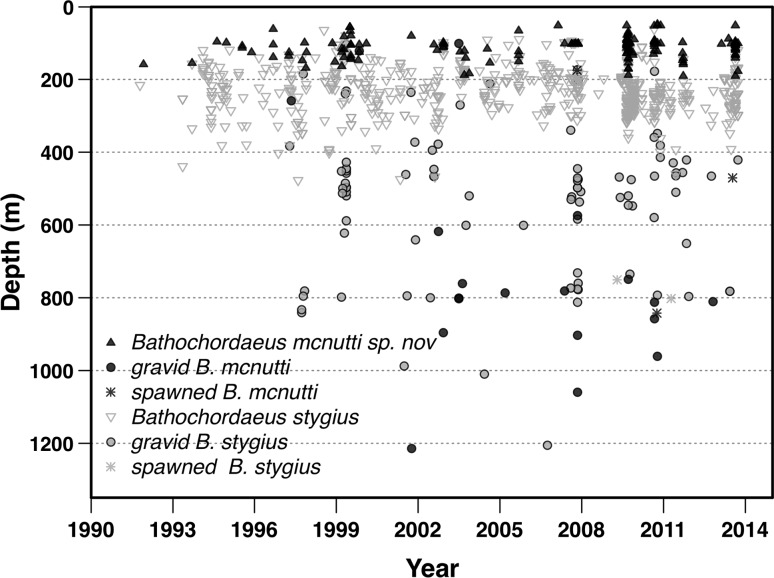
Depth distribution for Bathochordaeus mcnutti sp. nov. and B. stygius. Over MBARI’s 23-year video time series 64% of B. mcnutti (n = 161) have been found between 90 and 120 m. Although the depth distribution of the two species does overlap, only 28% of B. stygius (n = 672) were found above 190 m and 68% were observed between 200 and 300 m over the same time frame. For both species, the deepest animals found were always gravid or spent (had spawned their gametes) and were never found in houses (i.e., feeding)
Spiracles: The diameter of the spiracles is approximately equivalent at inner and outer openings for B. mcnutti and B. stygius (Fig. 5), i.e., neither species has funnel-shaped spiracles like B. charon (Sherlock et al. 2016). However, blue-tailed larvaceans have conspicuously smaller spiracles relative to the width of their pharynx than does B. stygius, and the spiracles of B. mcnutti appear more circular and less dorsoventrally flattened than those of B. stygius. In part, this is due to the robust pharynx of B. mcnutti versus the more slender, almost tubular pharynx of B. stygius. In longest dimension, the spiracle diameter of B. stygius exceeds the width of the pharynx at its mid-point. The diameter of the spiracles of B. mcnutti is clearly less than the width of the pharynx at their mid-point. When viewed either dorsally or ventrally, the outer spiracular opening of B. stygius is slightly cupped and appears flared. In contrast, the outer openings of the spiracles of B. mcnutti appear more flat than cupped (Fig. 5).
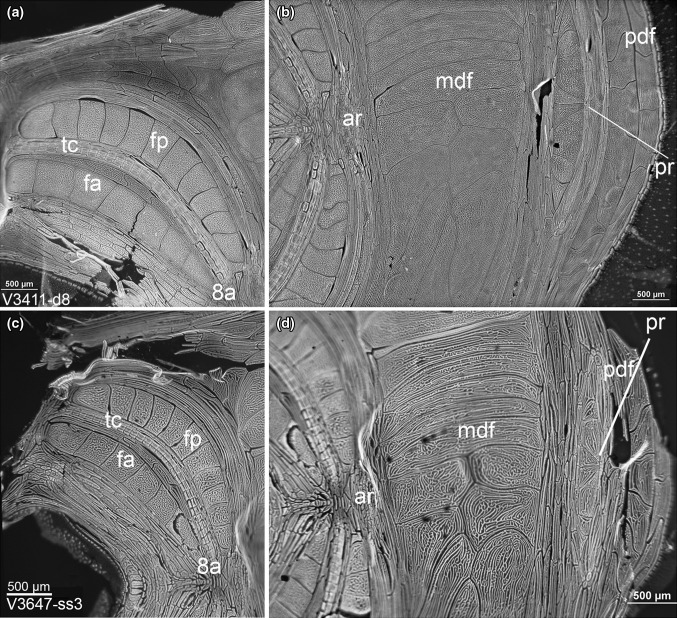
Oikoblastic region: The number of oikoblastic cells of all oikopleurids is thought to be constrained after the tail shift made during development (Lohmann 1898; Fenaux 1998b). However, the oikoblastic cells continue to grow as the animals age, and DNA replicates. This makes the oikoblastic cells of oikopleurids many times polyploid (Flood 2005). With Bathochordaeus, this endopolyploidy results in particularly large oikoblastic cells (Fig. 6). However, despite their size the oikoblastic cells are often difficult to see because they are colorless and can be easily damaged, and also because healthy Bathochordaeus spp. will begin to make a house very soon after capture, and the oikoblastic region can be obscured by the house-rudiment. Staining with DAPI or Hoechst 33258, both of which have an affinity for DNA, makes the polyploid cells of the oikoblastic region easily visible with a dissecting microscope, when illuminated by ultraviolet light.
Fig. 6.
DAPI-stained oikoblastic regions of Bathochordaeus stygius (a, b) and B. mcnutti sp. nov. (c, d) continue somatic growth after cell division ends; thus, the cells of which it is comprised are large and many times polyploid. Two conspicuous, crescent-shaped bands on either side of the mouth form Fol’s oikoplast. There are eight anterior Fol’s cells (fa) and 12 posterior cells (fp) that, with the trap cells (tc, also called “fibroblasts”), make the inner food-concentrating filter. Posterior to Fol’s oikoplast is the mid-dorsal field (mdf) that is composed of even larger cells. Unique to the genus Bathochordaeus, these cells are not bilaterally symmetrical. Since the oikoblastic cells are responsible for making the house with which these animals live and feed, it is expected that differences in the shapes of these cells will give rise to differences in the house structure. Scale 500 µm all plates
The most conspicuous cells of the oikoblastic region are the two pairs of dorsal bands that border either side of the mouth of Bathochordaeus, that Garstang called “moustache-shaped,” some cells of which can be close to a millimeter in longest dimension (Fig. 6). With the triple rows of evenly spaced, and much smaller trap cells in between, these paired bands comprise Fol’s oikoplast. In number, the giant cells of Fol’s oikoplast do not differ between B. mcnutti and B. stygius: eight for the anterior crescent and 12 for the posterior crescent. Chun (1900) described these as “moustache-like,” although he may have confused the anterior crescent with trap cells (or “fibroblasts”), because the anterior crescent of B. charon is greatly reduced in size compared to its congeners and is more similar in size to the band of much smaller (but more numerous) fibroblasts (Sherlock et al. 2016). When he described B. stygius with their much larger anterior Fol’s cells, Garstang (1937) was perplexed at how both Chun (1900) and Lohmann (1914) failed to see them when describing B. charon. In large part, these differences caused Garstang, reluctantly, to describe B. stygius in the first place. However, he thought that the posterior crescent was a modification of Martini’s field and that Lohmann was mistaken in calling them Fol’s cells. Instead Garstang (1937) referred to them as “Lohmann’s colloplasts” (Fig. 6). In most other oikopleurids, there are eight large Fol’s cells that are responsible for making the inner feeding filter of the house. The food-concentrating filters of B. mcnutti as well as B. stygius are made by all four bands of dorsal giant cells, and so it seems logical to refer to them as Fol’s posterior and Fol’s anterior when designating the twelve and eight-cell bands, respectively.
Behind Fol’s oikoplast are the even larger mid-dorsal field cells. Here the genus Bathochordaeus differs from all other oikopleurids in that the mid-dorsal field (mdf) is not bilaterally symmetrical. Apparently this difference was not noticed by Chun and was not well described by Lohmann (Garstang 1937). The posterior-dorsal field (pdf) is sandwiched between the mdf and the posterior rosette (also called Lohmann’s cross) that, at least for Bathochordaeus spp, looks neither like a cross nor a rosette. In number, the cells of the oikoblastic region appear similar for the two species, if not identical. But between B. stygius and B. mcnutti, there are consistent, if subtle differences in the whorls and interstices surrounding those cells (Fig. 6). Flood (2005) speculated that the patterns of cells in the oikoblastic region could provide a means of distinguishing between species, and we would agree; however, the importance of such distinctions is unclear. Such detailed examination of cellular arrangement requires time and equipment that may be outside the scope of many researchers (Hopcroft 2005), particularly when there are more conspicuous morphologic features that are also more clearly definitive, e.g., the digestive tract, spiracles and the blue outline of the tail.
House: Chun (1900) speculated that such large animals as Bathochordaeus must make “pumpkin”-sized houses. Barham (1979) first observed large larvaceans in their houses from a submersible and estimated their size at 30–100 cm. House size varies and is at least somewhat dependent on the size of the animal, but the giant larvaceans of Monterey Bay produce approximately one house per day (Silver et al. 1998; Robison et al. 2005) and that house can exceed a meter in greatest dimension (Hamner and Robison 1992).
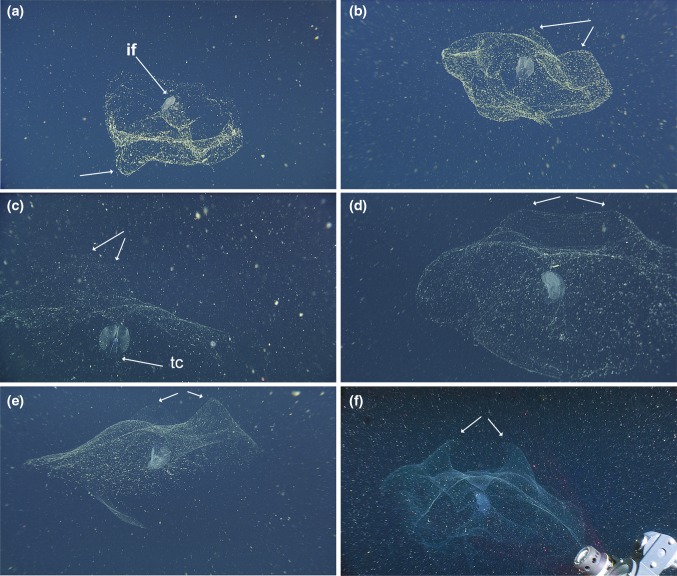
The oikoblastic cells of Bathochordeus spp. secrete the filters, or “houses” with which these animals feed. The house is acellular, made of mucopolysaccharides and consists of distinct inner and outer structures, with the innermost functioning to concentrate the larvacean’s food, and connected to the animal via a tube made of the same material as the house (Flood and Deibel 1998). Newly inflated houses are so transparent as to be almost invisible. With time, the house becomes easier to see as particles from the surrounding water accumulate and adhere to it, either passively as marine snow or actively as the house filters the water pumped through it (Silver et al. 1998). The inner filter (or filters) is bilobed and bilaterally symmetrical and also becomes clearly visible once filtered particles begin to adhere to it (Fig. 7).
Fig. 7.
Occupied, undisturbed houses of Bathochordaeus stygius (a, b) and B. mcnutti sp. nov. (c–f). The inner filter (if) is labeled (a) and appears in all plates as the relatively opaque, central structure. When recently formed (c) the outer house lacks a thick covering of marine snow but it becomes more visible as detritus accumulates, until the point where the outer house may begin to change shape or slough off, sometimes leaving the still-occupied inner filter. Depending on the vantage point, the undistorted houses of both species often show fairly conspicuous protrusions or humps (arrows). In (d) there is a continuous ridge. Their function is unknown, but these humps are typically more pronounced for B. mcnutti than B. stygius. In all plates except a and e the tail chamber (tc) of the animal is oriented in the same direction as the protrusions on the outer houses
The outer part of the house provides a protective barrier for the inner filter. Steinberg et al. (1994) showed that up to an order of magnitude more metazoans were found on houses than in surrounding waters. Some of these metazoans use the house as substrate. Invertebrates like medusae, ctenophores, and polychaete worms are commonly found trapped in the outer house. In the occupied houses of most other oikopleurids, some metazoans and large particles would be kept out of the inner food-concentrating filters by inlet filters, secreted by Eisen’s oikoplast (Flood and Deibel 1998); however, no Eisen’s cells have been reported in the genus Bathochordaeus. Despite this, the outer structure of the house does function as a roof-like barrier between the inner filter and the perpetual rain of marine detritus, as it filters large particles out of the water column and helps prevent metazoans from contacting and damaging the inner filter. As the outer house is coated and covered with particles, it begins to change shape.
The fragility of the outer house, along with the difficulty of observing one in situ makes the outer house a poor character by which to distinguish B. mcnutti from B. stygius. Nevertheless, one difference seen in the occupied and undisturbed outer house of B. mcnutti is a pair of prominent bumps, or a ridge, that if seen in B. stygius, is much more subtle (Fig. 7).
In contrast, the inner filter is well defined, more robust and more stereotypical in shape. However, the subtle differences in oikoblastic cells between B. mcnutti and B. stygius do not appear to generate differences in the inner filters of the houses we have studied, at least no apparent differences that can be discerned from the high-definition ROV video (online supporting video), although both differ markedly from the inner filter of B. charon (Sherlock et al. 2016).
Genital region: Larvaceans in the genus Bathochordaeus are simultaneous hermaphrodites. For the genus, gametogenesis begins near the midline of the ventral floor, close to the posterior end of the trunk. The eggs ultimately develop ventro-laterally while the testes spread posteriorly in wing-like tendrils that end up almost surrounding the eggs, as they expand dorso-laterally to envelop much of the trunk. During gametogenesis, the testes change from being virtually transparent (Fig. 1a) to an opaque white when mature (Fig. 1b–d). Among oikopleurids, gonad shape varies between genera and even between species (Fenaux 1998a). For Bathochordaeus spp., the size and wing-like shape of testes vary individually and perhaps between species (Fig. 1). The largest eggs we have measured have been 875 um in diameter, and we have counted as many as 640 eggs from one individual, although the number of eggs is likely relative to the size of the animal and/or the concentration of food present (Paffenhöfer 1973; Fenaux 1998a).
Spawning and reproduction
All oikopleurids except Oikopleura dioca are hermaphrodites (Fenaux 1998a). At least some abandon a final house, swim to the surface to spawn, and then die as gametes escape through their ruptured trunks (Alldredge 1982). In contrast to the smaller species, Bathochordaeus mcnutti and B. stygius abandon a final house then descend to darker depths, perhaps because their opaque, gamete-laden trunk is less apt to be seen by visual predators. Ultimately, it seems probable that gravid individuals make a final ascent in order to spawn in the shallower waters where they feed, but we have no observations of Bathochordaeus spp. spawning in situ. While it is possible that giant larvaceans descend and then spawn at depth, it seems unlikely that they would do so. Captured specimens have spawned in our samplers as the ROV ascended. The majority of eggs from those animals, kept in the water they were collected with, are near-neutral with a small percentage being either very slightly buoyant or sinking slowly. If giant larvaceans spawned at depth, it is difficult to fathom how the young would ascend the hundreds of meters necessary to reach the depth range of 100–300 m where they are most abundant. It is difficult to envision an adaptive benefit to spawning at depth. Furthermore, while we have sampled small individuals in the shallow mesopelagic and epipelagic from 50 to 150 m, below 450 m we have only encountered gravid or spent adults. We have also observed and collected large, gravid individuals in the shallow mesopelagic. Since these animals were swimming free of their houses, they were not feeding and it follows that they were ascending to spawn in the more productive surface waters.
From video, we have identified 833 non-gravid individuals to species level: 161 B. mcnutti and 672 B. stygius. From 1991 to 2014, the maximum depth observed for a non-gravid B. mcnutti was 189 m, with 64% of individuals found between 90–120 m (Fig. 8). In contrast, 28% of non-gravid B. stygius individuals were found above 190 m, while 68% of the individuals were found between 200–330 m.
Deeper occurring animals were more rare. Only 47 individuals from both species have been found in excess of 600 m. Of those, 42 animals were gravid and none were associated with a house (Fig. 8). The five non-gravid animals (three B. mcnutti, and two B. stygius) observed at depth were moribund, sinking, or swimming weakly downward. All were relatively large. None were associated with houses, and those that were observed closely appeared to have ruptured trunks. These animals appeared likely to have spawned in shallower waters and were either dead or dying as they sank back through the mesopelagic. In the laboratory, tails of spawned animals continued to beat reflexively, but weakly, several hours after the trunk had ruptured.
We observed a number of gravid and non-gravid B. stygius off the Oregon coast in summer of 2009. We did not encounter any B. mcnutti off Oregon or Washington; however, a single specimen was found at 86 m in the San Diego Trough off southern California (Latitude: 32.904325, Longitude: −117.782307). Animals from southern California, Oregon, and Washington are excluded from the depth comparison of B. mcnutti to B. stygius (Fig. 8).
Molecular diagnosis
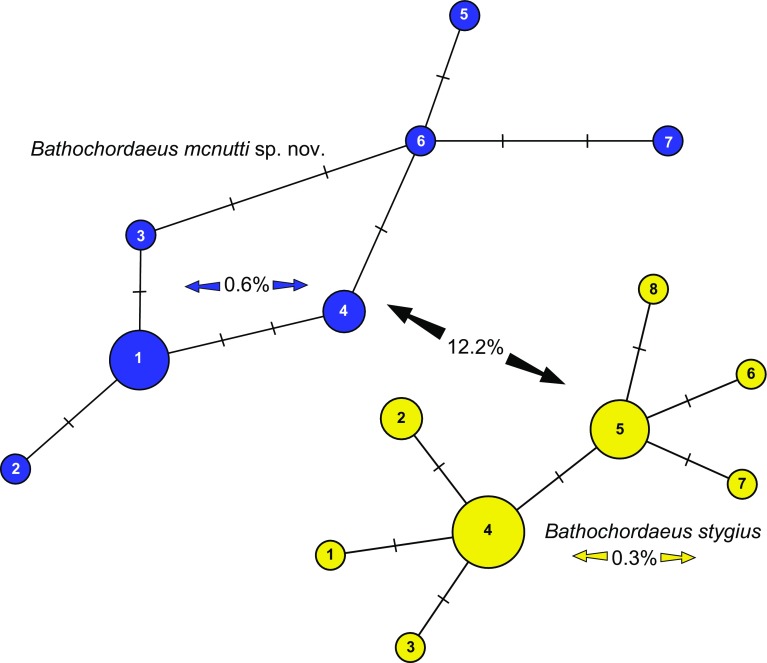
The 99.9% similarity of the 18S sequences (n = 3, one individual had one base-pair difference) confirmed what we had suspected based on morphological examinations: that blue-tailed giant larvaceans belong within the genus, Bathochordaeus. However, the 400-bp partial COI gene obtained from 17 individuals of B. stygius, and 11 individuals of B. mcnutti was more distinct. The genetic differences for COI between B. mcnutti and B. stygius are significantly greater than the haplotype differences found within each species (Fig. 9). For COI, the sequence difference between species averaged 12.2%. In comparison, the B. mcnutti haplotypes differed by an average of 0.6% within the group of 11 individuals, and the B. stygius haplotypes differed by an average of 0.3% for 17 individuals (Fig. 9).
Fig. 9.
Molecular data support morphological observations of the differences between Bathochordaeus mcnutti sp. nov. and B. stygius. For the 400-base-pair region of the COI gene, the sequence difference between B. mcnutti (n = 11) and B. stygius (n = 17) averaged 12.2%. In comparison, the B. mcnutti haplotypes differed by an average of 0.6% within the 11 individuals, and B. stygius by only 0.3%
Discussion
Larvaceans are among the most common zooplankton, and their importance to the epipelagic, mesopelagic and benthic environments of the world ocean has been described in some detail (Alldredge 1981; Deibel 1988; Hamner and Robison 1992; Steinberg et al. 1994; Gorsky and Fenaux 1998; Hopcroft and Robison 1999; Robison 2004; Robinson et al. 2010). Larvaceans can also be among the most abundant mesozooplankton found as prey in the guts of many animals from medusae to fish (Gorsky and Fenaux 1998; Purcell et al. 2005). As they feed, small larvaceans like Oikopleura dioica filter relatively large quantities of water, concentrating food particles in the submicron range and making those particles more readily available to larger animals in the form of fecal pellets (Deibel 1998; Wilson et al. 2013). Alldredge (1977) showed that larger oikopleurids filtered larger prey and that relatively large oikopleurids like Stegosoma magnum can filter 20 L of water or more per day, enough water to have significant grazing impact. With trunk lengths of 2–3 cm, the largest B. mcnutti we have collected (Fig. 3) are close to an order of magnitude larger than the S. magnum studied by Alldredge (1977). Calculations made by Morris and Deibel (1993) showed that filtration rates of oikopleurids are a function of tail length, width, and beat frequency, from which Silver et al. (1998) calculated that Bathochordaeus stygius could filter approximately 250 l day−1. The mouth of an adult Bathochordaeus varies in size from approximately 1–2 mm in diameter and limits the size of the largest particles on which these animals can feed. Whether Bathochordaeus spp. are also able to feed upon submicron-sized particles is not known.
It is important to remember that some of the particles in the water filtered by oikopleurids remain on their filters, or “houses” (Gorsky et al. 1984). These particles include diatoms, ciliates, protozoans, fecal pellets, bacteria, as well as small invertebrates like copepods (Steinberg et al. 1994; Gorsky and Fenaux 1998; Silver et al. 1998). When abandoned, the discarded houses provide a ready way to rapidly transport carbon to the seafloor. However, because of their diaphanous nature, they are not well sampled by traditional methods such as sediment traps (Steinberg et al. 1994; Silver et al. 1998; Robison et al. 2005). We have observed hundreds of discarded houses, or “sinkers,” from Bathochordaeus spp. in the water column as well as on the seafloor, at times being consumed by a diverse group of animals such as ctenophores, munnopsid isopods, holothurians, and even the archaic cephalopod, Vampyroteuthis infernalis (Hoving and Robison 2012).
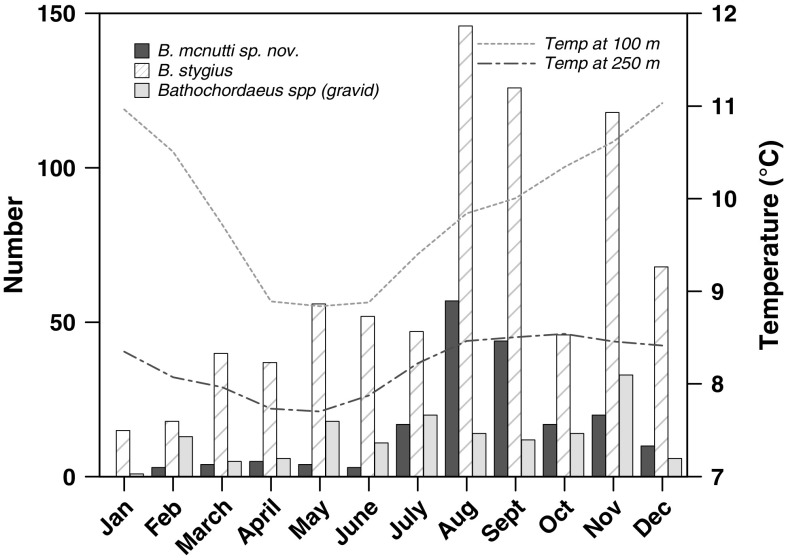
Hydrography likely controls the presence/absence of B. mcnutti in Monterey Bay, where they occur only during part of the year. They begin to arrive in the summer and disappear in winter (Fig. 10). This timing corresponds to the Oceanic season, which occurs after spring upwelling weakens and the California Current moves onshore, often into Monterey Bay, but before the winter storm (Davidson) season begins (Pennington and Chavez 2000; Collins et al. 2003). As upwelling declines, warmer offshore surface waters ebb back toward the coast, perhaps bringing B. mcnutti with them. Blue-tailed larvaceans subsequently seem to disappear from Monterey Bay in the winter months and that timing coincides approximately with the onset of the northward-flowing Davidson Current sometime in November or December (Pennington and Chavez 2000). Bathochordaeus mcnutti may be a species that is found in warmer waters. We have not observed any during surveys made off the coast of Oregon but have recorded one in infrequent surveys made off southern California waters. More extensive sampling will need to be done to determine the geographical range of B. mcnutti.
Fig. 10.
A twenty-three-year time series of collections and observations compressed into one calendar year. Bathochordaeus mcnutti sp. nov. is usually present in Monterey Bay for approximately 6 months. Typically, they begin to arrive in the summer as spring upwelling weakens and disappear sometime before the winter storm (Davidson) season begins. The abundance of both species peaks late summer to fall (September–November), but B. stygius tends to occur in higher numbers than B. mcnutti the rest of the year. Temperature data (averaged over the 23-year time series) show the cessation of upwelling during the summer, coincident with the highest abundance of B. mcnutti. The highest number of gravid individuals (both species) has been observed in late fall
Bathochordaeus stygius is also present in Monterey Bay during the fall and other mesopelagic fauna, micro- and macrozooplankton, have been linked to this biologically productive time (Robison et al. 1998; Kudela et al. 2008; Checkley and Barth 2009). However, B. stygius occurs in Monterey Bay throughout most of the year, perhaps because they live predominantly below (Fig. 9) the extent of the California Current, which shoals as it approaches shore (Castro et al. 2001).
Hydrography may also play a role in the life cycle of giant larvaceans and their seemingly unique (as a genus) habit of descending hundreds of meters while gravid (Fig. 8). Very little is known in general about the life histories of larvaceans, but their populations are generally thought to be tuned to event-scale (days to weeks), rather than seasonal-scale changes in environment (Deibel and Lowen 2012). Bathochordaeus spp., with their large size and growth to adulthood in water averaging <10 °C, may be an exception. Larvaceans are not known to have resting eggs or stages that over-winter, means by which some invertebrate taxa are able to survive lean times when their food is scarce (Strathmann 1987; Gorsky and Fenaux 1998). Over 23 years, more individuals of both species have been observed in the fall in Monterey Bay. These animals are large relative to specimens collected earlier in the year, and often they are gravid (Fig. 10). Presumably, they have been feeding and growing throughout the upwelling season and into the Oceanic season. Descending to colder waters where gametes will develop more slowly, before ascending to finally spawn, may be a reproductive strategy similar to over-wintering in that it could allow Bathochordaeus spp. to avoid some of the pitfalls of the Davidson season, with its northward, onshore, downwelling current, and relatively nutrient-poor waters (Pennington and Chavez 2000; Collins et al. 2003).
Molecular data support the morphological findings in this study. Mitochondrial DNA from larvaceans (and urochordates in general) is difficult to sequence using traditional DNA barcoding primers for the cytochrome c oxidase subunit I region (Hirose and Hirose 2009; Bucklin et al. 2011); however, an increasing number of tunicate COI sequences using the degenerate primers of Hirose et al. (2009) are published in GenBank for salps and tunicates.
DNA barcoding is an important tool in the determination of biodiversity in oceanographic studies (Bucklin et al. 2010). However, largely because of the high rates of mutation and genetic simplification, tunicates in general have proven difficult to study using molecular tools (Denoeud et al. 2010). As of May 2015, there are only two larvacean species with COI sequences in GenBank, both oikopleurids: Oikopleura dioica and Oikopleura intermedia. In order to make comparisons between species of larvaceans, more sequences need to be published and made available. Because these animals are fragile and difficult to collect intact by conventional methods, access to more molecular sequences will be instrumental in identifying new species of larvaceans. Molecular data in conjunction with morphological traits, ecological data, and in situ observations will provide more opportunities for species discoveries in these abundant and ecologically important animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (M4 V 70766 kb)
Acknowledgements
The careful collections made by our ROV pilots and ships’ crews made this paper possible, and we are grateful to them. For assistance at sea and/or in the laboratory, we thank Kim Reisenbichler, Russell Hopcroft, Tim Pennington, Kurt Buck, Lynne Christianson and Nancy Burnett. Ann Bucklin provided helpful comments regarding barcoding and primers. This work was funded through the generous support of the David and Lucile Packard Foundation.
Compliance with ethical standards
Conflict of interest
Robert Sherlock declares that he has no conflict of interest. Kristine Walz declares that she has no conflict of interest. Kyra Schlining declares that she has no conflict of interest. Bruce Robison declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Human and animal rights
This article does not contain any studies with human participants. Larvacean specimens were collected in accordance with the guidelines of the California Department of Fish and Wildlife and collecting permit SC-2506.
Footnotes
Reviewed by Undisclosed experts.
References
- Alldredge AL. House morphology and mechanisms of feeding in the Oikopleuridae (Tunicata, Appendicularia) J Zool. 1977;181:175–188. doi: 10.1111/j.1469-7998.1977.tb03236.x. [DOI] [Google Scholar]
- Alldredge AL. The impact of appendicularian grazing on natural food concentrations in situ. Limnol Oceanogr. 1981;26:247–257. doi: 10.4319/lo.1981.26.2.0247. [DOI] [Google Scholar]
- Alldredge AL. Aggregation of spawning Appendicularians in surface windrows. Bull Mar Sci. 1982;32:250–254. [Google Scholar]
- Appeltans W, Ahyong ST, Anderson G. The magnitude of global marine species diversity. Curr Biol. 2012;22:2189–2202. doi: 10.1016/j.cub.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Barham EG. Giant larvacean houses: observations from deep submersibles. Science. 1979;205:1129–1131. doi: 10.1126/science.205.4411.1129. [DOI] [PubMed] [Google Scholar]
- Bassham S, Postlethwait JH. The evolutionary history of placodes: a molecular genetic investigation of the larvacean urochordate Oikopleura dioica. Development. 2005;132:4259–4272. doi: 10.1242/dev.01973. [DOI] [PubMed] [Google Scholar]
- Bucklin A, Ortman BD, Jennings RM. A “Rosetta stone” for metazoan zooplankton: DNA barcode analysis of species diversity of the Sargasso Sea (northwest Atlantic Ocean) Deep Sea Res Pt II. 2010;57:2234–2247. doi: 10.1016/j.dsr2.2010.09.025. [DOI] [Google Scholar]
- Bucklin A, Steinke D, Blanco-Bercial L. DNA barcoding of marine metazoa. Ann Rev Mar Sci. 2011;3:471–508. doi: 10.1146/annurev-marine-120308-080950. [DOI] [PubMed] [Google Scholar]
- Bückmann A, Kapp H. Taxonomic characters used for the distinction of species of appendicularia. Mitt Hamb Zool Mus Inst. 1975;72:201–228. [Google Scholar]
- Castellanos IA, Morales-Ramírez, Suárez-Morales E (2009) Appendicularians (Urochordata). In: Marine biodiversity of Costa Rica, Central America. Springer, Netherlands, pp 445–452. doi:10.1007/978-1-4020-8278-8_41
- Castro CG, Chavez FP, Collins CA. Role of the California Undercurrent in the export of denitrified waters from the eastern tropical North Pacific. Global Biogeochem Cycles. 2001;15:819–830. doi: 10.1029/2000GB001324. [DOI] [Google Scholar]
- Checkley DM, Jr, Barth JA. Patterns and processes in the California Current system. Prog Oceanogr. 2009;83:49–64. doi: 10.1016/j.pocean.2009.07.028. [DOI] [Google Scholar]
- Chun C (1900) Aus den tiefen des weltmeeres. Gustav Fischer, Jena., pp 519–521
- Collins CA, Pennington JT, Castro CG, Rago TA, Chavez FP. The California Current system off Monterey, California: physical and biological coupling. Deep-Sea Res Pt II. 2003;50:2389–2404. doi: 10.1016/S0967-0645(03)00134-6. [DOI] [Google Scholar]
- Connor J (2005) Archiving, annotating and accessing video as data. In: Report of the national marine fisheries service workshop on underwater video analysis. pp 54
- Deibel D. Filter feeding by Oikopleura vanhoeffeni: grazing impact on suspended particles in cold ocean waters. Mar Biol. 1988;99:177–186. doi: 10.1007/BF00391979. [DOI] [Google Scholar]
- Deibel D. Feeding and metabolism of Appendicularia. The biology of pelagic tunicates. Oxford: Oxford University Press; 1998. pp. 139–149. [Google Scholar]
- Deibel D, Lowen B. A review of the life cycles and life-history adaptations of pelagic tunicates to environmental conditions. ICES J Mar Sci: J Conseil. 2012;69:358–369. doi: 10.1093/icesjms/fsr159. [DOI] [Google Scholar]
- Denoeud F, Henriet S, Mungpakdee S. Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate. Science. 2010;330:1381–1385. doi: 10.1126/science.1194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux R. Synonymie et distribution géographique des Appendiculaires. Bull Inst Océanog Mon. 1966;66:23. [Google Scholar]
- Fenaux R. The house of Oikopleura dioica (Tunicata, Appendicularia): structure and functions. Zoomorphology. 1986;106:224–231. doi: 10.1007/BF00312043. [DOI] [Google Scholar]
- Fenaux R. The classification of Appendicularia (Tunicata): history and current state. Bull Inst Océanog Mon. 1993;17:124. [Google Scholar]
- Fenaux R. Anatomy and functional morphology of the Appendicularia. In: Bone Q, editor. The biology of pelagic tunicates. Oxford: Oxford University Press; 1998. pp. 25–34. [Google Scholar]
- Fenaux R. Life history of the Appendicularia. In: Bone Q, editor. The biology of pelagic tunicates. Oxford: Oxford University Press; 1998. pp. 151–159. [Google Scholar]
- Fenaux R, Bone Q, Deibel D. Appendicularian distribution and zoogeography. In: Bone Q, editor. The biology of pelagic tunicates. Oxford: Oxford University Press; 1998. pp. 251–264. [Google Scholar]
- Flood PR. Toward a photographic atlas on special taxonomic characters of oikopleurid Appendicularia (Tunicata) In: Gorsky G, Youngbluth M, Deibel D, editors. Response of marine ecosystems to global change: Ecological impact of appendicularians. Paris: Contemporary Publishing International; 2005. pp. 59–85. [Google Scholar]
- Flood PR, Deibel D. The appendicularian house. The biology of pelagic tunicates. Oxford: Oxford University Press; 1998. pp. 105–124. [Google Scholar]
- Folmer RH, Folkers PJ, Kaan A. Secondary structure of the single-stranded DNA binding protein encoded by filamentous phage pf3 as determined by NMR. Eur J Biochem. 1994;224:663–676. doi: 10.1111/j.1432-1033.1994.00663.x. [DOI] [PubMed] [Google Scholar]
- Galt CP, Mackie GO. Electrical correlates of ciliary reversal in Oikopleura. J Exp Biol. 1971;55:205–212. [Google Scholar]
- Garstang W (1936) On a new appendicularian, Bathochordaeus sp., from Bermuda, with a revision of the genus. [Abstract.]. In: P Linn Soc London. pp 131–132
- Garstang W. On the anatomy and relations of the appendicularian Bathochordaeus, based on a new species from Bermuda (B. stygius, sp. N.) J Linn Soc Lond Zool. 1937;40:283–303. doi: 10.1111/j.1096-3642.1937.tb01683d.x. [DOI] [Google Scholar]
- Gorsky G, Fenaux R. The role of Appendicularia in marine food webs. The biology of pelagic tunicates. Oxford: Oxford University Press; 1998. pp. 161–169. [Google Scholar]
- Gorsky G, Fisher NS, Fowler SW. Biogenic debris from the pelagic tunicate, Oikopleura dioica, and its role in the vertical transport of a transuranium element. Estuar Coast Shelf S. 1984;18:13–23. doi: 10.1016/0272-7714(84)90003-9. [DOI] [Google Scholar]
- Hamner WM, Robison BH. In situ observations of giant appendicularians in Monterey Bay. Deep-Sea Res. 1992;39:1299–1313. doi: 10.1016/0198-0149(92)90070-A. [DOI] [Google Scholar]
- Hirose M, Hirose E. DNA barcoding in photosymbiotic species of Diplosoma (Ascidiacea: Didemnidae), with the description of a new species from the southern Ryukyus, Japan. Zool Sci. 2009;26:564–568. doi: 10.2108/zsj.26.564. [DOI] [PubMed] [Google Scholar]
- Hirose E, Oka AT, Hirose M. Two new species of photosymbiotic ascidians of the genus Diplosoma from the Ryukyu Archipelago, with partial sequences of the COI gene. Zool Sci. 2009;26:362–368. doi: 10.2108/zsj.26.362. [DOI] [PubMed] [Google Scholar]
- Hopcroft RR (2005) Diversity in larvaceans: How many species? In: Gorsky G, Youngbluth M, Deibel D (eds) Response of marine ecosystems to global change: ecological impact of appendicularians. Contemporary Publishing International, pp 45–57
- Hopcroft RR, Robison BH. A new mesopelagic larvacean, Mesochordaeus erythrocephalus, sp. nov., From Monterey Bay, with a description of its filtering house. J Plankton Res. 1999;21:1923–1937. doi: 10.1093/plankt/21.10.1923. [DOI] [Google Scholar]
- Hoving HJ, Robison BH. Vampire squid: detritivores in the oxygen minimum zone. Proc Biol Sci. 2012 doi: 10.1098/rspb.2012.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudela RM, Banas NS, Barth JA. New insights into the controls and mechanisms of plankton productivity in coastal upwelling waters of the Northern California Current system. Oceanography. 2008;21:46–59. doi: 10.5670/oceanog.2008.04. [DOI] [Google Scholar]
- Lohmann H. Das gehäuse der appendicularien: sein bau, seine funktion und seine entstehung. Schriften des naturwissenschaftlichen vereins fur Schleswig-Holstein. 1898;11:347–407. [Google Scholar]
- Lohmann H. Die Appendicularien der Valdivia expedition. Verh Dtsch Zool Ges. 1914;24:157–192. [Google Scholar]
- Lombard F, Selander E, Kiørboe T. Active prey rejection in the filter-feeding appendicularian Oikopleura dioica. Limnol Oceanogr. 2011;56:1504–1512. doi: 10.4319/lo.2011.56.4.1504. [DOI] [Google Scholar]
- Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16 s-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Morris CC, Deibel D. Flow rate and particle concentration within the house of the pelagic tunicate Oikopleura vanhoeffeni. Mar Biol. 1993;115:445–452. doi: 10.1007/BF00349843. [DOI] [Google Scholar]
- Paffenhöfer G-A. The cultivation of an appendicularian through numerous generations. Mar Biol. 1973;22:183–185. doi: 10.1007/BF00391782. [DOI] [Google Scholar]
- Pennington JT, Chavez FP. Seasonal fluctuations of temperature, salinity, nitrate, chlorophyll and primary production at station H3/M1 over 1989–1996 in Monterey Bay, california. Deep-Sea Res Pt II. 2000;47:947–973. doi: 10.1016/S0967-0645(99)00132-0. [DOI] [Google Scholar]
- Pimm S. Biodiversity: not just lots of fish in the sea. Curr Biol. 2012;22:R996–R997. doi: 10.1016/j.cub.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Purcell JE, Sturdevant MV, Galt CP, Gorsky G, Youngbluth MJ, Deibel D. A review of Appendicularians as prey of invertebrate and fish predators. Response of marine ecosystems to global changes: ecological impact of appendicularians. Paris: Contemporary Publishing International; 2005. pp. 359–435. [Google Scholar]
- Robinson C, Steinberg DK, Anderson TR. Mesopelagic zone ecology and biogeochemistry: a synthesis. Deep-Sea Res Pt II. 2010;57:1504–1518. doi: 10.1016/j.dsr2.2010.02.018. [DOI] [Google Scholar]
- Robison BH. Midwater research methods with MBARI’s ROV. Mar Technol Soc J. 1993;26:32–39. [Google Scholar]
- Robison BH. Deep pelagic biology. J Exp Mar Biol Ecol. 2004;300:253–272. doi: 10.1016/j.jembe.2004.01.012. [DOI] [Google Scholar]
- Robison BH, Reisenbichler KR, Sherlock RE, Silguero JMB, Chavez FP. Seasonal abundance of the siphonophore, Nanomia bijuga, in Monterey Bay. Deep-Sea Res Pt II. 1998;45:1741–1751. doi: 10.1016/S0967-0645(98)80015-5. [DOI] [Google Scholar]
- Robison BH, Reisenbichler KR, Sherlock RE. Giant larvacean houses: rapid carbon transport to the deep sea floor. Science. 2005;308:1609–1611. doi: 10.1126/science.1109104. [DOI] [PubMed] [Google Scholar]
- Robison BH, Sherlock RE, Reisenbichler KR. The bathypelagic community of Monterey Canyon. Deep-Sea Res Pt II. 2010;57:1551–1556. doi: 10.1016/j.dsr2.2010.02.021. [DOI] [Google Scholar]
- Schlining BM, Stout NJ. MBARI’s video annotation and reference system. OCEANS. 2006;2006:1–5. [Google Scholar]
- Sherlock RE, Walz KR, Robison BR. The first definitive record of the giant larvacean, Bathochordaeus charon, since its original description in 1900 and a range extension to the northeast Pacific Ocean. Mar Biodivers Rec. 2016;9(79):1–10. [Google Scholar]
- Silver MW, Coale SL, Pilskaln CH, Steinberg DR. Giant aggregates: importance as microbial centers and agents of material flux in the mesopelagic zone. Limnol Oceanogr. 1998;43:498–507. doi: 10.4319/lo.1998.43.3.0498. [DOI] [Google Scholar]
- Steinberg DK, Silver MW, Pilskaln CH, Coale SL, Paduan JB. Midwater zooplankton communities on pelagic detritus (giant larvacean houses) in Monterey Bay, California. Limnol Oceanogr. 1994;39:1606–1620. doi: 10.4319/lo.1994.39.7.1606. [DOI] [Google Scholar]
- Strathmann MF. Reproduction and development of marine invertebrates of the northern Pacific Coast: data and methods for the study of eggs, embryos, and larvae. Seattle: University of Washington Press; 1987. p. 670. [Google Scholar]
- Wilson SE, Ruhl HA, Smith KL., Jr Zooplankton fecal pellet flux in the abyssal northeast Pacific: a 15 year time-series study. Limnol Oceanogr. 2013;58:881–892. doi: 10.4319/lo.2013.58.3.0881. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (M4 V 70766 kb)